INTRODUCTION
Telomeres form the ends of eukaryotic chromosomes consisting of an array of tandem repeats of the hexanucleotide 5’-TTAGGG-3’. Their functions are protecting the ends of chromosomes against exonucleases and ligase, preventing the activation of DNA-damage checkpoints, and countering the loss of terminal DNA segments that occur during linear DNA is replicated[1,2]. Telomerase, a ribonucleoprotein enzyme, add the hexanucleotides to the ends of replicating chromosomes[3]. It is believed that telomerase plays a crucial role in cellular senescence and immortalization[3-5]. Telomerase is strongly repressed in most human somatic cells, and telomeres shorten progressively with each cell division[6]. Most cancer cells express telomerase activity[3,6-9]. The restoration of telomerase activity is considered to immortalize cells and to be an important step in carcinogenesis. Inhibition of telomerase can lead to telomere shorting and tumor cell death. Thus, specific inhibition of human telomerase is suggested to be an efficient means of tumor therapy. Nowadays, a limited number of means can be used experimentally to inhibit the activity of human telomerase including chemical agents[10], antisence oligonucleotide[11,12], peptide nucleic acid[13], and ribozyme[14-16]. Telomerase is composed of at least three subunits[17]. The RNA subunit and the catalytic subunit are the essential components for telomerase activity[18]. The RNA subunit of telomerase serves as the template for addition of short sequence repeats to the chromosome 3’ends[19]. And the catalytic subunit, telomerase reverse transcriptase (TERT), is the most important component in telomerase complex which is responsible of catalytic activity of telomerase. The expression of TERT correlates with the presence of telomerase activity[20]. These studies suggest that hTERT is a good target for cancer gene therapy.
Ribozymes are sis- or trans-acting, sequence-specific catalytic RNA molecules. Based on the studies about natural ribozymes, we can design site-specific trans-acting ribozymes to suppress the expression of genes by recognize and splice the mRNA. Hammerhead ribozyme and hairpin ribozyme are intensively studied because of their simple structures and dual activity of splicing as well as blocking[21]. Ribozymes may surpass the efficiency of the antisence oligonucleotide and are considered a promising means of gene therapy[22,23]. In this study, we designed a hammerhead ribozyme targeting hTERT and cloned the gene encoding the ribozyme for future study of this ribozyme in the treatment of malignancies.
MATERIALS AND METHODS
Materials
Plasmid vector pGEMEX-1, X-gal and IPTG were Promega products. T4 DNA ligase, Klenow fragment and restriction endonucleases used in this study were purchased from Sino-America Biotechnology Co.
Design of the hammerhead ribozyme
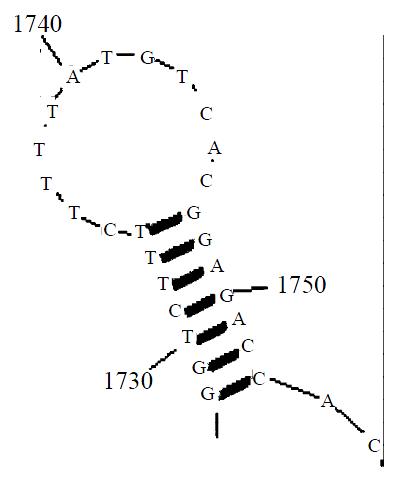
Using the software RNAstructure 3.6 (mFOLD for windows version), the full-length telomerase mRNA (GenBank NM003219) was analyzed and the second structure of telomerase mRNA was predicted. Since trans-acting hammerhead ribozymes preferentially recognize and cleave the GUC sequence, all the fragments containing GUC were candidate target sequences for ribozyme binding and cleavage. Considering the predicted local conformation near the GUC triplets and the nucleotide sequences flanking the GUCs, triplet GUC located at 1742 of the hTERT mRNA was chosen as the target site of ribozyme. According to the rules of hammerhead ribozyme design suggested by Haseloff et al[24], the ribozyme was primarily designed. Then, bimolecular fold between the ribozyme and substrate RNA was predicted and modification of the ribozyme sequence was made according to the prediction to improve the binding between the ribozyme and the substrate. The hammerhead ribozyme designed was comprised of 39 nucleotides, including 22 nt catalytic core and 17 nt (5’ 7 nt and 3’ 10 nt) flanking antisence sequence. The sequence is 5’-TCTCCGTCTGATGAGTCCGTGAGGACGAAACATAAAAGA-3’ (The underlined are nucleotides complementary to the substrate).
Synthesis of the ribozyme gene
DNA synthesis was carried out by Shanghai Sangon Biotechnology Co. LTD. with 3900 DNA autosynthesis instrument. The primers used for the ribozyme were as follows: 5’-CCCAAGCTTCTCCGTCTGATGAGTCCGTGAGGACGAAAC-3’ (strand A) and 5’-GGAATTCATCGATAGATCTTTTATGTTTCGTCCTCA-3’ (strand B). Twelve nucleotides from the 3’ends of the two strands were complementary to each other. A Hind III restriction site was introduced into the 5’ end of strand A. And restriction sites of Bgl II, ClaIand EcoR I were introduced into the 5’ end of strand B. Equal molecule of the two strands were mixed and annealed to form a hemiduplex. The hemiduplex was extended with Klenow fragment to form full duplexes. The reaction mixture contained 50 mM Tris-HCl (pH7.2), 10 mM MgSO4, 0.1 mM DTT, 0.4 mM dNTP mixture, 4 μg each of the two single strand oligonucleotides, 20 U Klenow fragment in a final volume of 50 μL. The reaction was carried out at 37 °C for an hour. The reaction mixture was extracted with phenol-chloroform and precipitated with sodium acetate ethanol (The methods referred to Sambrook et al[25]).
Cloning of ribozyme gene
The double strand DNA was digested with Hind III and EcoR I. The product was electrophoresed in 2% agrose for purification followed by electroelution in a dialysis bag for DNA recovery. The recovered fragment was inserted into the Hind III/EcoR I site of plasmid vector pGEMEX-1 with T4 DNA ligase. E. coli JM109 was transformed with the recombinant plasmid and cultured on a solid medium containing ampicillin, X-gal and IPTG. Six white colonies were picked and plasmid was extracted by alkaline lysis. The plasmid was digested by Hind III, Not I, EcoR I, Bgl II, respectively. Endonuclease digests of plasmid DNA were analyzed on 1% agarose. DNA sequencing was carried out to further confirm the inserted sequence of ribozyme gene (The methods referred to Sambrook et al[25]).
RESULTS
Selection of cleavage site and design of the ribozyme
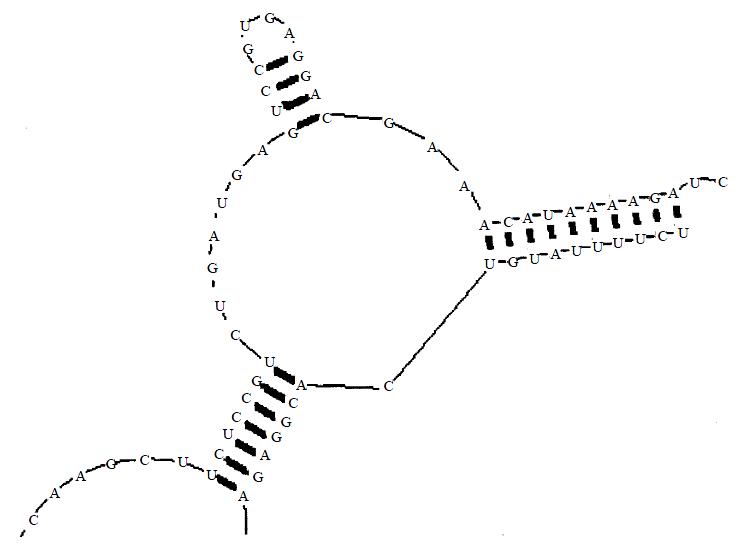
Aided by computer prediction of RNA secondary structure, we selected the triplex GUC situated at 1742 of the hTERT mRNA as the cleavage site (Figure 1). The ribozyme could co-fold with the substrate RNA to form a desired conformation in computer-aided prediction (Figure 2).
Figure 1 Predicted second structure of the substrate RNA surrounding the selected cleavage site
Figure 2 Predicted bimolecular co-fold between the hammer-head ribozyme and the substrate RNA
Analysis of the transformants
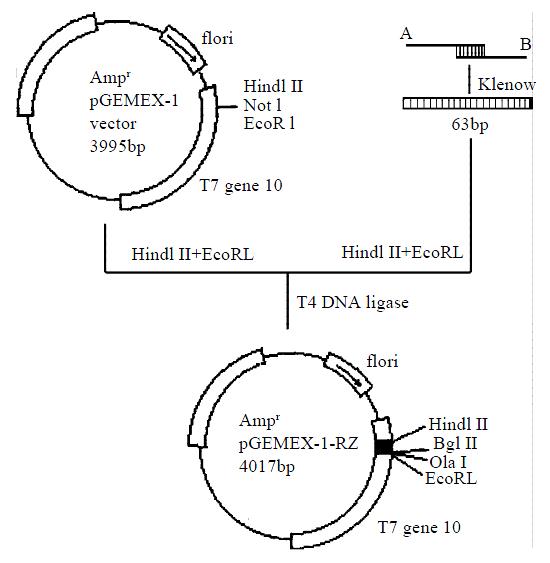
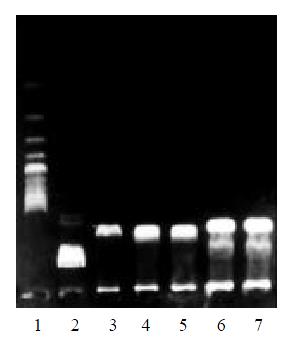
Six white colonies were picked. All the plasmids were cleaved into two fragments (1.0 kb + 3.0 kb) by Bgl II. The plasmid from colony number 6 was digested with Hind III, Not I and EcoR I respectively. The plasmid DNA was linearized by HindIII or EcoR I but not by Not I. This result suggested plasmid from colony number 6 was a correct recombinant (Figure 3, Figure 4).
Figure 3 Diagram of the cloning of the hammerhead ribozyme
Figure 4 Gel analysis of the plasmid containing the ribozyme gene.
Lane 1: 200 bp DNA ladder; lane 2: λDNA/Hind III marker; lane 3: pGEMEX-1-RZ/Bgl II; lane 4: pGEMEX-1-RZ/Hind III; lane 5: pGEMEX-1-RZ/EcoR I; lane 6: pGEMEX-1-RZ/Not I; lane 7: pGEMEX-1-RZ
DNA sequencing
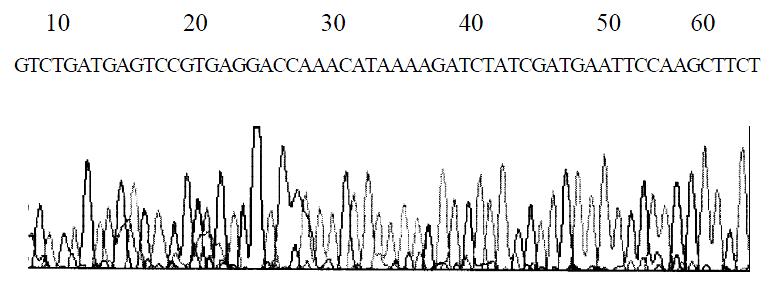
The DNA sequencing of the plasmid DNA from colony number 6 confirmed that the ribozyme gene was correctly inserted into the vector pGEMEX-1. This recombinant was named pGEMEX-1-RZ. The diagram of DNA sequencing is shown in Figure 5.
Figure 5 DNA sequencing of ribozyme gene
DISCUSSION
A hammerhead ribozyme is composed of a 22 nt or 23 nt catalytic core and the flanking complementary sequences. The catalytic core determines the cleavage activity of the ribozyme. And the nucleotide sequence of the catalytic core is conserved. Whereas the flanking sequences determine the recognition specificity of the ribozyme[24]. The catalytic mechanisms of hammerhead ribozyme and related factors have been intensively studied[26]. Hammerhead ribozyme can cleave the triplet sequence NUH (N is anyone of A, U, C or G, and H could be C, U or A), but the triplet GUC is most efficiently cleaved[27]. The length of complementary sequence also influences the catalytic activity. Short complementary sequence will decrease the recognition specificity of the ribozyme and the annealing between ribozyme and substrate. In contrary, long complementary sequence will influence the dissociation of ribozyme from the substrate and hence the turnover of the ribozyme. Commonly, the proper length of each of the flanking sequence is 6-8 nt[28,29] although some studies suggested that longer antisense flanking arms have higher intracellular cleavage efficacy[30,31]. This opinion is also supported by the estimation that stretches of 11-15 nucleotides define unique sequences for cellular RNA[32]. It was reported that asymmetric ribozymes (longer helix III, shorter helix I) have higher cleavage activity than symmetric ribozyme[33]. In this study, we designed a asymmetric hammerhead ribozyme which had 7 nt helix Iand 10 nt helix III.
The premise of ribozyme cleavage is the base pairing between flanking sequence of the ribozyme and the substrate mRNA[34]. This step is a kin to the antisense oligodeoxyribonucleotides used for the same purpose. And the base pairing is determined by the secondary structure of the target site, Triplet NUH situated in a loop, protruding or linker of the substrate is advantageous to the annealing between ribozyme and substrate and then the cleavage of the substrate RNA by the ribozyme[35]. The selection of the cleavage sites is most important in the design of ribozymes. Nowadays, two approaches are available in the selection of the cleavage sites of ribozymes. One approach is to use in vitro accessibility assays. The other is to use theoretical prediction, that is, computer-aided prediction. A number studies aimed to compare the two approaches in their consistency have suggested that theoretical prediction is positively correlate with the intracellular accessibility of the target sites in mRNA[35-37]. So we performed computer-aided analysis of the secondary structure of the substrate RNA. mFOLD is a commonly used software for RNA secondary structure[38], so we used mFOLD to predict the secondary structure of hTERT mRNA previous to designing the ribozyme. We choose 1742 GUC as the cleavage site of ribozyme in total 57 triplet GUC in hTERT mRNA. The reasons included: (1) 1744 triplet RNA is located in coding region of an essential motif of telomerase activity-T motif, and T motif is unique to telomerase in all kinds of reverse transcriptase[39]. (2) 1742 triplet GUC is situated in a rather large loop of the predicted secondary structure of telomerase RNA where comparatively more unpaired bases are present near the cleavage triplet. It was reported this kind of structure was correlated with the hybridization accessibility for hammerhead ribozymes[35]. (3) The sequence surrounding 1742 triplet GUC is rich of adenosine and uridine. It is reported that for efficient catalytic turnover, the free energy of the ribozyme-substrate duplex should be less than -16 kal/mol, that is, higher ratio of A + U will be advantageous to the annealing between ribozyme and substrate[28]. Next, we performed a computer-aided bimolecular fold prediction between the ribozyme and the substrate and modified the complementary sequences until the ribozyme and the substrate can co-fold into a correct conformation in computer-aided prediction.
Commonly, cloning of short double-strand DNA is carried out by synthesizing the full-length sense strand and antisense strand, annealing and then inserting into a cloning vector. In this study, both the sense strand and antisense strand synthesized were partial of the ribozyme gene which were 12 nucleotides complementary to each other on the 3’ends. After annealing to form a hemiduplex, the hemiduplex was extended with Klenow fragment to obtain full-length double-strand DNA. The reliability of this method is proven by the present study. The advantages of this method including comparative simplicity, higher precision and lower cost to synthesize shorter single-strand DNA.
Since the hammerhead ribozyme gene is only about 50 bps in length, primary analysis of the recombinant relies on PAGE conventionally. Because PAGE is comparatively complex, in this study, we introduced Bgl IIand ClaIrestriction sites to the 3’end of the ribozyme gene for the convenience of restriction analysis. As shown in figure, correct recombinants can be linearized by ClaIas well as cleaved into two fragments(1.0 kb + 3.0 kb) by Bgl II. Also, because Not I restriction site in pGEMEX-1 had been removed, correct recombinant could not be cleaved by Not I. Then agarose electrophoresis can be used to analyze the recombinants. One of the six recombinants selected by this strategy was sequenced, and the sequence of the inserted ribozyme gene was confirmed. The result suggests that this strategy is applicable. The recombinant plasmid pGEMEX-1-RZ also would be used as an in vitro transcription vector to test the in vitro cleavage activity of the ribozyme. The recovered 2.0 kb fragment after Bgl II restriction would be used as the transcription template. The transcribed product would have two additional nucleotides on the 3’end of the ribozyme, but these two additional nucleotides have no influence on the secondary structure of the ribozyme predicted by computer.
Design and cloning of a ribozymes are the essential work of studies about ribozymes. In this study, we designed and cloned the anti-hTERT ribozyme successfully. Next, we will test the in vitro cleavage activity of the ribozyme and will investigate the growth inhibition effect of this ribozyme on colonic tumor cell lines.