Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.562
Revised: April 13, 2002
Accepted: April 25, 2002
Published online: June 15, 2002
AIM: Cytokine release by macrophages critically determines the type of immune response to an antigen. Therefore, we studied hepatitis C virus (HCV)-specific induction of interleukins-1β, -10, -12 (IL-1β, IL-10, IL-12), and tumor necrosis factor-α (TNF-α) in monocytes.
METHODS: Intracellular cytokine expression was studied by flow cytometry in 23 patients with chronic hepatitis C, 14 anti-HCV seropositives without viremia and 11 controls after stimulation of peripheral blood mononuclear cells with recombinant core, NS3, NS4, NS5a and NS5b proteins.
RESULTS: Patients with HCV viremia revealed greater spontaneous expression of IL-1β, TNF-α, and IL-10. Furthermore, greater than twofold higher IL-10 expression was induced by the HCV antigens in chronic hepatitis C than in the other two groups (P < 0.05). In contrast, neither IL-12 nor TNF-α was induced preferentially.
CONCLUSION: In chronic hepatitis C antigen-specific cytokine induction in monocytes is apparently shifted towards predominant IL-10 induction - not counterbalanced by antiviral type 1 cytokines. This may contribute to persistent viral replication.
- Citation: Woitas RP, Petersen U, Moshage D, Brackmann HH, Matz B, Sauerbruch T, Spengler U. HCV-specific cytokine induction in monocytes of patients with different outcomes of hepatitis C. World J Gastroenterol 2002; 8(3): 562-566
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.562
Resistance or susceptibility to viral infections is critically linked to cytokine release, which can be polarized towards a type 1 (IFN-γ, TNF-α, IL-2) or type 2 (IL-4, IL-10, IL-13) pattern in helper as well as cytotoxic T lymphocytes[1]. Type 1 and type 2 T lymphocytes are not derived from different lineages but develop from the same precursors, and their differentiation is influenced by the environment during priming. The most important signals are cytokines themselves: IL-12 produced by activated macrophages is the principal cytokine inducing type 1 responses, whereas the development of type 2 T lymphocytes is induced by IL-4 and IL-10.
Hepatitis C virus (HCV) infection frequently leads to persistent viral replication, which may be facilitated by selective alterations in the host’s immune response[2]. In this context, studies of T cell functions in patients with chronic hepatitis C have indicated an inappropriately low production of antiviral type 1 cytokines in response to HCV antigens[3-7] possibly facilitating persistent infection. However, it is not clear, whether the imbalance in the cytokine pattern is due to direct alterations of T cell function or a consequence of altered T cell priming. Because of their critical role for the type of immune reaction triggered in response to an antigen, we used flow cytometric detection of intracytoplasmic cytokines to study at the single cell level the HCV-specific induction of IL-1β, IL-10, IL-12 and TNF-α in peripheral blood monocytes.
Three groups of patients were included into this study: Group 1 consisted of 23patients with chronic hepatitis C (male, n = 20; female, n = 3; median age 33, range 21-61), elevated liver enzymes and detectable HCV-RNA in the serum. The mean virus load was 6.3 × 106 copies/mL (SD 1.0 × 106 copies/mL) (QuantiplexTM HCV RNA 2.0 assay, Chiron, Emeryville, CA). Group 2 consisted of 14 carefully selected patients with previous HCV infection (male, n = 12; female, n = 2; median age 28.5, range 18-63), who had consistently normal aminotransferases without detectable viral RNA on repeated examination over at least 2 years. Finally, 11 anti-HCV negative volunteers (male, n = 5; female, n = 6; median age 30, range 24-66) served as a control group (group 3).
There were no significant differences between the two anti-HCV groups with respect to total immunoglobulin levels, and all were free from cryoglobulins or autoantibodies. None of the individuals in this study had hepatitis B virus or human immunodeficiency virus co-infection. The study was approved by the local ethical committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
HCV antibodies were detected with a microparticle enzyme immunoassay (MEIA) (Axsym, Abbott, Wiesbaden, Germany) according to the instructions of the manufacturer. Positive results were confirmed by dot immunoassay (Matrix, Abbott, Wiesbaden, Germany). HCV RNA was detected with a nucleic acid purification kit (Viral Kit, Qiagen, Hilden, Germany) followed by reverse transcription and nested polymerase chain reaction as described elsewhere[8]. Quantitative determination of HCV RNA copies was done via branched DNA technology (Chiron, Emeryville, CA). In group 1, genotypes of the infecuing HCV strains were determined by the INNO-LiPA HCV II test (Innogenetics, Zwijndrecht, Belgium) except one patient, who could not be genotyped. This group revealed the following isolates: 1a (n = 8), 1b (n = 9), 2b (n = 1), 3a (n = 1), and mixed (n = 3; genotypes 1b/2b; 2a/2c; 4c/4d).
Patients of group 2 were characterized by genotype-specific antibodies to NS4 (Murex, Abbott Wiesbaden, Germany). Serotypes 1 and 4 were found in 10 and 1 patients, respectively. Three patients had indeterminate serotypes.
The purified recombinant proteins [r-core (truncated): aa 1-115, r-NS3: aa 1007-1534, r-NS4: aa 1616-1862, r-NS5: aa 2007-2268] derived from the HCV-1 prototype sequence[9] were purchased from Mikrogen, Munich, Germany. The bacterial lipopolysaccharide content of the proteins was between 4.0-20 pg/μg recombinant protein as determined by the Limulus assay. Lipopolysaccharide from E. coli OH101 (Sigma, Munich, Germany) was used in the control experiments.
Fluoresceinisothiocyanate (FITC)- and phycoerythrin (PE)-labelled antibodies were purchased from the following companies: FITC-and PE-labelled-anti-CD14 (mouse IgG2b, clone MÖP9) from Becton Dickinson (Heidelberg, Germany); FITC- and PE-labelled anti-IL-1β (mouse IgG1, clone H9.5) was purchased by Holtzel Diagnostica (Cologne, Germany). Anti-IL-10/PE (rat IgG2a, clone JES3-19F1), anti-IL-12/PE (mouse IgG1, clone C11.5.14), anti-TNF-α/PE (mouse IgG1, clone MAb11) as well as appropriate isotype controls from Pharmingen (Hamburg, Germany). Unlabelled mAbs for blocking experiments were purchased from Pharmingen (Hamburg, Germany) except for IL-1β that was a gift of Holtzel Diagnostica (Cologne, Germany).
PBMC (1.1 × 106/ml) isolated from fresh EDTA blood by Ficoll density-gradient centrifugation (Biochrom, Berlin, Germany) were resuspended in low endotoxin level culture medium (RPMI 1640, Biochrom, Berlin, Germany) containing 10% autologous human serum, 100 units/ml penicillin, 100 units/ml streptomycin and incubated at 37 °C with 5% CO2 in 96 well microtiter plates (Sarstedt, Berlin, Germany) in the presence of recombinant HCV proteins (1 μg/ml) or LPS (10-200 ng/ml). Kinetic experiments showed that the antigen-specific cytokine induction indicated a maximum after 12 hours of stimulation. Further experiments revealed 1.0 μM monensin (Sigma, Munich Germany) for 12 hours to be optimal to enhance the signal/noise ratio as well as to exclude relevant toxicity[10-12].
CD14 and iotracytoplasmic cytokines were detected by direct immunofluorescence using a paraformaldehyde (PFA)-saponin procedure with 4% PFA as fixative and 0.2% saponin for permeabilisation. In brief, cultured cells were washed twice in Hank’s balanced salt solution (HBSS) (Gibco, Eggenstein, Germany) and stained for the surface markers (20 minutes incubation at 4 °C in the dark). After one further wash, the cells were fixed in ice cold HBSS containing 4% PFA for 5 minutes and washed again. Cells were resuspended in HBSS containing 0.2% saponin (saponin buffer). Then cytokine specific antibodies diluted in saponin buffer were added at a concentration of 0.5-3.0 μg/ml and incubated for 30 minutes at room temperature in the dark. Cells were washed in saponin buffer and analyzed by dual-colour flow cytometry on a FACSort flowcytometer (Becton Dickinson, Heidelberg, Germany). Forward and side scatter as well as gating for CD14+, cells were used to identify monocytes. The data were analyzed with the CellQuest (software (Becton Dickinson) after counting 5000 CD14+ cells. All experiments were performed in triplicate.
To ensure specificity of the cytokine staining procedures, the binding of each mAb was blocked with an excess of unlabelled mAb.
Results are given as median and range. Differences between the groups were analyzed by the Kruskal-Wallis test, Mann-Whitney U test and Wilcoxon signed rank test, as appropriate. All calculations were performed on a personal computer with Statview 4.5 software (Abacus Concepts inc., Berkeley CA, USA). P values < 0.05 were regarded as significant.
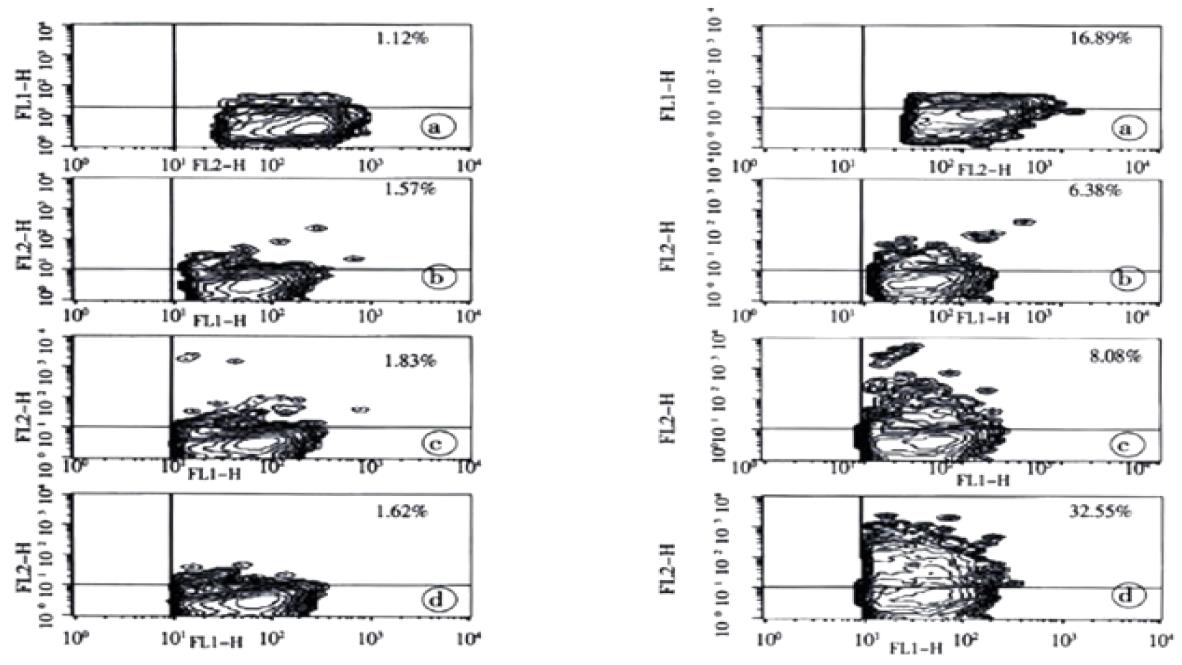
Using flowcytometric detection of intracytoplasmic cytokines in combination with CD14 staining, we were able to specifically measure spontaneous as well as HCV antigen-induced production of IL-1β, IL-10, IL-12 and TNF-α in peripheral blood monocytes (Figure 1). Following stimulation with LPS, intracytoplasmic expression of the cytokines did not show statistically significant differences between the study groups for the cytokines with the exception of IL-1β, which was higher in patients with chronic hepatitis C when compared to the controls (Table 1). Cytokine production after 12 hours of HCV-specific stimulation together with the corresponding spontaneous and LPS-induced production is summarized in Table 1.
| Chronic hepatitis C (n = 23) | Aviremic anti-HCV seropositives (n = 14) | Controls (n = 11) | ||||
| % CD14+monocytes | § Significance | % CD14+monocytes | § Significance | % CD14+monocytes | § Significance | |
| IL-1β | ||||||
| Spontaneous expression | 13.6 [0.3-82.1] | a, b | 1.9 [0.4-65.5] | 2.1 [0.6-4.7] | ||
| Expression after stimulation with | ||||||
| Core | 21.5 [0.9-84.8] | a, b, c | 3.1 [0.1-69.6] | c | 2.8 [0.6-8.3] | |
| NS3 | 42.0 [1.1-91.1] | a, b, c | 7.0 [0.6-71.8] | c | 6.1 [1.6-31.6] | c |
| NS4 | 28.3 [0.4-82.4] | a, b, c | 4.6 [0.2-81.8] | 6.2 [1.6-15.5] | c | |
| NS5a | 34.3 [0.4-87.2] | a, b, c | 3.3 [0.1-77.8] | c | 8.3 [1.7-14.5] | c |
| NS5b | 27.6 [1.7-89.9] | a, b, c | 6.2 [0.8-75.3] | c | 4.8 [0.8-30.3] | |
| LPS | 55.5 [7.6-96.5] | b | 36.2 [4.0-97.0] | 24.1 [9.4-48.7] | ||
| IL-10 | ||||||
| Spontaneous expression | 1.4 [0.2-8.6] | a, b | 0.4 [0.1-6.1] | 0.7 [0.1-1.3] | ||
| Expression after stimulation with | ||||||
| Core | 2.6 [0.3-24.4] | a, b, c | 0.6 [0.1-8.3] | c | 1.0 [0.2-1.8] | |
| NS3 | 2.4 [0.2-20.2] | a, b, c | 0.8 [0.2-9.8] | c | 0.8 [0.2-2.3] | c |
| NS4 | 2.0 [0.2-18.8] | a, b, c | 0.5 [0.0-7.7] | 0.9 [0.2-2.1] | c | |
| NS5a | 2.0 [0.2-16.9] | a, b, c | 0.7 [0.1-7.9] | c | 1.0 [0.1-3.2] | c |
| NS5b | 3.7 [0.1-22.4] | a, b, c | 0.7 [0.1-12.6] | c | 0.7 [0.2-5.4] | |
| LPS | 12.4 [0.2-39.8] | 6.4 [0.3-25.0] | 6.0 [0.5-19.1] | |||
| IL-12 | ||||||
| Spontaneous expression | 0.8 [0.2-5.1] | 0.5 [0.2-3.1] | 0.6 [0.1-2.3] | |||
| Expression after stimulation with | ||||||
| Core | 2.0 [0.2-8.1] | a | 0.7 [0.1-3.1] | 1.1 [0.3-3.2] | ||
| NS3 | 1.8 [0.5-16.3] | c | 1.1 [0.2-5.3] | c | 1.6 [0.2-12.2] | c |
| NS4 | 1.0 [0.3-6.1] | c | 0.8 [0.8-4.5] | 4.5 [0.3-5.2] | c | |
| NS5a | 1.5 [0.3-9.1] | c | 0.8 [0.1-3.7] | c | 1.5 [0.2-3.7] | c |
| NS5b | 2.2 [0.3-40.1] | b, c | 1.0 [0.3-5.2] | c | 0.9 [0.2-6.1] | |
| LPS | 18.7 [0.7-61.8] | 6.2 [0-12.7] | 11.8 [2.4-33.5] | |||
| TNF-α | ||||||
| Spontaneous expression | 3.2 [0.1-39.5] | 0.8 [0.2-12.0] | 1.3 [0.4-3.4] | |||
| Expression after stimulation with | ||||||
| Core | 6.9 [0.3-51.3] | b, c | 1.5 [0.1-12.8] | c | 1.7 [0.3-6.1] | c |
| NS3 | 5.4 [0.2-40.7] | 2.9 [0.4-16.4] | c | 3.7 [0.9-23.3] | c | |
| NS4 | 3.1 [0.2-21.9] | 2.7 [0.1-17.7] | c | 2.9 [1.1-14.6] | c | |
| NS5a | 4.30 [0.1-22.7] | 1.8 [0.2-11.1] | c | 4.0 [1.3-8.3] | c | |
| NS5b | 10.9 [0.7-53.8] | a, b, c | 2.3 [0.4-23.2] | c | 2.0 [0.9-9.6] | c |
| LPS | 35.0 [1.50-91.2] | 21.3 [1.5-51.4] | 31.5 [12.1-47.9] | |||
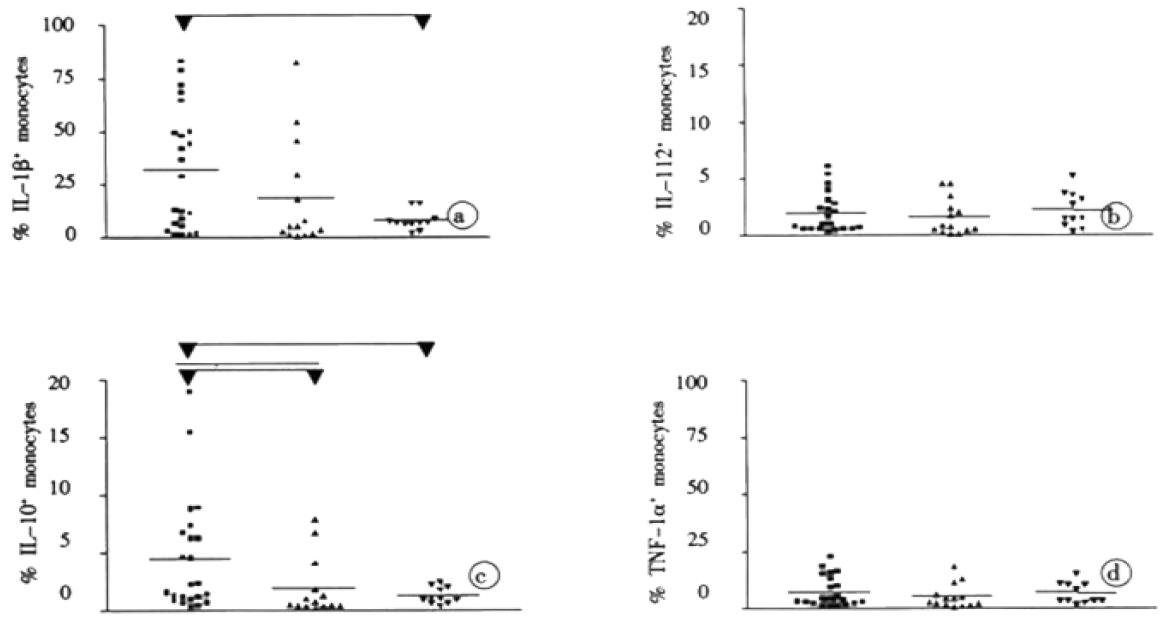
With respect to spontaneous production of IL-1β, TNF-α, IL-10, and IL-12, there was considerable individual variability which was most pronounced in the group with chronic hepatitis C. The numbers of monocytes with detectable spontaneous production of IL-12 were low in general (0.1%-5.1%) and on average not significantly different between the three study groups. In contrast, average numbers with detectable spontaneous production of IL-1β, TNF-α, and IL-10 were higher in patients with chronic hepatitis C than in aviremic anti-HCV seropositives. However, due to considerable inter-individual variations, these differences between the groups reached statistical significance only for IL-1β and IL-10 (P < 0.05).
Antigen-specific stimulation of peripheral blood mononuclear cells resulted in increased numbers of monocytes with production of IL-1β, TNF-α, IL-10, and IL-12 in each study group for most of the tested HCV proteins (Table 1). However, after stimulation with the HCV proteins core, NS3, NS4, NS5a, and NS5b, the numbers of IL-1β and IL-10 producing monocytes were at least twofold higher in patients with chronic hepatitis C than in aviremic anti-HCV seropositives and the controls (P < 0.05 for each HCV protein). In contrast, statistically significant differences (P < 0.05) in the numbers of TOF-α and IL-12 producing monocytes between patients with chronic hepatitis C and the other groups were only seen after stimulation with HCV core and NS5b (Table 1). The marked difference in the balance between IL-1β and IL-10 producing monocytes, and TNF-α and IL-12 producing ones among the patients with chronic hepatitis C is illustrated in Figure 2, which shows the stimulation experiments with recombinant NS4 as a representative example. Since stimulation of monocytes was performed in the presence of T cells, we undertook control experiments with untouched isolated monocytes. When these purified monocytes were stimulated with HCV antigens or LPS, the cells showed a similar behaviour although the number of cytokine producing macrophages was strongly reduced compared to the PBMC assays (data not shown).
Impaired function of macrophages has been described repeatedly in infection with HCV and also the closely related Dengue virus[13-17]. Our study adds to these observation that in chronic hepatitis C altered macrophage function may also comprise the pattern of cytokines produced in response to HCV antigens. Of note, the various cytokines appeared to be involved differentially. Despite considerable individual variability, we found significantly greater average numbers of monocytes with spontaneous IL-1β and IL-10 production in patients with chronic hepatitis than that in aviremic anti HCV seropositives or the HCV-naive controls. This finding appears to be compatible with in vivo pre-activation of the monocytes from patients with chronic hepatitis C. However, the numbers of monocytes with spontaneous IL-12 production did not reveal any conspicuous differences between our study groups. Thus far, studies on the cytokine production in hepatitis C have produced conflicting results for macrophages, mainly because monocytes were assessed only indirectly by measuring cytokines in the supernatants of peripheral blood mononuclear cells after stimulation with mitogen or LPS. This flowcytometric study is the first investigation, which provides HCV-specific data for monocytes at the single cell level in a more or less physiological environment that enables natural interactions to take place between the monocytes and other peripheral blood mononuclear cells, e.g. CD4+ T lymphocytes. Control experiments with untouched isolated monocytes showed that these interactions were crucial for an effective cytokine induction. Despite similar behaviour after stimulation the amount of cytokine producing macrophages was strongly reduced (data not shown). With the flowcytometric approach, we could demonstrate that the numbers of IL-1β+ and IL-10+ monocytes increased significantly upon stimulation with the HCV antigens, whereas a similar induction of TNF-α and IL-12 was not observed.
After exposure to the HCV proteins a slight increase in the number of monocytes with expression of IL-1β, TNF-α, IL-10, and IL-12 was also seen in the HCV-naive control group. This finding most likely indicates a non-specific stimulatory effect of the HCV antigens on the monocytes, probably due to small amounts of contaminating endotoxin. Compared to this non-specific effect this results indicate marked (2-7 fold) antigen-specific induction of IL-1β and IL-10 in response to all HCV antigens, but a less prominent induction of IL-12 and TNF-β in response to HCV core and NS5b in the group with chronic hepatitis C.
Similar responses were occasionally seen in few patients of our aviremic anti-HCV seropositive group. However, we cannot exclude completely that HCV replication below the detection limit of PCR technique was present in some of these patients, despite the fact that the group of aviremic anti-HCV seropositives was selected carefully to ensure that these patients had gained immune-mediated control of their HCV infection. Thus persistent low level viremia might be an explanation for the occasional altered cytokine responses in this group.
In general, our findings are in line with a previous report by Kakumu et al[18], who reported increased IL-10 levels but unaltered IL-12 production in mononuclear cells of patients with chronic hepatitis C. Furthermore, increased spontaneous TNF-α expression in patients with chronic hepatitis C is in line with previous work published by Kishihara and co-workers[19]. As our flowcytometric approach enabled interactions between the monocytes and other immunoregulatory cells, which are important for the generation of antigen- specific responses, it was not unexpected that our results differed from those studies, which used purified monocytes[20]. Thus, we could not confirm reduced numbers of IL-1β and TNF-α monocytes in chronic hepatitis C, as has been reported for purified monocytes from patients with chronic hepatitis C by Mendoza and co-workers[21].
The reasons for altered macrophage functions in chronic hepatitis C are unclear at present. Although HCV RNA has been detected in macrophages of a variable proportion of patients with chronic hepatitis C[22], the reported percentages of HCV-RNA positive monocytes seem to be too low as to explain the altered cytokine induction by a direct infection of the monocytes[23]. Persistent antigenic stimulation due to chronic hepatitis C viremia provides a better alternative explanation. For instance, continued production of HCV antigens may lead to the formation of antigen-antibody complexes, which can bind to Fcγ receptors on immunocompetent cells. Fcγ receptor ligation on monocytes will then result in reversal of macrophage pro-inflammatory responses as IL-10 up-regulation together with a reciprocal inhibition of IL-12 production can result from such Fcγ receptor triggering[24]. Likewise, the blunted TNF-α responses observed in our in vitro stimulation experiments are likely to reflect altered immunoregulation, because loss of inducible TNF-α secretion has been well documented in pre-activated monocytes[25].
Irrespective of the underlying cause, altered cytokine production by macrophages in chronic hepatitis C is likely to have important functional consequences. The high proportion of macrophages, which produce IL-1β either spontaneously or after antigen-specific challenge may indicate that these macrophages are pre-conditioned towards pro-inflammatory reactions. However, proinflammatory cytokines required for efficient antiviral responses such as IL-12 and TNF-α show blunted responses to the HCV antigens. On the contrary, cytokine production appeared to be markedly biased towards IL-10 already at the level of the macrophages. Due to the pivotal role of monocytes and macrophages for the initiation of immune responses, the uniform up-regulation of IL-10 in response to HCV antigens, not counterbalanced by IL-12, may explain poor HCV-specific type I cytokine responses, which have been observed repeatedly in chronic hepatitis C[3-7]. Thus, altered cytokine production by monocytes and macrophages might contribute to HCV persistence, because these cells may not support sufficiently effective antiviral immune responses.
We gratefully acknowledge Eva-Maria Althausen, Department of Internal Medicine I and Bettina Kochan, Institute of Medical Microbiology and Immunology for excellent technical assistance. This work was supported by a generous grant of the Joachim Kuhlmann AIDS-Stiftung.
Edited by Zhang ZJ
| 1. | Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3292] [Cited by in RCA: 3186] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 2. | Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Lechmann M, Woitas RP, Langhans B, Kaiser R, Ihlenfeldt HG, Jung G, Sauerbruch T, Spengler U. Decreased frequency of HCV core-specific peripheral blood mononuclear cells with type 1 cytokine secretion in chronic hepatitis C. J Hepatol. 1999;31:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Malaguarnera M, Di Fazio I, Laurino A, Pistone G, Restuccia S, Trovato BA. Decrease of interferon gamma serum levels in patients with chronic hepatitis C. Biomed Pharmacother. 1997;51:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Osna N, Silonova G, Vilgert N, Hagina E, Kuse V, Giedraitis V, Zvirbliene A, Mauricas M, Sochnev A. Chronic hepatitis C: T-helper1/T-helper2 imbalance could cause virus persistence in peripheral blood. Scand J Clin Lab Invest. 1997;57:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Reiser M, Marousis CG, Nelson DR, Lauer G, González-Peralta RP, Davis GL, Lau JY. Serum interleukin 4 and interleukin 10 levels in patients with chronic hepatitis C virus infection. J Hepatol. 1997;26:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Woitas RP, Lechmann M, Jung G, Kaiser R, Sauerbruch T, Spengler U. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J Immunol. 1997;159:1012-1018. [PubMed] |
| 8. | Woitas RP, Rockstroh JK, Beier I, Jung G, Kochan B, Matz B, Brackmann HH, Sauerbruch T, Spengler U. Antigen-specific cytokine response to hepatitis C virus core epitopes in HIV/hepatitis C virus-coinfected patients. AIDS. 1999;13:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Farci P. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome [Science 1989; 244: 359-362]. J Hepatol. 2002;36:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4657] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 10. | Tartakoff AM. Perturbation of the structure and function of the Golgi complex by monovalent carboxylic ionophores. Methods Enzymol. 1983;98:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 698] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408-1419. [PubMed] |
| 13. | Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 15. | Mathew A, Kurane I, Green S, Vaughn DW, Kalayanarooj S, Suntayakorn S, Ennis FA, Rothman AL. Impaired T cell proliferation in acute dengue infection. J Immunol. 1999;162:5609-5615. [PubMed] |
| 16. | Hiasa Y, Horiike N, Akbar SM, Saito I, Miyamura T, Matsuura Y, Onji M. Low stimulatory capacity of lymphoid dendritic cells expressing hepatitis C virus genes. Biochem Biophys Res Commun. 1998;249:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Kakumu S, Okumura A, Ishikawa T, Iwata K, Yano M, Yoshioka K. Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;108:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kishihara Y, Hayashi J, Yoshimura E, Yamaji K, Nakashima K, Kashiwagi S. IL-1 beta and TNF-alpha produced by peripheral blood mononuclear cells before and during interferon therapy in patients with chronic hepatitis C. Dig Dis Sci. 1996;41:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1539] [Cited by in RCA: 1549] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 21. | Mendoza EC, Paglieroni TG, Zeldis JB. Decreased phorbol myristate acetate-induced release of tumor necrosis factor-alpha and interleukin-1 beta from peripheral blood monocytes of patients chronically infected with hepatitis C virus. J Infect Dis. 1996;174:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Bouffard P, Hayashi PH, Acevedo R, Levy N, Zeldis JB. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992;166:1276-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Mellor J, Haydon G, Blair C, Livingstone W, Simmonds P. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Haas JG, Baeuerle PA, Riethmüller G, Ziegler-Heitbrock HW. Molecular mechanisms in down-regulation of tumor necrosis factor expression. Proc Natl Acad Sci USA. 1990;87:9563-9567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |