Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.1050
Revised: May 30, 2002
Accepted: June 3, 2002
Published online: December 15, 2002
AIM: To evaluate the function of the longer transcripts LPTS-L in hepatocellular carcinoma cell line SMMC-7721.
METHODS: SMMC-7721 cells were transfected with LPTS-L expression construct and stably transfected cells were selected by G418. Multiple single clones formed and were checked for their phenotype. In the study of the effect on telomerase activity of LPTS-L in vitro, GST-LPTS-L fusion protein was expressed in E.coli and purified by glutathione-agarose column. Telomeric repeat amplification protocol (TRAP) assays were performed to study the influence of telomerase activity in SMMC-7721 cells.
RESULTS: Over-expression of LPTS-L induced SMMC-7721 cells into crisis. LPTS-L could inhibit the telomerase activity in SMMC-7721 cells in vitro.
CONCLUSION: LPTS-L is a potent telomerase inhibitor. Over-expression of LPTS-L can induce hepatoma cells into crisis due to the reduction of telomerase activity.
- Citation: Liao C, Zhao MJ, Zhao J, Jia D, Song H, Li ZP. Over-expression of LPTS-L in hepatocellular carcinoma cell line SMMC-7721 induces crisis. World J Gastroenterol 2002; 8(6): 1050-1052
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/1050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.1050
Telomeres are a specialized structure composed of deoxyribonucleic acid (DNA) with associated proteins in the ends of chromosomes in the nuclei of all eukaryotic organisms[1]. Telomeric DNA consists of long head-to-tail arrays of repeated DNA sequences and the repetitive hexanucleotide in human is TTAGGG[2]. Telomeres provide a mechanism to compensate for under-replication of the linear DNA ends, distinguish true chromosome ends from breaks in DNA, and protect chromosome ends from fusing with others or broken chromosomes. In some cells, telomeres also control the positions of chromosomes within the nucleus[3,4].
Telomerase, a special reverse transcriptase enzyme,extends the telomeric DNA using intrinsic RNA template and helps stabilization of telomere length in human stem cells, reproductive cells and cancer cells[5-7]. Telomerase activity has been found in almost all human tumors, while in most somatic tissues, including the liver, it cannot be detected, and telomere length is shortened by 50-200 nucleotides with each cell division[8-10].
The telomere and telomerase serve as a kind of clock controlling life span, which forms the telomere and telomerase hypothesis of cell aging and tumorigenesis. The prominent hypothesis states that a signal is initiated to cease cell dividing and induce cell senescence when the telomere is shortened with cell doubling and reach to a certain threshold level. The maintenance of telomere stability is required for the long-term proliferation of tumor cells.Thus, escaping from cell senescence and becoming immortal by activating telomerase, or an alternative mechanism to maintain telomeres, constitutes an additional step in tumorigenesis[8,9].
LPTS gene is a human novel liver-related putative tumor suppressor gene (LPTS), identified by our laboratory[10]. It is located in chromosome 8p23, a hot spot of tumor suppressor in hepatocellular carcinoma (HCC) with a high frequency of loss of heterozygosity (LOH). LPTS gene is transcribed to two isoforms of mRNA. The longer transcript, referred to as LPTS-L, encodes a 328-a.a. protein and is down-regulated in HCC cell lines and tissues at both RNA and protein levels (unpublished data). LPTS-L is highly homologous with PinX1, identified as a telomerase inhibitor recently[12], with quite different sequences in 3’ untranslational region.
In this study, we determined the function of LPTS-L through transfecting the LPTS-L into hepatoma cell line SMMC-7721, and analyzing its effect of cell proliferation and morphology. We also detected the effect of LPTS-L to the telomerase activity. The results clearly showed that LPTS-L is a potent telomerase inhibitor and could induce hepatoma cells into crisis.
The previously characterized hepatoma cell line SMMC-7721 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI medium 1640 (Life Technologies Inc.) plus 10% new-born calf serum at 37 °C at a 5% carbon dioxide atmosphere in a humidified incubator.
The coding region of LPTL was subcloned into eukaryotic expression vector pcDNA3 ( Invitrogen, Inc., Carlsbad CA) and constructed pcDNA3-LPTS-L. The plasmids were purified with QIAGEN Plasmid Kit (QIAGEN Inc.) and transfected with 2 mg of plasmid with LipofectAMIN (Life Technologies, Inc.) according to the manufacturer’s protocols. Twenty-four hours after transfection, G418 (Life Technologies, Inc.) was added to the medium at a final concentration of 700 mg/L to kill untransfected cells.
The coding sequence of LPTS-L was cloned in frame into the E.coli GST fusion expression vector pGEX-4T2 (Amersham Pharmacia Biotech Inc.), then the construct was transfected into E.coli BL21(DE3). The protein was expressed with IPTG induction for 3 h and purified by glutathione-agarose (Sigma) column.
HCC cells SMMC-7721were lysed in lysis buffer (10 mM TrisHCl pH 7.5, 1 mM MgCl2, 1 mM EGTA, 0.1 mM PMSF, 5 mM 2-mercaptoethanol, 0.5% CHAPS, 10% glycerol) on ice for 30 min and centrifuged at 13000 rpm for 30 min, the telomerase was contained in the suspension of cell extract. The protein of LPTS-L was incubated with cell extract for 10 min at 4 °C before subjecting to telomerase extension according the reference[11]. Telomerase products were separated on 10% polyacrylamide gels, which were stained with silver.
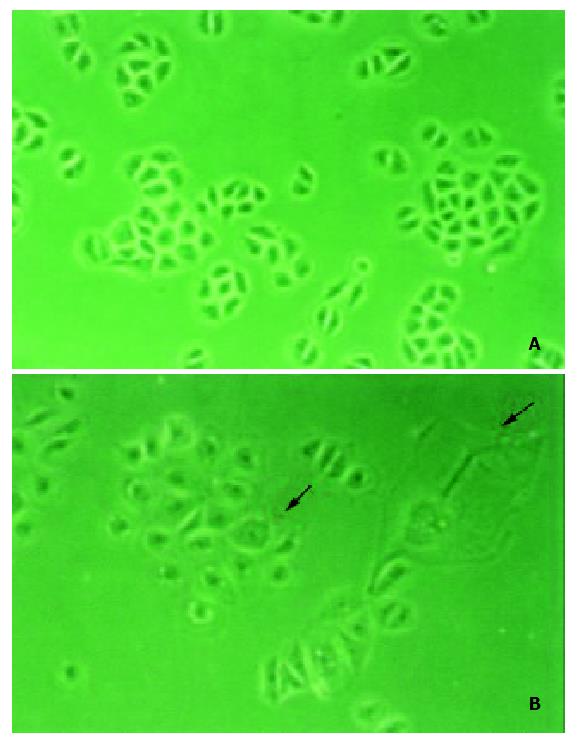
To investigate the function of LPTS-L in hepatoma cells, 987-bp coding region of LPTS-L was subcloned into pcDNA3 eukaryotic expression vector to construct pcDNA3-LPTS-L, then the construct was transfected into SMMC-7721 cell lines, with pcDNA3 vector as control. The growth of hepatoma cells transfected with LPTS-L became slow, possibly due to an increase rate of cell death, as compared with control. In most cases, the cells transfected with LPTS-L exhibited increased size and flattened-morphology, with the characteristics associated with crisis (Figure 1).
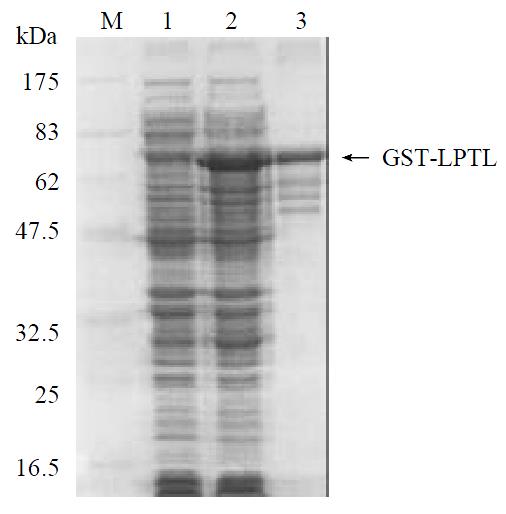
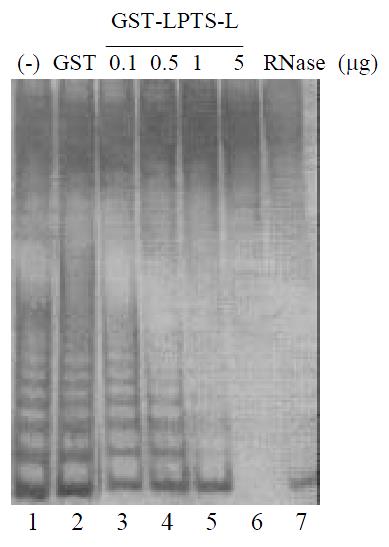
To study the mechanism of the induction of the hepatoma cells into crisis by LPTS-L, we used telomerase activity assay in vitro to determine the correlation between LPTS-L protein and telomerase. Firstly, we subcloned the coding sequence of LPTS-L into GST fusion protein expression vector in frame and purified the GST-LPTS-L fusion protein by affinity chromatography (Figure 2). The purity of the fusion protein was enough for the next telomerase activity assay. We next added the GST-LPTS-L protein into the cell extract of SMMC-7721 and then performed the TRAP assay. The results showed clearly that LPTS-L inhibited telomerase activity greatly, 1 mg fusion protein could almost inhibit the telomerase activity, while 10 μg GST protein had no effect at all (Figure 3).
Normal hepatocytes seldom divide, which is reflected by a fact that their telomeres length does not decrease with age. Telomerase activity is absent in the resting liver but transiently reappears during hepatic regeneration. The telomere length of HCC cells is relatively shorter than that of the normal hepatocytes, but is stable due to the activation of telomerase activity[14,15]. The telomerase activity could be detected in all HCC tissue samples smaller than 3 cm, regardless of the patient’s age, sex, viral marker and degree of tumor differentiation[16]. Telomerase activity was also detectable in hepatoma cell line SMMC-7721 as assayed by TRAP assay. Over-expression of LPTS-L in SMMC-7721 cells could inhibit telomerase activity, and induce cells into crisis. From the results above, we can conclude that LPTS-L encoded by LPTS gene is expressed in normal liver cells and serves as a telomerase inhibitor. In the HCC cells, LPTS-L was down-regulated or absent, while transformed LPTS-L into HCC cells could stop the cell proliferation and the cells became large, flat and dead. LPTS gene must be inactivated to depress the telomerase inhibitory activity during hepatocarcinogenesis.
Recently, the correlation between telomerase and immortalization of tumor cells, makes the telomere and telomerase a hotspot in tumor molecule pathological study. Reactivation of telomerase appears to be a universal and obligatory step towards cell immortalization. Telomerase activity has been found in about 85%-90% of all human tumors, including liver cancer, but not in adjacent normal cells. As a result, telomerase has become a novel target not only for cancer diagnosis but also for the development of novel therapeutic agents[17,18]. LPTS-L encoded by LPTS gene, which could inhibit the activity of the telomerase directly, is an important protein inhibiting telomerase activity. LPTS gene might be a new target in the “battle” against hepatocellular carcinoma, and could be used in tumor gene therapy through reactivation of its expression in tumor cells.
Edited by Ma JY
| 1. | Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2511] [Cited by in RCA: 2637] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 2. | Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622-6626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1603] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 3. | Blackburn EH. Telomeres: structure and synthesis. J Biol Chem. 1990;265:5919-5921. [PubMed] |
| 4. | Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1601] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 5. | Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2342] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 6. | Nugent CI, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 323] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1606] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 8. | Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol. 2000;18:2626-2634. [PubMed] |
| 11. | Liao C, Zhao M, Song H, Uchida K, Yokoyama KK, Li T. Identification of the gene for a novel liver-related putative tumor suppressor at a high-frequency loss of heterozygosity region of chromosome 8p23 in human hepatocellular carcinoma. Hepatology. 2000;32:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Zhou XZ, Lu KP. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell. 2001;107:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 227] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5234] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 14. | Miura N, Horikawa I, Nishimoto A, Ohmura H, Ito H, Hirohashi S, Shay JW, Oshimura M. Progressive telomere shortening and telomerase reactivation during hepatocellular carcinogenesis. Cancer Genet Cytogenet. 1997;93:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Huang GT, Lee HS, Chen CH, Chiou LL, Lin YW, Lee CZ, Chen DS, Sheu JC. Telomerase activity and telomere length in human hepatocellular carcinoma. Eur J Cancer. 1998;34:1946-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Erlitzki R, Minuk GY. Telomeres, telomerase and HCC: the long and the short of it. J Hepatol. 1999;31:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] |
| 18. | Yakoob J, Hu GL, Fan XG, Zhang Z. Telomere, telomerase and digestive cancer. World J Gastroenterol. 1999;5:334-337. [PubMed] |