Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.64
Revised: March 6, 2001
Accepted: March 12, 2001
Published online: February 15, 2002
AIM: To investigate the relationship between hepatocarcinogenesis and the expression of connexin32 (cx32), connexin43 (cx43) mRNAs and proteins in vitro.
METHODS: Gap junction genes cx32 and cx43 mRNA in hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG were detected by in situ hybridization (ISH) with digoxin-labeled cx32, and cx43 cDNA probes. Expression of Cx32 and Cx43 proteins in the cell lines was revealed by indirect immuno-fluorescence and flow cytometry (FCM).
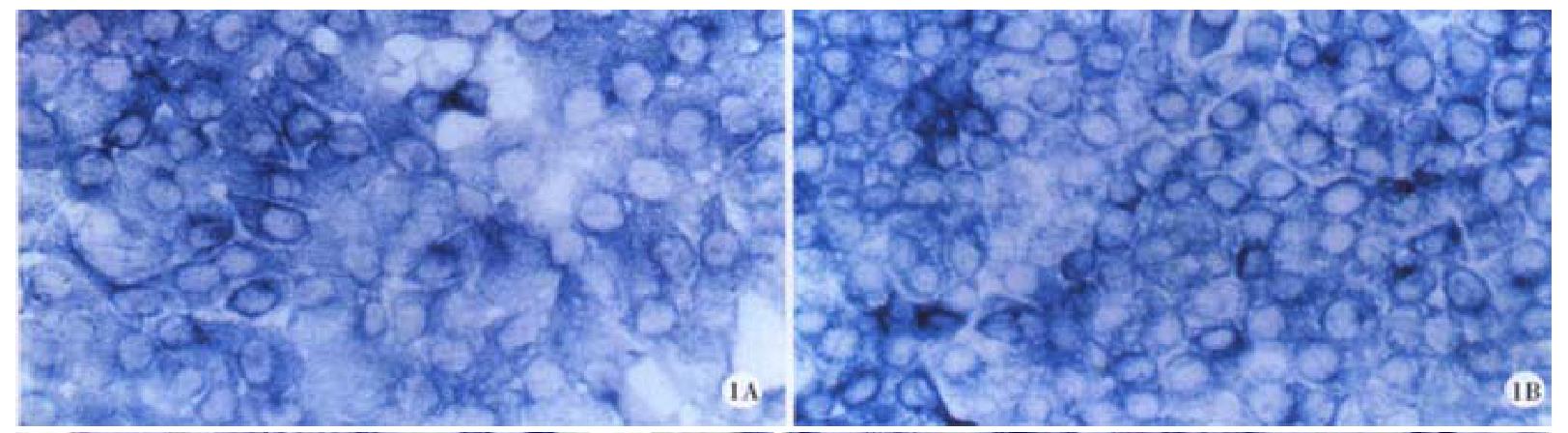
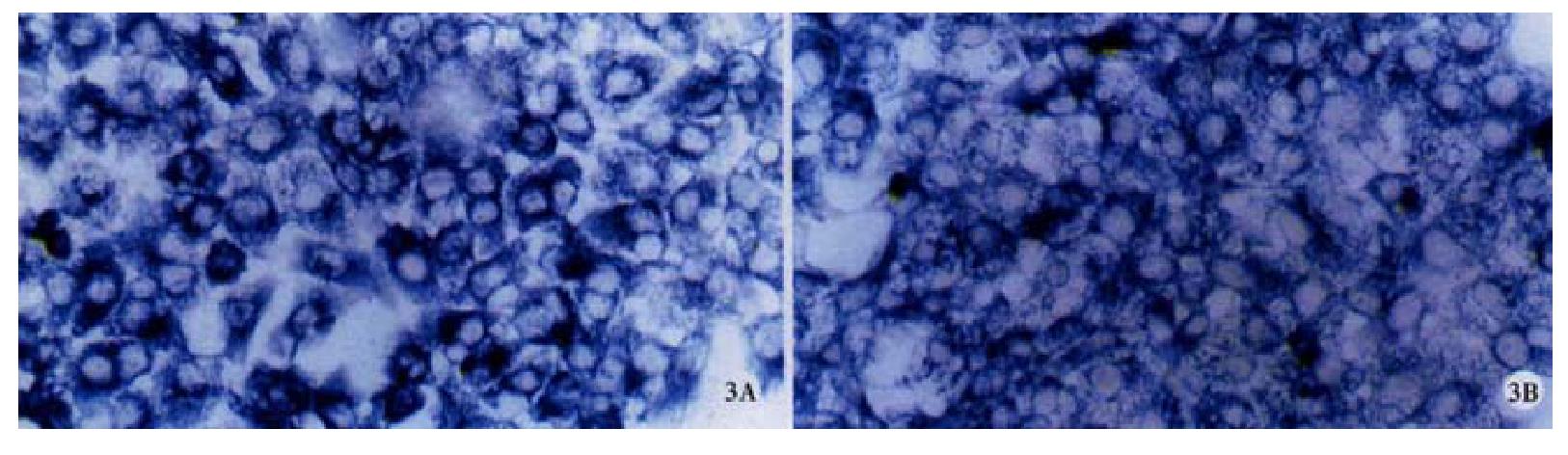
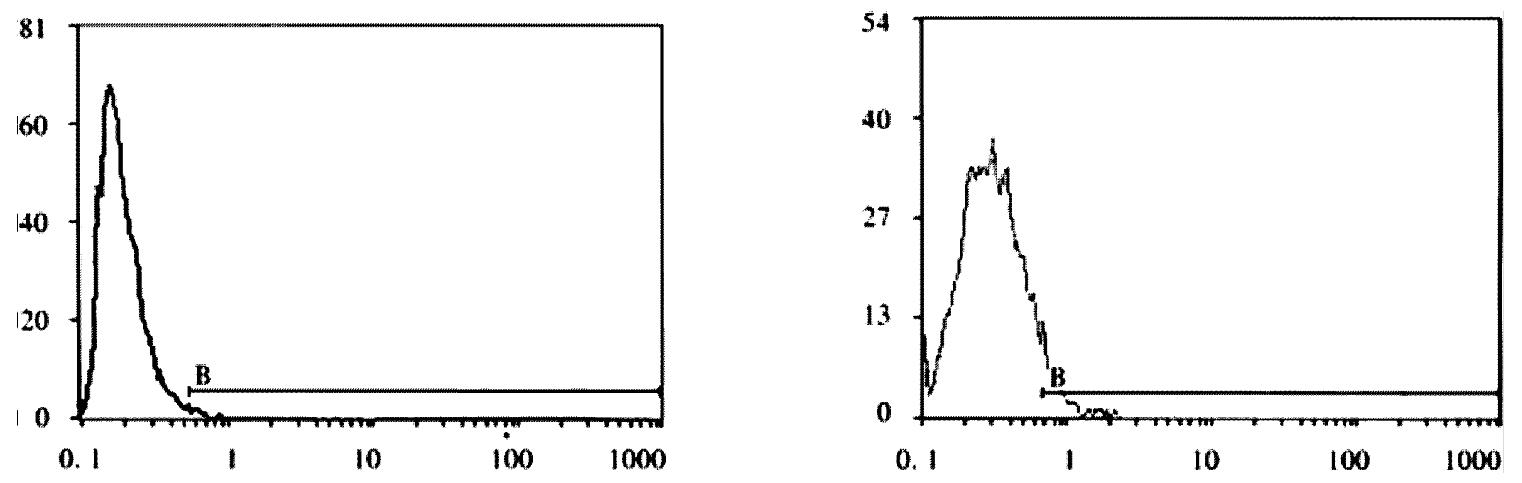
RESULTS: Blue positive hybridization signals of cx32 and cx43 mRNAs detected by ISH with cx32 and cx43 cDNA probes respectively were located in cytoplasm of cells of HHCC, SMMC-7721 and QZG. No significant difference of either cx32 mRNA or cx43 mRNA was tested among HHCC, SMMC-7721 and QZG (P = 2.673, HHCC vs QZG; P = 1.375, SMMC-7721 vs QZG). FCM assay showed that the positive rates of Cx32 protein in HHCC, SMMC-7721 and QZG were 0.7%, 1.7% and 99.0%, and the positive rates of Cx43 protein in HHCC, SMMC-7721 and QZG were 7.3%, 26.5% and 99.1% respectively. Significant differences of both Cx32 and Cx43 protein expression existed between hepatocellular carcinoma cell lines and normal liver cell line (P = 0.0069, HHCC vs QZG; P = 0.0087, SMMC-7721 vs QZG). Moreover, the fluorescent intensities of Cx32 and Cx43 proteins in HHCC, SMMC-7721 were lower than that in QZG.
CONCLUSION: Hepatocellular carcinoma cell lines HHCC and SMMC-7721 exhibited lower positive rates and fluorescent intensities of Cx32, Cx43 proteins compared with that of normal liver cell line QZG. It is suggested that lower expression of both Cx32 and Cx43 proteins in hepatocellular carcinoma cells could play pivotal roles in the hepatocarcinogenesis. Besides, genetic defects of cx32 and cx43 in post-translational processing should be considered.
- Citation: Ma XD, Ma X, Sui YF, Wang WL. Expression of gap junction genes connexin 32 and connexin 43 mRNAs and proteins, and their role in hepatocarcinogenesis. World J Gastroenterol 2002; 8(1): 64-68
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/64.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.64
Carcinoma of the liver is one of the most common malignant tumors which seriously threatens to human life[1-14]. About 90% of carcinoma of the liver are hepatocellular carcinoma (HCC)[15-19]. In each year, more than 250 thousands of new cases of HCC were reported around the world, among which 110000 cases (44.7%) occurred in China[20-24]. Due to the difficulty in early diagnosis and treatment, the tumor molecular mechanisms, early diagnosis and effective methods of clinical treatment for HCC have been a major research project all over the world.
Gap junctions are intercellular channels formed by the interaction of two hemichannels—connexons, one of which is composed of six protein subunits[25,26]. Connexins (Cx) are subunits of gap junctional channels[27,28], by which neighboring cells can exchange low molecular weight ions and molecules, i.e., gap junctional intercellular communication (GJIC)[29,30]. GJIC mediated via gap junctions plays important roles in embryogenesis, cell proliferation, tissue homeostasis and in carcinogenesis[31,32]. Connexins are encoded by a gene family of at least 16 members, which have been divided into two groups based on primary amino acid sequence homology. Connexin32 (Cx32) and connexin43 (Cx43) are the major gap junction forming proteins in liver tissues. Decreased expression of Cx genes and disordered signal transduction pathway of Cx genes contribute to abnormal gap junctional intercellular communication between contacting cells[33]. Uncontrolled tumor cell growth because of the loss of gap junctional intercellular communication due to the down-regulated expression of Cx genes appears to bean important event in cell transformation[34-37]. In this study, the hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG were employed to investigate the relationship between hepatocarcinogenesis and cx32, cx43 mRNA and their protein expression.
Hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG, were kindly provided by Professor Chen in the 863 Research Group. The cells were cultured on slides in RPMI 1640 medium (Gibco BRL, USA), supplemented with 100 mL·L-1 fetal bovine serum (Gibco BRL, USA), incubated in a humidified atmosphere of 950 mL·L-1 air and 50 mL·L-1 CO2 at 37 °C. The cells were passaged by trypsinization twice a week.
pGEM-cx32 plasmid containing cx32 cDNA 1.5 kb, pSG5-cx43 plasmid containing cx43 cDNA 1.1 kb were gifts from Professor Li in Hunan Medical University. After amplification, isolation and purification, pGEM3-cx32 plasmid was digested by EcoR I (Gibco BRL, USA) and pSG5-cx43 by BamH I (Gibco BRL, USA). The digested plasmids were electrophorated on 7 g·L-1 agrose gel with DNA/Hind III + EcoR I Marker (Gibco BRL, USA). cx32, cx43 cDNAs from gel were extracted and purified as the protocol of PCR-pure kit (Clontech, USA), and labeled using Dig DNA labeling and detection kit (Boehringer Mannheim, Germany).
Slides of varied cells were incubated in 0.2 mL·L-1 DEPC at RT for 10 min , then in 0.2 mL·L-1 HCl for 10 min, 5 mg·L-1 PK at 37 °C for 10 min. The digestion reaction was stopped in 0.1 mol·L-1 glycine and the slides were fixed in 40 g·L-1 PFA for 10 min, dehydrated in ethanol and air dried in sequence. Prehybridization was performed at 42 °C for 30 min. The labeled cDNA probes were denatured in hybridization buffer at 100 °C for 10 min, then at -20 °C for 3 min, then added on tissues and coverslipped at 42 °C overnight. Washing of sections was done with 2 × SSC, 1 × SSC, 0.5 × SSC and Buffer I. The slides were incubated in NSS at 37 °C for 30 min, and then Dig-Ap (Boehringer Mannheim, Germany, 1: 500) for 2 h, finally detected with NBT/BCIP of Dig DNA labeling and detection kit (Boehringer Mannheim, Germany). Positive signals were visualized as intensive blue granules in the cytoplasm. Control sections were used. All results were verified by χ2 test.
Total 106·L-1 cells of HHCC, SMMC-7721 and QZG were collected, and blocked with normal serum (Vector, USA) for 30 min at 4 °C, then added mouse-anti Cx32 McAb (Zymed, USA, 1:1000) and mouse-anti Cx43 McAb (Zymed, USA, 1:1000) respectively for 30 min at 4 °C, whereas IgG was added for control. FITC-IgG (Jackson,1:100) was added in the cells for 30 min at 4 °C, precipitated and washed by 0.01 mol·L-1 pH 7.5 PBS. Detective rates and the fluorescent intensity of Cx32, Cx43 proteins in the cells were measured by ELITE ESP flow cytometer (Coulter, USA) and phoenix software (Coulter, USA).
The cx32, cx43 mRNA in hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG were detected by Dig-labeled cx32, cx43 cDNA probes. After in situ hybridization, blue positive hybridization signals of mRNA were located in cytoplasm of the cells. The results showed that bright blue specific hybridization signals of cx32 mRNA and cx43 mRNA were detected in hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver line QZG. χ2 tests did not show any significant difference (P > 0.05) between them. The results were showed in Figures 1, 2 and 3.
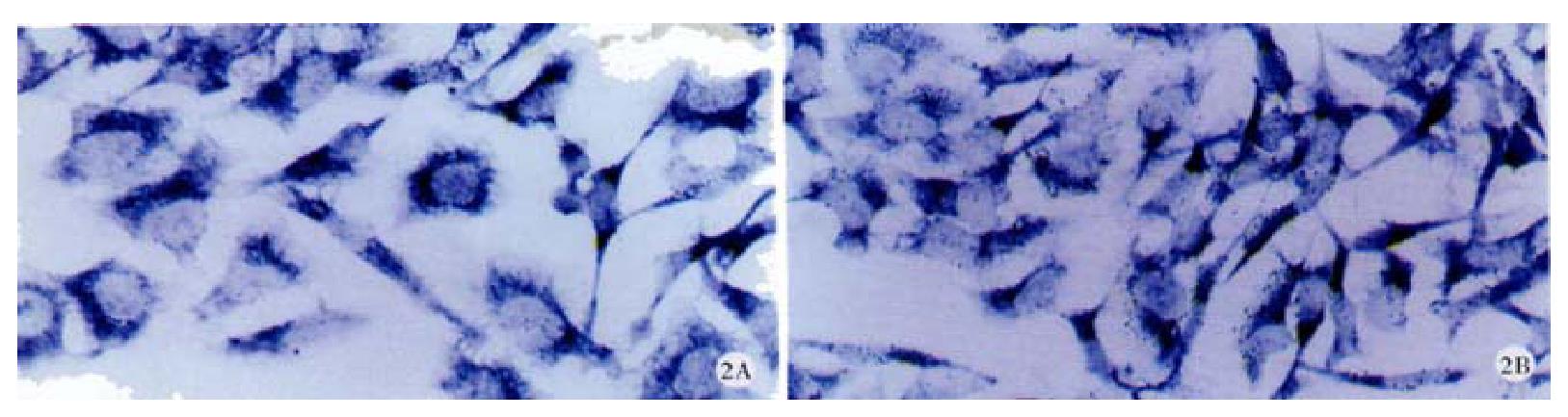
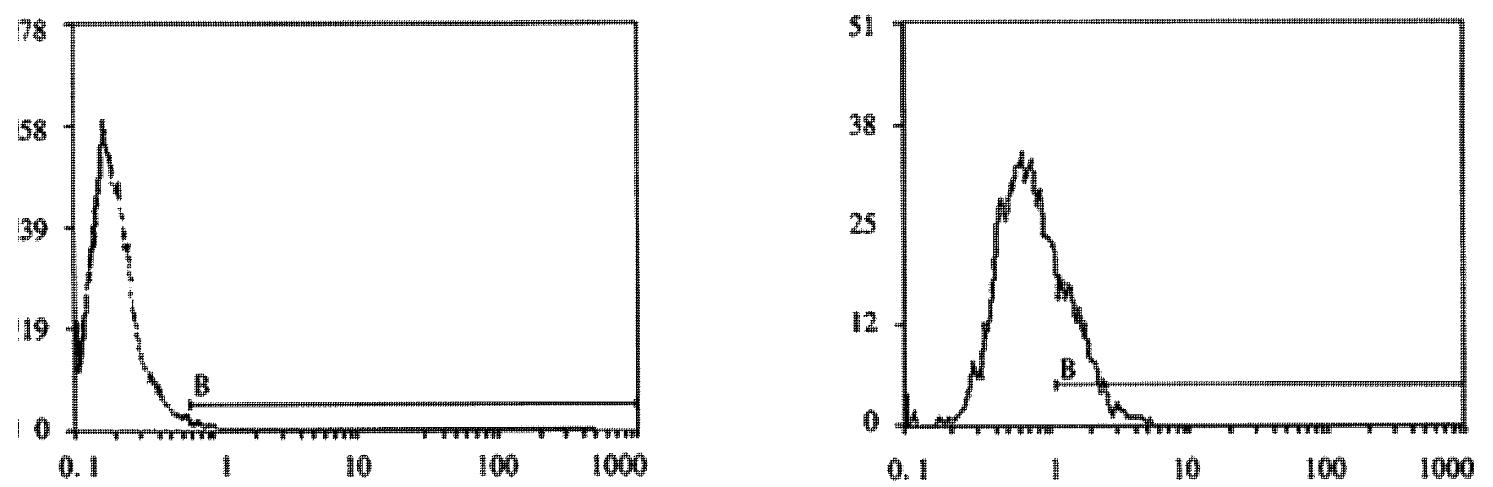
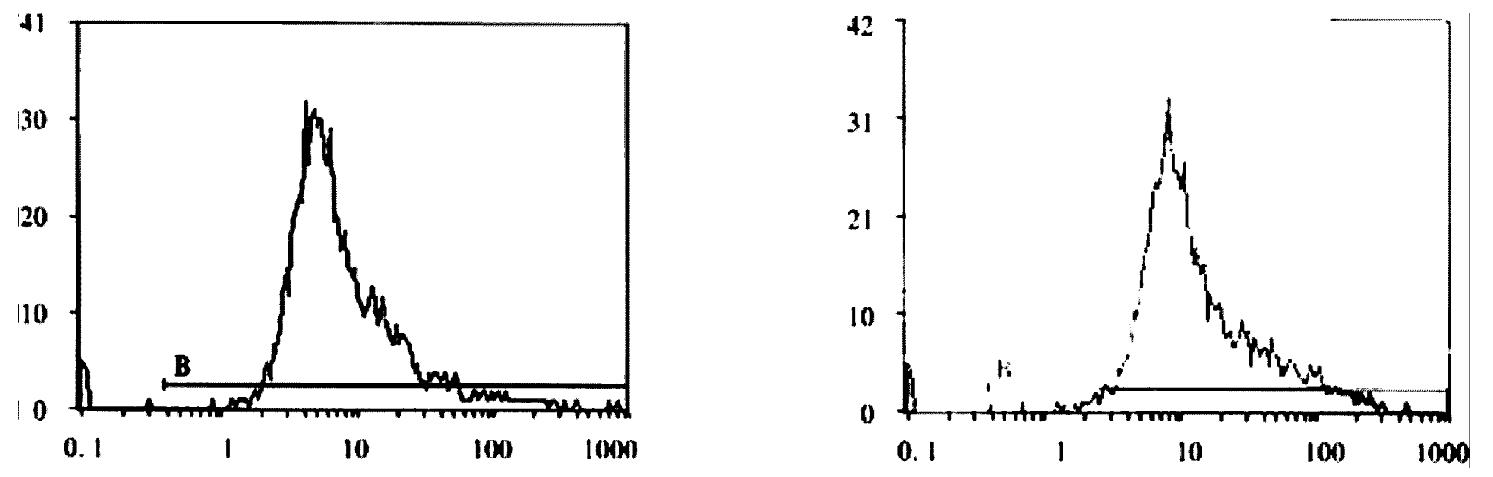
Expression of Cx32, Cx43 proteins in cultured hepatocellular carcinoma cell lines HHCC, SMMC-7721 and normal liver cell line QZG were detected by FCM after immunoreaction with mouse-anti Cx32 McAb, mouse-anti Cx43 McAb. FCM examined both positive rates of Cx proteins expression and their quantities in each cell line.
FCM assay showed that positive rates of Cx32 protein expression in HHCC, SMMC-7721 and QZG were 0.7%, 1.7% and 99.0%, and those of Cx43 protein were 7.3%, 26.5% and 99.1% respectively. The fluorescent intensity of Cx32 protein and Cx43 protein in HHCC, SMMC-7721 were lower than those in QZG. QZG cells showed both higher positive rates for Cx32, Cx43 proteins and strong fluorescent intensity. The detection rates of Cx32, Cx43 proteins were showed in Figure 4, 5, 6 and Table 1.
Gap junction intercellular communication (GJIC) mediated by gap junction channels[38,39] has been postulated to be an important tool for the maintenance of tissues homeostasis, metabolism, control growth and differentiation[40,41]. Carcinogenesis is one of the pathological processes in which disorders of GJIC may play an important role. The inhibited GJIC in many kinds of tumor cells has been found, which could make important contributions to neoplastic progression by allowing tumor cells to escape by either systemic or local control mechanisms[42-44].
Cx32, Cx43 are widely expressed in many tissues, especially in normal liver. This study revealed that Cx32 and Cx43 proteins expressed at a high level in normal liver cell line but at low level in hepatocellular carcinoma cell lines[45-47].
Hepatocarcinogenesis is dramatically enhanced in liver neoplasm tissues lacking of Cx32 and Cx43 proteins, as we shown previously[48]. The results showed that cx32 and cx43 mRNAs and their proteins were highly expressed in normal liver tissues and cell lines, and had significantly decreased in hepatocellular carcinoma tissues and cell lines except expression of Cx43 protein in hepatoma cell line SMMC-7721. cx32 is the specific expression gene in human normal liver tissues and cell line whereas cx43 is a kind of variable expressing gene in either normal liver or hepatoma cell lines.Decrease of Cx proteins related with abnormal function of GJIC between hepatocellular carcinoma cells and surrounding normal cells finally results in hepatocarcinogenesis. Aberrant localization of Cx32 and Cx43 may be not only essential for the reduced GJIC in HCC[49], but also disturb the mechanism of a bystander effect[50]. Expression of the gap junctional proteins is often decreased in tumor tissues, but recruited expression could suppress malignant phenotypes of the tumor cells. The mechanism is that enhanced GJIC on basis of normal gap junctional protein expression regulates homologous and heterologous communication between tumor cells, surrounding normal hepatocytes and other cells[51].
Many observations demonstrate that the lower expression of Cx32 and Cx43 may be involved in the development of hepatocellular carcinoma[52]. It is interesting, moreover, that our results have showed even lower expression of Cx32, Cx43 proteins in hepatocellular carcinoma cell lines HHCC, SMMC-7721 than that in normal liver cell line QZG, but ISH results have shown no decreases of cx32 and cx43 mRNA in hepatocellular carcinoma compared with normal liver cell line. Therefore, it appears that cx32 and cx43 genes transcription is not responsible for aberrant expression of Cx32 and Cx43 proteins during human liver tumorigenesis. Besides, the results have indicated that cx32 and cx43 genes, the specific genes expressing in normal liver tissues, are expected to be the potential unmutated tumor suppressor genes.
Some abnormal regulatory events promote carcinogenesis[55,56], through multiple mechanisms, including post translation process and other potential mechanisms[53-56]. The carcinogenesis and development of hepatocellular carcinoma are related with the abnormal expression of cx genes, signal transduction disorders[57,58], such as reduce of [Ca2+][59,60] and post-translational phosphorylation on tyrosine of Cx proteins, which are associated with dramatic changes in gap junctional intercellular communication and carcinogenesis[61]. The possibility is that defects in post-translational processing of Cx32 and Cx43 proteins may be obstacle for their transportation to cell membranes[62]. Furthermore, phosphorylation on tyrosine of Cx protein can affect the structure of Cx proteins, which is related to channel properties[63]. Post-translational phosphorylation on tyrosine of Cx32 and Cx43 may be important factors controlling the GJIC in hepatocellular carcinoma and substantially responsible for the assembly and function of these proteins as well. Further investigation is expected to understand the mechanism in detail.
Edited by Zhang JZ
| 1. | Ma XD, Sui YF, Wang WL. Expression of gap junction genes connexin 32, connexin 43 and their proteins in hepatocellular carcinoma and normal liver tissues. World J Gastroenterol. 2000;6:66-69. [PubMed] |
| 2. | Wang Y, Liu H, Zhou Q, Li X. Analysis of point mutation in site 1896 of HBV precore and its detection in the tissues and serum of HCC patients. World J Gastroenterol. 2000;6:395-397. [PubMed] |
| 3. | Mei MH, Xu J, Shi QF, Yang JH, Chen Q, Qin LL. Clinical significance of serum intercellular adhesion molecule-1 detection in patients with hepatocellular carcinoma. World J Gastroenterol. 2000;6:408-410. [PubMed] |
| 4. | Qin Y, Li B, Tan YS, Sun ZL, Zuo FQ, Sun ZF. Polymorphism of p16INK4a gene and rare mutation of p15INK4b gene exon2 in primary hepatocarcinoma. World J Gastroenterol. 2000;6:411-414. [PubMed] |
| 5. | Wang XZ, Chen XC, Yang YH, Chen ZX, Huang YH, Tao QM. Relationship between HBxAg and Fas/FasL in patients with hepato-cellular carcinoma. World J Gastroenterol. 2000;6:17-20. |
| 6. | Zhao CY, Li YL, Liu SX, Feng ZJ. Changes of IL6 and relevant cytokines in patients with hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2000;6:33-36. |
| 7. | Su ZJ, Zhang YF, Shi MY, Zeng QX, Chen ML. A prospective study of nosocomial infection in 848 cases of liver diseases. World J Gastroenterol. 2000;6:38-40. |
| 8. | Li JY, Huang Y, Lin MF. Clinical evaluation of several tumor mark-ers in the diagnosis of primary hepatic cancer. World J Gastroenterol. 2000;6:39-41. |
| 9. | Yin ZZ, Jin HL, Yin XZ, Li TZ, Quan JS, Jin ZN. Effect of Boschniakia rossica on expression of GST-P, p53 and p21(ras)proteins in early stage of chemical hepatocarcinogenesis and its anti-inflammatory activities in rats. World J Gastroenterol. 2000;6:812-818. [PubMed] |
| 10. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] |
| 11. | Jiang Z, Liu L, Fang W, Shou WZ, Zhang DS, Dai MM. Local radio-active treatment of hepatocellular cancer with phosphorus32 glass microspheres to enhance the efficacy of hepatic artery chemoembolism and possibly related with MDR expressed glycoprotein. World J Gastroenterol. 2000;6:59-62. |
| 12. | Liu LX, Jiang HC, Zhu AL, Zhou J, Wang XQ, Wu M. Gene expres-sion profiles in liver cancer and normal liver tissues. World J Gastroenterol. 2000;6:85-88. |
| 13. | Wang CF, Shao YF, Zhang HZ. Surgical treatment for patients with stage IVa hepatic carcinoma and related studies. World J Gastroenterol. 2000;6:86-89. |
| 14. | Lin NF, Tang J, Ismael HS. Study on environmental etiology of high incidence areas of liver cancer in China. World J Gastroenterol. 2000;6:572-576. [PubMed] |
| 15. | Tian DY, Yang DF, Xia NS, Zhang ZG, Lei HB, Huang YC. The serological prevalence and risk factor analysis of hepatitis G virus infection in Hubei Province of China. World J Gastroenterol. 2000;6:585-587. [PubMed] |
| 16. | Zhong DR, Ji XL. Hepatic angiomyolipoma-misdiagnosis as hepatocellular carcinoma: A report of 14 cases. World J Gastroenterol. 2000;6:608-612. [PubMed] |
| 17. | Riordan SM, Williams R. Transplantation of primary and reversibly immortalized human liver cells and other gene therapies in acute liver failure and decompensated chronic liver disease. World J Gastroenterol. 2000;6:636-642. [PubMed] |
| 18. | Li Y, Su JJ, Qin LL, Yang C, Luo D, Ban KC, Kensler T, Roebuck B. Chemopreventive effect of oltipraz on AFB(1)-induced hepatocarcinogenesis in tree shrew model. World J Gastroenterol. 2000;6:647-650. [PubMed] |
| 19. | Xu HY, Yang YL, Gao YY, Wu QL, Gao GQ. Effect of arsenic trioxide on human hepatoma cell line BEL-7402 cultured in vitro. World J Gastroenterol. 2000;6:681-687. [PubMed] |
| 20. | Zang GQ, Zhou XQ, Yu H, Xie Q, Zhao GM, Wang B, Guo Q, Xiang YQ, Liao D. Effect of hepatocyte apoptosis induced by TNF-alpha on acute severe hepatitis in mouse models. World J Gastroenterol. 2000;6:688-692. [PubMed] |
| 21. | Huang XF, Wang CM, Dai XW, Li ZJ, Pan BR, Yu LB, Qian B, Fang L. Expressions of chromogranin A and cathepsin D in human primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:693-698. [PubMed] |
| 22. | Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721-724. [PubMed] |
| 23. | Chen YP, Liang WF, Zhang L, He HT, Luo KX. Transfusion transmitted virus infection in general populations and patients with various liver diseases in south China. World J Gastroenterol. 2000;6:738-741. [PubMed] |
| 24. | Ma XD, Sui YF, Wang WL. The expression of gap junction protei n connexin32 in human hepatocellular carcinoma, cirrhotic and viral hepatitis liver tissues. Aizheng. 1999;18:133-135. |
| 25. | Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1356] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 26. | Windoffer R, Beile B, Leibold A, Thomas S, Wilhelm U, Leube RE. Visualization of gap junction mobility in living cells. Cell Tissue Res. 2000;299:347-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J. 2000;79:1954-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Falk MM. Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol. 2000;79:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Ruch RJ, Trosko JE. The role of oval cells and gap junctional intercellular communication in hepatocarcinogenesis. Anticancer Res. 1999;19:4831-4838. [PubMed] |
| 31. | Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 341] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Krutovskikh V, Yamasaki H. The role of gap junctional intercellular communication (GJIC) disorders in experimental and human carcinogenesis. Histol Histopathol. 1997;12:761-768. [PubMed] |
| 33. | Hahn AF, Ainsworth PJ, Naus CC, Mao J, Bolton CF. Clinical and pathological observations in men lacking the gap junction protein connexin 32. Muscle Nerve Suppl. 2000;9:S39-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Moennikes O, Buchmann A, Romualdi A, Ott T, Werringloer J, Willecke K, Schwarz M. Lack of phenobarbital-mediated promotion of hepatocarcinogenesis in connexin32-null mice. Cancer Res. 2000;60:5087-5091. [PubMed] |
| 35. | Moennikes O, Buchmann A, Willecke K, Traub O, Schwarz M. Hepatocarcinogenesis in female mice with mosaic expression of connexin32. Hepatology. 2000;32:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Piechocki MP, Toti RM, Fernstrom MJ, Burk RD, Ruch RJ. Liver cell-specific transcriptional regulation of connexin32. Biochim Biophys Acta. 2000;1491:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Ma XD, Sui YF, Wang WL. Expression of gap junction genes connexin32, connexin43 and their proteins in human hepatocellular carcinoma cell lines and normal liver cell line. Shiyong Aizheng Zazhi. 1999;14:161-163. |
| 38. | Ma XD, Sui YF, Wang WL, Xu QL. A flow cytometric study of gap junction cx32,cx43 in human hepatocellular carcinoma cell lines. Xibao Yu Fenzi Mianyixue Zazhi. 1999;15:51-54. |
| 39. | Ma XD, Sui YF, Wang WL, Wang CM. Expression of gap junction proteins Cx32, Cx43 in human hepatocellular carcinoma cell lines and normal liver cell line: Study with laser scanning confoc al microscope. Zhongguo Zhongliu Zazhi. 2000;10:1-2. |
| 40. | Juul MH, Rivedal E, Stokke T, Sanner T. Quantitative determination of gap junction intercellular communication using flow cytometric measurement of fluorescent dye transfer. Cell Adhes Commun. 2000;7:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Nielsen M, Ruch RJ, Vang O. Resveratrol reverses tumor-promoter-induced inhibition of gap-junctional intercellular communication. Biochem Biophys Res Commun. 2000;275:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Krutovskikh VA, Troyanovsky SM, Piccoli C, Tsuda H, Asamoto M, Yamasaki H. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene. 2000;19:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Christ GJ. Gap junctions and ion channels: relevance to erectile dysfunction. Int J Impot Res. 2000;12 Suppl 4:S15-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Piechocki MP, Burk RD, Ruch RJ. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Ma XD, Sui YF, Wang WL. Expression of gap junction protein connexin32 in human hepatocellular carcinoma, pericancerous liver and normal liver tissues. Disi Junyi Da xue Xuebao. 1999;20:48-50. |
| 46. | Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Wang Y, Rose B. An inhibition of gap-junctional communication by cadherins. J Cell Sci. 1997;110:301-309. [PubMed] |
| 48. | Krutovskikh V, Yamasaki H. Connexin gene mutations in human genetic diseases. Mutat Res. 2000;462:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon AM, Mesnil M, Berger F, Partensky C, Yamasaki H. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994;56:87-94. [PubMed] |
| 50. | Kawamura K, Bahar R, Namba H, Seimiya M, Takenaga K, Hamada H, Sakiyama S, Tagawa M. Bystander effect in uracil phosphoribosyltransferase/5-fluorouracil-mediated suicide gene therapy is correlated with the level of intercellular communication. Int J Oncol. 2001;18:117-120. [PubMed] |
| 51. | Ren P, Ruch RJ. Inhibition of gap junctional intercellular communication by barbiturates in long-term primary cultured rat hepatocytes is correlated with liver tumour promoting activity. Carcinogenesis. 1996;17:2119-2124. [PubMed] |
| 52. | Omori Y, Krutovskikh V, Mironov N, Tsuda H, Yamasaki H. Cx32 gene mutation in a chemically induced rat liver tumour. Carcinogenesis. 1996;17:2077-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Neveu MJ, Hully JR, Babcock KL, Hertzberg EL, Nicholson BJ, Paul DL, Pitot HC. Multiple mechanisms are responsible for altered expression of gap junction genes during oncogenesis in rat liver. J Cell Sci. 1994;107:83-95. [PubMed] |
| 54. | DeoCampo ND, Wilson MR, Trosko JE. Cooperation of bcl-2 and myc in the neoplastic transformation of normal rat liver epithelial cells is related to the down-regulation of gap junction-mediated intercellular communication. Carcinogenesis. 2000;21:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Kolaja KL, Engelken DT, Klaassen CD. Inhibition of gap-junctional-intercellular communication in intact rat liver by nongenotoxic hepatocarcinogens. Toxicology. 2000;146:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III. 1999;322:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Niessen H, Harz H, Bedner P, Krämer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113:1365-1372. [PubMed] |
| 58. | Niessen H, Willecke K. Strongly decreased gap junctional permeability to inositol 1,4, 5-trisphosphate in connexin32 deficient hepatocytes. FEBS Lett. 2000;466:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Suadicani SO, Vink MJ, Spray DC. Slow intercellular Ca(2+) signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H3076-H3088. [PubMed] |
| 60. | Røttingen J, Iversen JG. Ruled by waves Intracellular and intercellular calcium signalling. Acta Physiol Scand. 2000;169:203-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Chen BL, Ma XD, Xin XY, Wang DT, Wang CM. The regulation on signal transduction pat hway of gap junction gene connexin43 in HeLa cell line by all-trans-retinoic a cid. Shiyong Aizheng Zazhi. 2000;15:337-340. |
| 62. | Ma XD, Chen BL, Wang DT, Xin XY. Expressio n of antioncogene connexin43 mRNA and its protein in human cervical carcinomace ll line HeLa. Xiandai Fuchanke Jinzhan. 1999;8:317-319. |
| 63. | Guan X, Ruch RJ. Gap junction endocytosis and lysosomal degradation of connexin43-P2 in WB-F344 rat liver epithelial cells treated with DDT and lindane. Carcinogenesis. 1996;17:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |