INTRODUCTION
Apoptosis is an active cell death process, which requires specific gene regulation. A critical role for p53 in the execution of some forms of apoptosis has been suggested[1-6]. This protein is a sequence-specific DNA-binding protein, active as a transcription factor. It has been proposed that p53 may be involved in the cellular response to DNA damage, producing arrest in the G1 phase of the cell cycle to allow efficient repair of DNA before entry to S phase, or cell death if the damage is too large to be repaired[7,8]. Another gene implicated in apoptosis is bcl-2. The bcl-2 gene product functions as an anti-apoptotic signal, suppressing apoptosis induced by a wide variety of stimuli, including chemotherapeutic drugs and γ radiation[9-13]. The exact mechanism of bcl-2 in preventing apoptosis is still not clear. However, bcl-2 has been implicated in cellular control of their redox state[14].
Previous studies have demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) given in vivo to rodents and human can inhibit tumor growth[15,16]. JTE-522 is a novel NSAIDs, which is a specific inhibitor of cyclooxygenase-2 (COX-2) with significant anti-inflammatory and analgestic properties[17]. Some reported that JTE-522 possesses strong chemopreventive activity against colon carcinogenesis[18], but the precise mechanism by which JTE-522 inhibits colon carcinogenesis is not clear. It is often attributed to specific inhibition of arachidonic acid metabolism via coxenzymes. However, recent studies showed that the antitumor effect had little connection with NSAIDs inhibitory activity against cyclooxygenase, and was not prevented by exogenous supplementation of 16, 16-dimethyl prostaglandin E2. Several groups have shown that certain NSAIDs induce apoptosis of tumor cell line, which is associated with the generation of reactive oxygen species (ROS)[19,20]. However, the signaling pathway leading to apoptotic cell death remains unclear.
ROS can play a central role in regulating cell proliferation and cell death. Evidence has been obtained that ROS such as superoxide and hydrogen peroxide can influence cell death triggered by internal cues (p53-mediated), external cues (TGF-beta-mediated) and immunogenic signals (TNF-alpha)[21,22]. In other instances, however, generation of ROS can inhibit apoptosis. Although the mechanism involved is still controversial, redox status and/or hydrogen peroxide have both been proposed as critical factors[19]. Therefore it is possible that ROS may play a role in regulating apoptosis in gastric epithehum.
The purpose of the present study is to identify whether JTE-522 can induce apoptosis in AGS cells and ROS are also involved in the process, and to investigate the changes in NF-κB, p53, bcl-2 and caspase in the apoptosis process.
MATERIALS AND METHODS
Cell line and reagents
Human gastric adenocarcinoma cell line AGS was provided by Cancer Institute, Zhongshan University. Cells were grown in RPMI-1640 medium and supplemented with 10% new bovine serum, penicillin G (100 kU·L-1) and kanamycin (0.1 g·L-1) at 37 °C in a 5% CO2-95% air atmosphere. Antibodies used in this study included p53, bcl-2, IkBα and Beta actin were obtained from Santa Cruz. All other chemicals were purchased from Sigma Chemical Co (St. Louis, MO, USA).
MTT assay
AGS cells growing on 96-well plates were treated with JTE-522 (0.1 mmol/L-1 mmol/L) for 72 h, untreated cells served as a control. 10 μL of the 2.5 g·L-1 stock solution of 3-[4,5-dimethylthiaolyl]-2,5-diphenyl-tetrazolium bromide (MTT) was added to each well. After 1 h of incubation at 37 °C, the medium was removed, 50 µl of the extraction buffer (10% Trition-X100; 0.1 mol/L HCl) was added, and plates were gently shaken for 30 min at room temperature. The optical densities were measured at 570 nm.
Morphological and biochemical analysis of apoptosis
Morphological changes in the nuclear chromatin of cells undergoing apoptosis were detected by electron microscopy (EM). Cells were peleted and fixed with 30 mL/L glutaraldehyde in PBS. EM analysis was performed as described previously[23]. Oligonucleosomal cleavage of genomic DNA was detected by agarose gel electrophoresis. In brief, genomic DNA isolated as previously described[24] was subjected to 1.5% agarose gel electrophoresis, followed by ethidium bromide staining.
Assay for reactive oxygen species production
Generation of ROS was assessed using lucigenin. AGS cells grown in 75 cm2 culture flasks were incubated for 6 h with JTE-522 (0.1-1 mmol/L) in the presence or absence of 100 µmol/L pyrrolidine dithiocarbamate (PDTC). The cells were then scraped off and washed in cold Hank’s buffer. An aliquot containing 1 × 106 cells in 100 µL of Hank’s buffer was mixed in microtitrator wells with 100 µl of lucigenin prepared at a concentration of 40 µmol/L. Light emission was detected using a Berthold LB96V luminometer for 3 min.
Assessment of caspase activity
Caspase-3 activity was measured using a caspase assay kit according to the supplier’s instruction. In brief, caspase-3 fluorogenic substrates (Ac-DEVD-AMC or Ac-IETD-AMC) were incubated with JTE-522-treated with cell lysates for 1 h at 37 °C, then AMC liberated from Ac-DEVD-AMC or Ac-IETD-AMC was measured using a fluorometric plate reader with an excitation wavelength of 380 nm and an emission wavelength of 420-460 nm.
Western blot analysis
The cells were lysed in lysis buffer (25 mmol/L hepes, 1.5% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.5 mol/L NaCl, 5 mmol/L EDTA, 50 mmol/L NaF, 0.1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 0.1 g·L-1 leupeptin (pH7.8) at 4 °C with sonication. The lysates were centrifuged at 15000 g for 15 min and the concentration of the protein in each lysate was determined with Coomassie brilliant blue G-250.Loading buffer (42 mmol/L Tris-HCl, 10% glycerol, 2.3% SDS, 5% 2-mercaptoethanol and 0.002% bromophenol blue) was then added to each lysate, which was subsequently boiled for 3 min and then electrophoresed on a SDS-polyacrylamidel gel. Proteins were transferred to nitrocellulose and incubated sequentially with antibodies against IkBα, p53 and bcl-2 and then with peroxidase-conjugated secondary antibodies in the second reaction. Detection was performed with enhanced chemiluminescence reagent.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from AGS cells treated with JTE-522. Synthetic double-strand oligonucleotides of consensus NF-κB binding sequence, GATCCCAACGGCAGGGGA, were end-labeled with [γ32] ATP using T4 polynucleotide kinase. Nuclear extract was incubated with the labeled probe in the presence of poly (dI-dC) in a binding buffer containing 20 mM N-2-hydrocyethylpiperazine-N’-2-ethanesulfonic acid at room temperature for 30 min. For supershift assays, a total of 0.2 µg of antibodies against p65 subunit of NF-κB were included in the reaction. DNA-protein complexes were resolved by electrophoresis in a 5% non-denaturing polyacrylamide gel, which was dried and visualized by autoradiography.
RESULTS
Effect of JTE-522 on cell proliferation and apoptosis
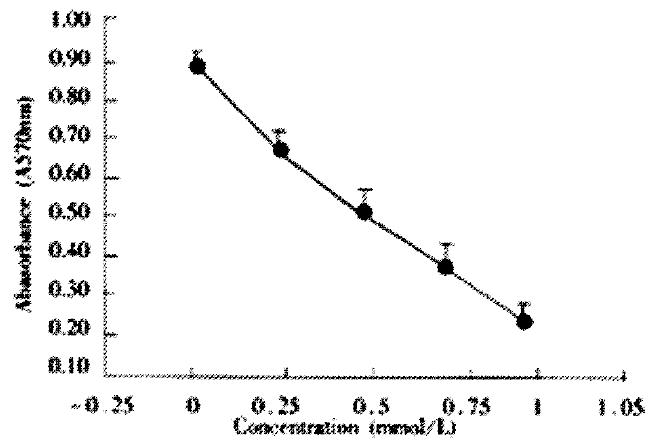
AGS cells were incubated with various does of JTE-522 for 72 h. Analysis of cell viability using to MTT assay showed that JTE-522 significantly inhibited cell viability. The inhibition of cell viability was dependent on the dose of JTE-522 used (Figure 1).
Figure 1 Effect of JTE-522 on cell growth in AGS cells.
The cells were treated with various concentrations of JTE-522 for 72 h. The antiproliferative effect was measured by MTT assay. Results are the means ± SD from three independent determinations.
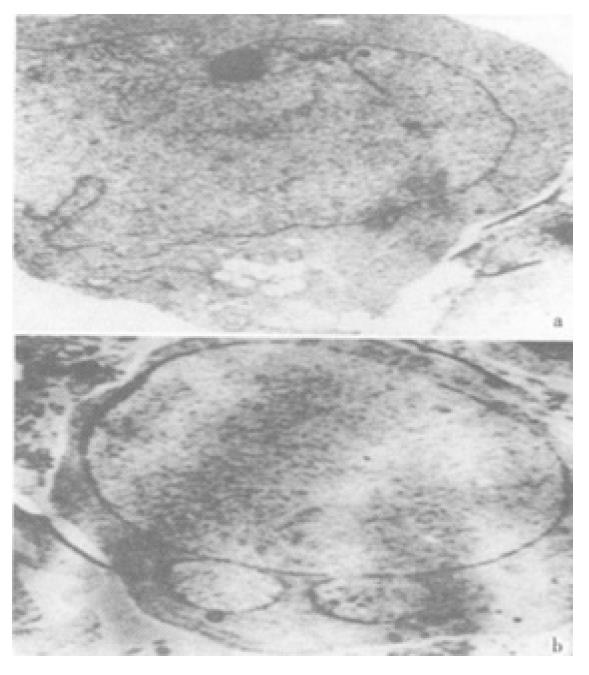
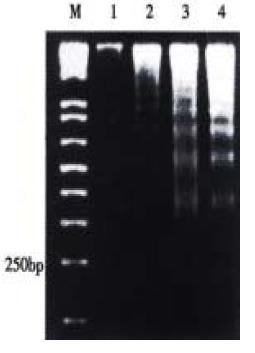
The effect was due to apoptosis as demonstrated by EM and electrophoresis of genomic DNA. JTE-522-treated cells showed compacted nuclear chromatin with finely granular masses marginated against the nuclear envelopeard condensed cytoplasm, the nuclear outline was convoluted and the organelles were preserved (Figure 2) and led to oligonucleosomal cleavage of genomic DNA (Figure 3), which were hallmarks of apoptosis.
Figure 2 Electro micrographs of JTE-522-treated AGS cells.
Control AGS cells (A), or treated with 1 mmol/L (B) JTE-522 for 72 h, were examined by EM as in "Materials and Methods". Magnification: × 4000
Figure 3 DNA ladder pattern formation of AGS cells.
Cells treated with different concentrations of JTE-522 for 72 h and uhe formation of oligonucleosomal fragments was determined by 1.5% agarose gel electrophoresis. M, DNA markers; lanes 1-4, AGS cells treated with 0, 0.25, 0.50, 1 mmol/L of JTE-522
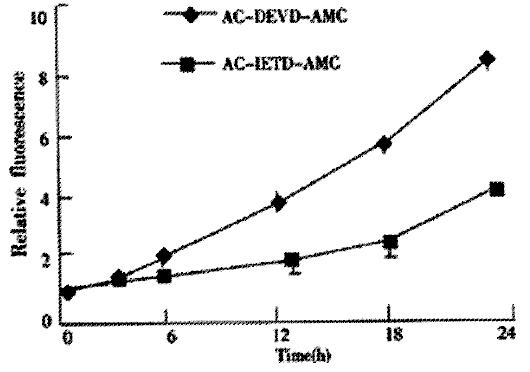
We next investigated whether the activaton of caspase was involved in JTE-522-induced apoptosis of AGS cells. JTE-522-induced apoptosis of AGS cells was accompanied by the induction of caspases activity as demonstrated by the cleavage of Ac-DEVD-AMC and Ac-IETD-AMC, respectively (Figure 4). These results indicated that JTE-522-induced cell death of AGS cells was a typical apoptosis associated with caspase activation.
Figure 4 Activation of caspase-3 activities by JTE-522 in AGS cells for indicated time period.
JTE-522 treatment (0.75 mmol/L) induced cleavage of Ac-DEVD-AMC and Ac-IETD-AMC, indicating activation of caspase-3 activity, respectively
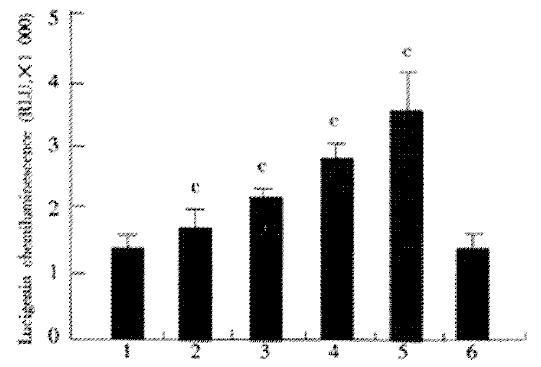
Effect of JTE-522 on the production of ROS in AGS cells
The effect of JTE-522 on the production of ROS in AGS cells, as assessed with lucigenin chemiluminesecence, was shown in Figure 5. Chemiluminesecence was significantly enhanced by incubation with various doses of JTE-522. This enhancement was prevented by co-incubation with PDTC at 100 μmol/L. These results demonstrated the generation of reactive oxygen species in cells under incubation with JTE-522.
Figure 5 Effect of JTE-522 on the generation of ROS.
AGS cells were incubated for 6 h with JTE-522 (0.25-1 mmol/L) in the presence or absence of PDTC at 100 μmol/L. Lucigenic-associated chemiluminescence was mea-sured for 3 min with a lumirometer. (A). Lane 1: control; lane 2-5: AGS cells treated with 0.25, 0.5, 0.75, 1 mmol/L of JTE-522; lane 6: JTE-522 (1 mmol/L) +PDTC (100 μmol/L) cP < 0.01 vs control. Results are the means ± SD from three independent determinations.
Effect of JTE-522 on the expression of p53, bcl-2, IkBα and the activation of NF-κB
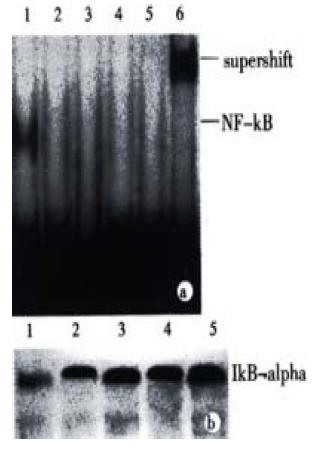
NF-κB plays a complex role in regulating programmed cell death. In many instances the inhibition of NF-κB activaty can sensitize cells to death inducers[25]. In other instances, however, NF-κB activation has been found to play an important role in the induction of apoptosis. To determine whether the treatment with JTE-522 have any effect in the NF-κB transcriptional factors. We performed EMSA with nuclear extracts prepared from control or treated cells exposed to JTE-522 for various concentrations for 6 h. The NF-κB specific complexes found in this cell line were almost complete inhibited in comparision with untreated cells (Figure 6A). In accordance with result, an analysis of IkBá proteins level by Western blot demonstrated that the degradation of this protein was greatly inhibited during the apoptotic process (Figure 6B).
Figure 6 Effect of JTE-522 on NF-κB binding activity and IkBα degradation.
Cells were treated with JTE-522 for 6 h. Cells were harvested and EMSA was performed as described (A). Lane 1: control; lane 2-5: AGS cells treated with 0.25, 0.50, 0.75, 1 mmol/L of JTE-522. The identity of DNA-complexed pro-teins was confirmed by supershift assays using antibodies against p65 sub-unit of NF-κB (lane 6). Immunobolt analysis of IkBα of corresponding cytosilic supernatant (B). Representative results from four independent experiments.
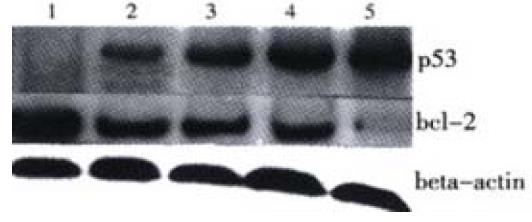
Additionally, by using Western blot we confirmed that the level of bcl-2 was decreased, whereas p53 showed a great increase following JTE-522 treatment. Their changes were in a dose-dependent manner (Figure 7).
Figure 7 P53, bcl-2 protein levels in AGS cells treated with JTE-522.
Cell lysates were collected and processed at 6 h. The whole cellular protein was electrophoresed in SDS-PAGE gel, Western blot was performed using antibodies against p53, bcl-2. Beta actin was used as a lane-loading control. (1) control; (2) 0.25 mmol/L; (3) 0.5 mmol/L; (4) 0.75 mmol/L; (5) 1 mmol/L. Representative results from three independent experiments.
DISCUSSION
Using cultured AGS human gastric adenocarcinoma cells, the observations described in this study demonstrate that JTE-522, a novel NSAIDs inhibits the growth of AGS cells and induces apoptosis in a concentration-dependent manner.
The onset of apoptosis is associated with the proteolytic activation of caspases. Caspases, a family of cysteine proteases, play a critical role in the execution of apoptosis[26-31]. They are synthesized as proenzymes that are processed by self-proteolysis and/or cleavage by another protease to their active forms in cells undergoing apoptosis Caspase-3 is a major executioner of apoptosis. It is promoted during the early stage of apoptosis and the activated form is a marker for cells undergoing apoptosis[32]. After activation by initiators, the proform (p32) is cleaved to the active forms p20, p17, or p11, respectively[33]. Therefore, activation of caspase-3 in AGS cells in this study not only indicated the occurrence of apoptosis, but also implied the involvement of caspase-3 in JTE-522-induced apoptosis.
ROS have been found to play a central role in regulating apoptosis in numerous instances. Given that reduced rates of apoptosis may contribute to carcinogenesis, the regulation of cellular ROS production may be an important variable in the development of neoplasias. Other studies have also suggested a potential role for ROS in cancer suppression. For example, the p53 tumor suppressor protein activates the expression of ROS-generating proteins that increase cellular ROS production and eventually trigger apoptosis[34,35]. Increased ROS generation by chemopreventive agents may serve to compensate the lower levels of ROS generation in p53 null cells (AGS cells are p53 null)[36,37]. Animal studies have also implicated ROS in regulating carcinogenesis. Mice with elevated levels of glutathione peroxidase are more sensitive to skin carcinogenesis than their wild type counterpart[32]. A similar correlation has been made in the colon, where strains with higher levels of glutathione peroxidase activity have a higher cancer risk. The role of ROS in carcinogenesis is however likely to be complex given the potential mutagenicity of ROS. For example, the NSAIDs inhibition of cyclooxygenase has been proposed to suppress carcinogenesis by suppressing the production of peroxyl radicals and the subsequent formation of mutagenic lipid peroxidation breakdown products[38]. The role of ROS in carcinogenesis may depend on the relative levels of different ROS generated, and where and when they are present. However, as demonstrated in this paper, we showed that JTE-522 increased ROS generation in AGS cells, and this increased ROS generation might contribute to the induction of AGS cells to apoptosis.
The bcl-2 protooncogene is unique among cellular genes for its ability in many contexts to block apoptotic cell death. A mechanism has been proposed in which bcl-2 regulates antioxidant pathways at sites of free radical generation[39]. The protein of bcl-2 also protects against apoptosis by blocking cytochrome C release hence this protein may have an antioxidant function[40]. In our experiment, the expression of bcl-2 decreased, and the p53 protein upregulated following JTE-522 treatment, these changes may implies that intracellular ROS may interfere with the expression of bcl-2 and p53, thereby contributing to inducing apoptosis in AGS cells.
In studying the mechanism by which the NSAIDs influenced cell death, common effects on the transcription factor NF-κB were noted. The NF-κB transcription factor is ubiquitous and can be detected under its inactive form in the cytoplasm of almost all cell types[41,42]. It supresses the expression of cytokines, chemokines, growth factors, cell adhesion molecules, and some acute phase proteins in health and various pathological states[43,44]. Experimental data clearly indicate that NF-κB is a major regulator of the inflammatory reaction by controlling the expression of pro-inflammatory molecules in response to cytokines oxidatives stress and infectious agents[45,46]. NF-κB is maintained under such an inactive cytoplasmic form by virtue of its association with an inhibitory molecule named IkB. IkBα is the best characterized member of this family. In our current work, EMSA revealed that JTE-522 inhibited NF-κB activation. Western blot experiments demonstrated that this effect was mediated by inhibition of IkBα degradation. Determining the role of NF-κB in gastric carcinogenesis could help to guide the development of improved chemoprevention and treatment strategies.
In summary, JTE-522 inhibited cell growth and induced apoptosis in AGS cells. Increased ROS may play an important role in this caspase-3 mediated apoptotic process. The inhibition of NF-κB by JTE-522 may be mediated by preventing IkBα degradation. The precise relationship and importance of each of these factors in the apoptotic process should be established by more direct and profound analysis.