Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.162
Revised: October 12, 2001
Accepted: November 5, 2001
Published online: February 15, 2002
AIM: To isolate the proteins involved in pharmacologic action of senna extract (SE) from mouse gastrointestinal tract and to explore the molecular mechanism of gastrointestinal motility change induced by SE.
METHODS: SE was administrated to mice by different routes. Gastrointestinal motility of mice was observed using cathartic, gastrointestinal propellant movement experiments and X-ray analysis. Mouse model for gastrointestinal motility enhancement was established through continuous gastric administration of SE at progressively increased dose. At 3 h and week 3, 4, 6 and 10, morphological changes of gastrointestinal tissues were found under light microscope. Ultrastructural changes of intestinal and colonic tissues at week 6 were observed under transmission electron microscope. The colonic proteomic changes in model mice were examined by two-dimension polyacrylamide gel electrophoresis with immobilized pH gradient isoelectric focusing to screen the differentially expressed proteins, and their molecular masses and isoelectric points were determined. Two N-terminal sequences of the samples were also determined by mass spectrometry.
RESULTS: SE (0.3 g) caused diarrhea after gastric administration in 1-6 h and enhanced gastrointestinal propellant (65.1% ± 7.5%; 45.8% ± 14.6%,P < 0.01) in mice, but intramuscular and hypodermic injection had no cathartic effect. X-ray analysis of gastrointestinal motility demonstrated that gastric administration of SE enhanced gastric evacuation and gastrointestinal transferring function. At 3 h and week 3 and 4 after gastric administration of SE, light microscopic examination revealed no apparent change in gastrointestinal mucosal tissues, but transmission electron microscopic examination revealed inflammatory changes in whole layer of intestinal and colonic wall. Twenty differential proteins were detected in the colonic tissues of the model mice by two-dimensional electrophoresis, and the N-terminal amino acid sequences of two proteins were determined.
CONCLUSION: SE causes diarrhea and enhances gastrointestinal motility through digestive tract administration. Long-term gastric administration of SE induces inflammatory changes and cell damage in the whole gastrointestinal tract. The differential proteins screened from the colonic tissues of the model mice might mediate the enhancing effect of SE on gastrointestinal motility.
- Citation: Wang X, Zhong YX, Lan M, Zhang ZY, Shi YQ, Lu J, Ding J, Wu KC, Jin JP, Pan BR, Fan DM. Screening and identification of proteins mediating senna induced gastrointestinal motility enhancement in mouse colon. World J Gastroenterol 2002; 8(1): 162-167
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.162
Senna, a traditional Chinese medicine, has potent cathartic effect[1-4]. Its extract(SE), composed of a few dozens of chemical substances, possesses multiple pharmacological activities. Especially, it can promote the motility and secretion of gastrointestinal tract. However, its application is greatly restricted due to its toxicity[5-17]. Much attention is being paid to the effect of traditional Chinese medicine on the regulation of gastrointestinal motility[18-37]. We analyzed the role of senna in gastrointestinal motility and in diarrhea of mice and observed its action pattern, the ultrastructural changes in the active sites, and the changes in protein expression. In doing so we intended to screen from the gastrointestinal tissues the biological molecules mediating diarrhea and the enhancement of gastrointestinal movement, to elucidate the mechanism of catharsis induced by senna at the molecular level, and to lay a foundation for the development of pharmaceutical agents enhancing gastrointestinal motility.
Imported senna (Shaanxi Medicine Corporation) was used, and its quality was confirmed by the Institute of Pharmaceutics, Fourth Military Medical University. The extract was obtained through solvent recovery and stored at -20 °C. Acrylamide, bisacrylamide, SDS, Tris base,PMSF (all from Sino-America Biotechnology Co); ultrapure urea (Shanghai Biotechnology Co); ampholyte pH 3-10 L, IPG dry strips pH 3-10 L, IPGphorTM Isoelectric Focusing System (Pharmacia Co); ultradispersor GF-1 (Jiangsu Qilin Medical Equipment Factory); two-dimensional electrophoresis bath DYY-III 26, gradient mixer (Beijing 61 Factory); electrophoresis apparatus (Bio-Rad Co) were used.
Thirty Balb/c mice (both sexes, 8 wk, 18 g-23 g) were used. Before experimental manipulation, the mice was fasted without water deprivation for 24 h, and each was kept in a single cage re-based with filter paper. After being divided into different groups, the mice were administered 0.3 g SE in different routes, and the diarrhea status was examined.
Effect of SE on gastrointestinal motility for carbon Thirty Balb/c mice (both sexes, 6-8 wk, 18-23 g) were divided randomly into 3 groups: Group I, II, and Control Group. SE of 0.3 g was delivered to each mouse in Group I through gastric administration; 0.3 g SE was im injected into each mouse in Group II; and the same volume of 9 g·L- 1 NaCl was delivered through gastric administration to the mice in control group. After 20 min, 0.1 mL of 100 g·L-1 mixture of Arabic gum and charcoal powder was administrated to all mice. Then, all mice were killed after 20 min. Whole gastrointestinal tract was taken out and pulled straight. The length of gastrointestinal tract from pylorus to anus and that from pylorus to the anterior extremity of carbon powder were measured. Percentage of migration distance of carbon powder out of whole length was calculated.
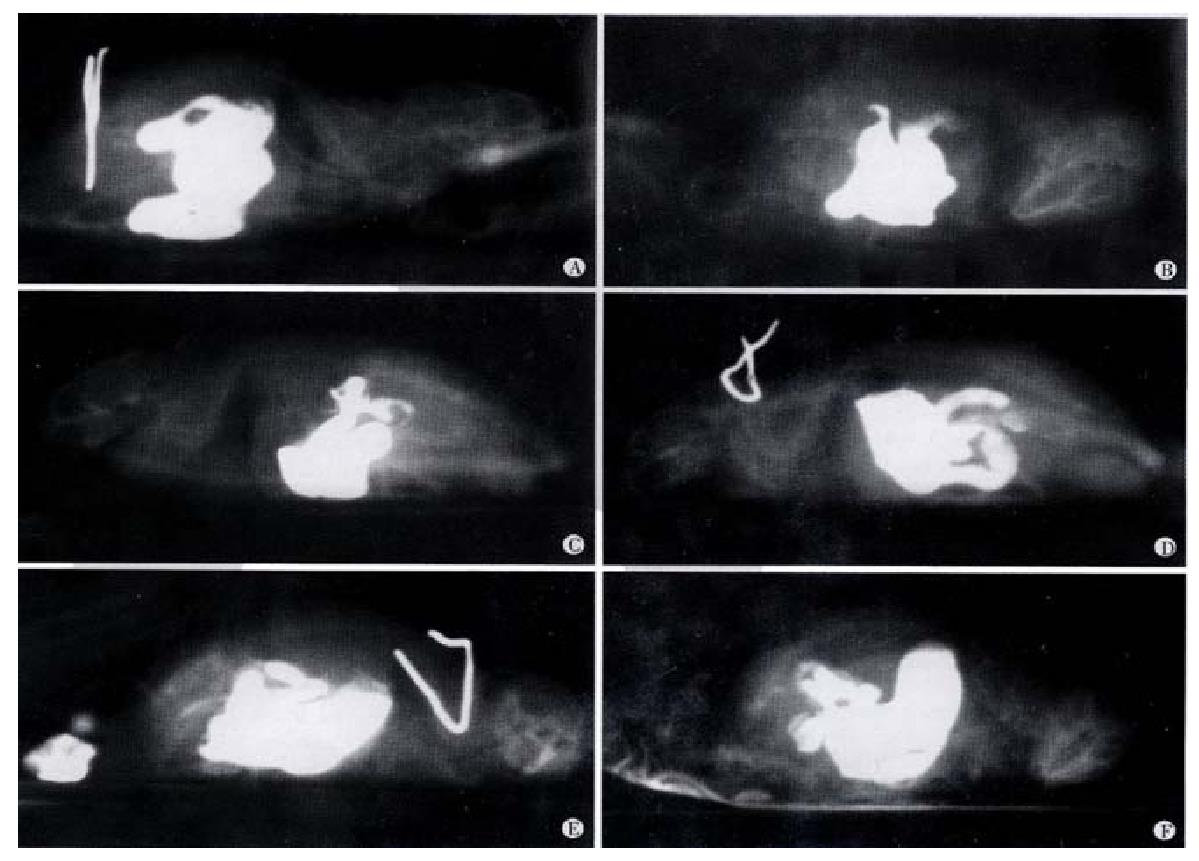
Effect of SE on gastrointestinal motility for barium Eight Balb/c mice (both sexes, 8 wk, 18-20 g), fasting without water deprivation within 24 h prior to the experimental manipulation, were divided randomly into 2 groups. SE of 0.3 g was given through gastric administration to each mouse in Group I, and the same volume of 9 g·L-1 NaCl to each mouse in the control group. After 20 min, 0.8 mL of 1.6 kg·L-1 barium sulfate suspension was given to each mouse. The mice were put into cloth bags that could restrict their movement, and the bags were placed on the flat of the X-ray digital machine. The migration of barium in the gastrointestinal tract was visualized and X-ray photos were taken at 5 and 40 min, 1, 1.5, 2, and 3 h after barium administration[38].
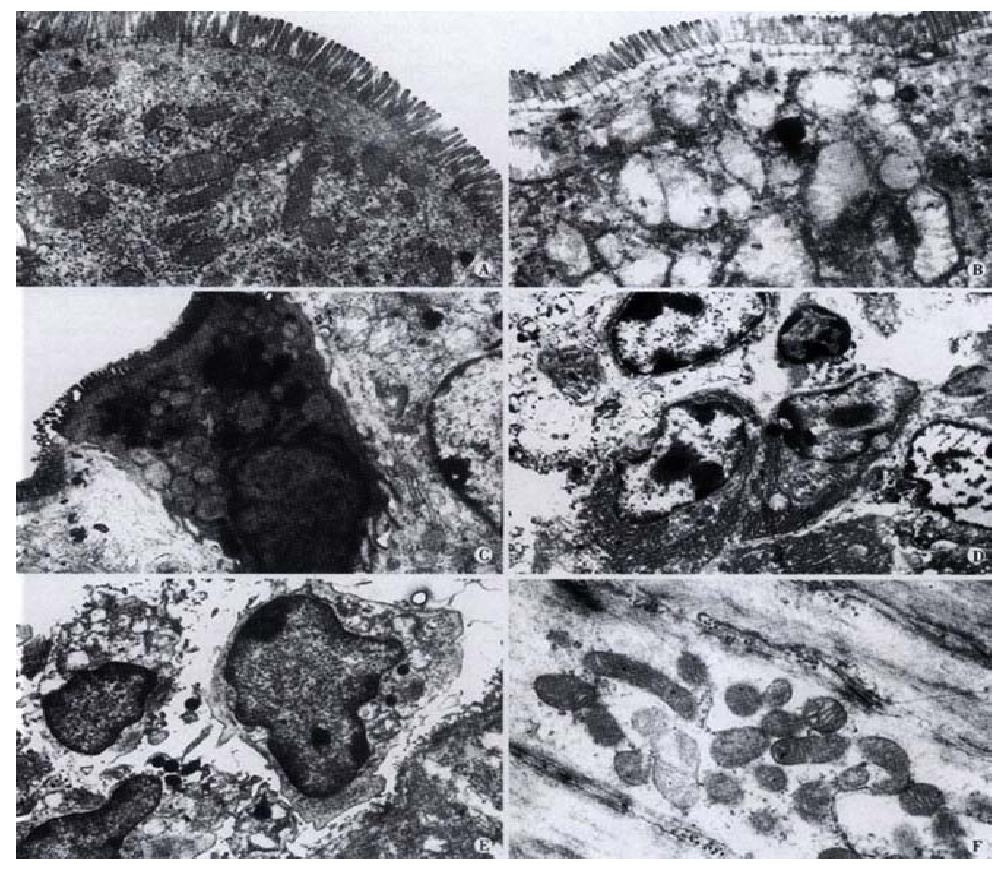
Thirty Balb/c mice (both sexes, 8 wk, 18-23 g) were fed separately according to their sexes. Ten were classified into the control group, and 20 into the experimental group. SE was given through gastric administration to the mice in the experimental group once a day. The frequency of drug delivery increased progressively from 1·d-1 to 4·d-1 and the dosage increased progressively from 0.1 g·d-1 to 0.8 g·d-1 in 6 wk. The same volume of 9 g·L-1 NaCl was given to the control group. Four mice in the experimental group and 2 in the control group were killed at 3 h, 3, 4 and 6 wk after reagent administration. Gastric, intestinal and colonic tissues were obtained and fixed in 40 g·L-1 paraformaldehyde and embedded routinely with paraffin. Sections were made. Morphological changes were examined under light microscope after HE staining. Intestinal and colonic tissues at week 6 were collected and fixed with 30 g·L-1 glutaraldehyde for 6 h at 4 °C. Ultrathin sections were routinely made. Cellular ultrastructural changes in the whole layer of the intestinal wall were examined under transmission electron microscope.
Establishment of animal model for chronic gastrointestinal motility enhancement Fourty Balb/c mice (both sexes, 8 weeks, 18-20 g) were selected. Twenty five mice in the experimental group received SE through gastric administration once a day, with the frequency and dosage increasing progressively from 0.1 g, 1·d-1 to 0.8 g, 4·d-1. Fifteen mice in the control group received the same volume of 9 g·L-1 NaCl.
Preparation of protein sample from intestinal tissue of mice Tweenty mice were killed at 6 weeks after receiving gastric administration of SE (10 mice) and 9 g·L-1 NaCl (10 mice). The colons were collected and put immediately into ice-cold 9 g·L-1 NaCl containing 0.1 mmol·L-1 PMSF. The colonic tracts were dissected and colonic contents were washed out. The moisture on the tissues was absorbed with filter paper, and the tissues were put into liquid nitrogen immediately. The whole procedure must be finished in 5 min. Then, the tissues were either stored at -70 °C until used again, or taken for immediate use. In the latter case, the tissues were weighed on electric scale, and lysed by adding tissue lytic solution (9.5 mol·L-1 urea, 20 g·L-1 NP, 402 g·L-1 ampholyte pH 3-10, 20 g·L-1 2-ME, 1.5 mmol·L-1 EDTA, 40 mmol·L-1Tris and ion-free water) by a mass volume ratio of 1︰5. Tissue homogenate was prepared with high-speed disperser in ice-bath (3500 r·min-1× 5 s × 5), and DNase and RNase (both 0.4 g·L-1) were added into the homogenate. After the incubation for 20 min in ice-bath and addition of PMSF (0.1 mmol·L- 1), the homogenate was centrifuged at 10000 × g for 10 min. The supernatant was harvested and stored in designated volume at -70 °C until used again. The total protein concentration in the supernatant was measured by Bradford method[40-42].
Solid-phase pH gradient isoelectric focusing For each group, 100 μg protein sample was solved in 250 μL mixture of deuteroxide solution and IPG buffer, placed at room temperature for 1h, and then applied to sample tank which was placed with IPG dry gel strip (13 cm in length), and covered with mineral oil. The isoelectric focusing program was: deuteroxidization for 12 h; isoelectric focusing 0-300 V, 1 h; 300-500 V, 1 h; 500-1000 V, 1 h; 1000-2000 V, 1 h; 2000-4000 V, 1 h; 4000-8000 V, 4 h. All operations above were performed at 20 °C.
Equilibration and transfer of IPG slab gels The slab gels were collected and equilibrated with SDS-balanced buffer (50 mmol·L-1 Tris, 6 mol·L-1 urea, 300 g·L-1 glycerin, 10 g·L-1 DTT, ion-free water) for 15 min followed by a second equilibration with balanced buffer (50 mmol·L-1 Tris, 6 mol·L-1 urea, 300 g·L-1glycerin, 25 g·L-1 idocetamide, ion-free water) for another 15 min. Two pieces of 125 g·L-1 separation gel (17 cm × 17 cm × 0.15 cm) was prepared in absence of sticking gels. IPG slab gels were fixed on the top of the seperation gel using 5 g·L-1 agarose. Protein marker was applied at the other terminus.
Second dimension SDS-PAGE Two pieces of 125 g·L-1 SDS-PAGE (17 cm × 17 cm × 0.15 cm) were prepared and underwent polymerization for 2 h in absence of sticking gels; IPG slab gels were fixed onto the top of SDS-PAGE gels; electrophoresis was performedfor 11 h at room temperature (15 °C-20 °C) with constant electric current (20 mA/gel, 40mA in total) and terminated when the marker arrived at the bottom.
Silver staining of gels The gels were collected and stained with silvery salt based on a modified protocol. The gels were fixed with the fixing solution (300 g·L-1 ethanol, 0.5 mol·L-1 sodium acetate, 5 g·L-1 glutaraldehyde, 2 g·L-1 sodium thiosulfate), rinsed with ion-free water for 15 min × 3, stained with 1 g·L -1 AgNO3 and 0.1 g·L-1formaldehyde for 20 min, washed with ion-free water for 30sec, and colored with 25 g·L-1 sodium carbonate, 0.5 g·L-1 sodium thiosulfate and 0.1 g·L-1 formaldehyde. The color development was terminated with 10 g·L-1 acetic acid.
Analysis of protein map on two-dimensional gels After silvery salt staining, the gels were scanned with transmission laser scanner at 500 bpi resolution and analyzed with image pattern analyzer under identical conditions. The position, shape and density information for each detected spot was compared to screen obviously differentially expressed proteins and to determine their molecular masses and isoelectric points.
Identification of the screened proteins by sequencing[43] One mg colonic proteins from the animal model was sampled and underwent solid-phase pH gradient isoelectric focusing and second dimension SDS-PAGE and transferred from gel slabs to polyvinylidene difluoride (PVDF) membranes (transferring buffer: CAPS, DTT, methanol and ion-free water, pH 11.0; transferring condition: 350 mA,4.5 h, 10 °C). The proteins on the PVDF membranes were stained with 1 g·L-1 Coomassie brilliant blue. PDVF membrane was air-dried at room temperature. The protein spots stained with Coomassie brilliant blue were excised from the membrane, and their amino acid sequences at the N-termini were determined by mass spectrometry.
Student’s t test and χ2 test were used for data measurement and enumeration, respectively.
One to 1.5 h after gastric administration of SE, mice started to suffer from diarrhea, defecating water-thin feces, which lasted 4-5 h. When the mice received im and sc injection of extract of doubled dosage, they did not develop diarrhea within 6 h (Table 1).
Effect of SE on gastrointestinal propellant movement While ig administration of SE could enhance gastric motility, im delivery did not show such effect (Table 2).
| Group | Dose/g | Administration routes | n | Propellant rate/% | |
| I | Normal saline | 0.3 | ig | 10 | 45.8 ± 14.6 |
| II | SE | 0.3 | ig | 10 | 65.1 ± 7.5b |
| III | SE | 0.3 | im | 10 | 48.3 ± 12.4 |
X-ray analysis of SE effect on gastrointestinal motility In mice with gastric administration of SE (0.3 g), barium sulfate was excreted at 40 min, and more at 2 h. The migration speed was significantly higher than that shown in the control group (Figure 1).
Morphological effects of SE on gastrointestinal tissue No pathological changes were observed in gastrointerestinal tissues under light microscopy examination at 3 h, 3, 4 and 6 wk after gastric administration of SE. However, at week 6, it was observed under transmission electron microscope that some small intestine epithelial cells underwent degeneration and necrosis. Although the microvilli were normal, the mitochondria in the cells were slightly swollen, with part of the cristae broken. In the mucosa, macrophages containing phagocytic particles were found to increase, and so were plasmacytes. Cell degeneration and necrosis occurred more frequently in the mucosal epithelial cells in colon than those in the small intestine (Figure 2). The mitochondrial abnormalities mentioned above were observed in both small intestine and colon smooth muscle cells, along with the occurrence of vacuolation.
Establishment of mice model for chronic gastrointestinal movement enhancement Gastrointestinal propellant movement experiment and X-ray analysis of gastrointestinal motility demonstrated that gastric administration of SE could significantly enhance the gastrointestinal motility in Balb/c mice. Therefore, continuous gastric administration of SE might keep the gastrointestinal tract in constant enhanced motility. At week 6, the weight of the mice in the model group was significantly lower than that in the control group.
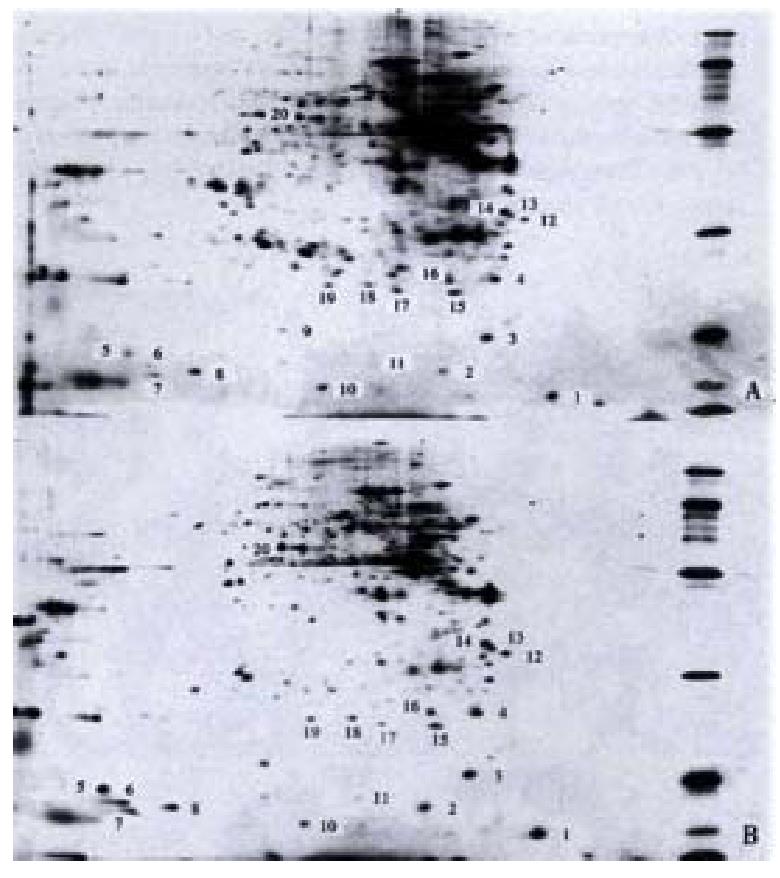
Differential expression of gastrointestinal tissue proteins in mice In the first dimension IPG isoelectric focusing, pH gradient was pH 3-10 L, 100 μg protein sample was added. In the second dimension IPG -PAGE 125 g·L-1 uniform gel was used, the gels were stained with silver salt (Figure 3).
Identification of differentially expressed proteins The colonic proteins of one control mouse and one model mouse were screened and compared, and the process repeated for 5 such pairs. Twenty proteins were found different in abundance (Table 3).
| No. | Molecular mass/ku | Isoelectric point/pI | No. | Molecular mass/ ku | Isoelectric Point/pI |
| 1 | 15.0 | 3.9 | 11 | 18.0 | 5.9 |
| 2 | 17.5 | 5.2 | 12 | 33.0 | 4.3 |
| 3 | 19.0 | 4.7 | 13 | 34.0 | 4.5 |
| 4 | 26.0 | 4.6 | 14 | 35.0 | 4.6 |
| 5 | 18.0 | 8.8 | 15 | 24.0 | 5.1 |
| 6 | 17.5 | 8.6 | 16 | 26.0 | 5.2 |
| 7 | 16.5 | 8.5 | 17 | 24.5 | 5.7 |
| 8 | 17.0 | 8.0 | 18 | 25.0 | 6.1 |
| 9 | 20.0 | 7.0 | 19 | 25.0 | 6.5 |
| 10 | 16.0 | 6.6 | 20 | 52.0 | 7.0 |
Identification of differential proteins by sequencing The N-terminal amino acid sequences of protein No. 4 and No 12 were determined by sequencing (No. 4: N-MIX/IYR-C; No 12: N-GFXDX/L-C). Protein database search showed that these two proteins were unknown proteins.
Senna is a traditional Chinese medicine containing various chemicals, the effective components of which are sennoside A, B, C and D. It has been shown that sennosides are decomposed into Rhein anthrone by bacteria in colon, and then take effects on the colonic smooth muscle, significantly promoting colonic motility in animals and humans. The mechanism is that sennoside and its active forms affect on the intestinal mucosal epithelium and submucosal nerve bundles, stimulating prostaglandin (PG) synthesis and endogenous acetylcholine release, and subsequently enhancing colonic smooth muscle contraction. [44-46]Meanwhile, sennosides may affect directly the colonic smooth muscle, evoking its spontaneous spike potential and promoting its contraction[47]. In addition, sennosides can stimulate the colonic mucosa to release PG, NO and 5-HT, efficiently inducing the excretion of water and electrolytes by epithelial cells[48-50]. Our study showed that 1-1.5 h after gastric administration of SE, mice started to suffer from diarrhea, defecating water-thin feces, which lasted 4-5 h. When the mice received im injection of extract of doubled dosage, they did not develop diarrhea within 6 h, but after 6 h, 3 mice had light diarrhea. It suggested that the possible pathway might be that the drug delivered through im injection entered blood circulation and was excreted into the intestinal tract with bile. With hypodermic injection of larger dosage of extract, no mice developed diarrhea. However, no enhanced gastrointestinal motility was observed after im injection of SE. These results indicate that the cathartic effect of SE can only be exerted through the digestive tract rather than other pathways.
Our study also indicated that SE could promote the propellant movement in mice, a result in agreement with the reports of other researchers. Although most researchers believe that the active components of senna mainly work on colon and promote colonic motility. We think that it may also affect small intestine, as our gastrointestinal propellant movement experiment has revealed that it promoted the motility of mice small intestine in mice. In order to locate the effective site of the drug, we continuously observed the movement of marked liquid (BaSO4) in the gastrointestinal tract after administration of SE, and discovered that it in hibited rather than promoted gastric empty; however, it promoted small intestinal motility, and especially colonic motility.
We made histological examinations of mouse gastrointestinal tracts at different stages after gastric administration of SE. No apparent change was found under light microscope, but transmission electron microscopy showed lesions and degenerative changes of small intestinal mucosal epithelial cells, increase in submucosal macrophages and plasmacytes, minor degeneration of smooth muscle cells, and severe extensive degeneration and necrosis of colonic epithelial cells. Mengs et al[10] observed the changes in guinea pig colonic epithelial cells after continuous gastric administration of sennoside for 2 weeks, and discovered epithelial cell degeneration. These results show that SE can injure the intestinal cells.
Using improved two-dimensional electrophoresis of proteomic analysis[42,51,52], we compared the protein expression in the colon tissues of promoted gastrointestinal motility model mice and that of normal controls. The conditions in the whole process were identical for the two groups, and the location, shape, size and density of many protein spots were similar on the two-dimensional electrophoresis maps. Hence, the two groups were comparable. Image analysis showed differential proteins in the colon tissues of the model animals; the differences were primarily the increase or decrease in the amount of protein expression. Most of the differential proteins had moderate or low molecular mass, as shown in Table 3, which are probably regulatory proteins induced or affected by SE. Two of the proteins were sequenced with mass spectrometry and were confirmed to be novel ones through protein database search. However, the pharmacological functions they mediate remain to be discovered.
SE administered through the gastrointestinal tract promotes diarrhea and gastrointestinal motility, especially that of the small intestine and the colon; it also injures the digestive tract mucosa and smooth muscles, and promotes differential protein expression in the colon tissues of model animals. We have isolated and identified some of the molecules that may be involved in mediating the motility promotion and secretion effect of senna, and will continue to investigate the molecular mechanism of the aforementioned effects so as to lay the foundation for further research.
Edited by Ma JY
| 1. | Arezzo A. Prospective randomized trial comparing bowel cleaning preparations for colonoscopy. Surg Laparosc Endosc Percutan Tech. 2000;10:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Chilton AP, O'Sullivan M, Cox MA, Loft DE, Nwokolo CU. A blinded, randomized comparison of a novel, low-dose, triple regimen with fleet phospho-soda: a study of colon cleanliness, speed and success of colonoscopy. Endoscopy. 2000;32:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Krumbiegel G, Schulz HU. Rhein and aloe-emodin kinetics from senna laxatives in man. Pharmacology. 1993;47 Suppl 1:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Valverde A, Hay JM, Fingerhut A, Boudet MJ, Petroni R, Pouliquen X, Msika S, Flamant Y. Senna vs polyethylene glycol for mechanical preparation the evening before elective colonic or rectal resection: a multicenter controlled trial. French Association for Surgical Research. Arch Surg. 1999;134:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lan M, Wang X, Wu HP, Fan DM. Biological effects of senna extract on human intestinal epithelial cells. Shijie Huaren Xiaohua Zazhi. 2001;9:555-559. |
| 6. | Stickel F, Seitz HK, Hahn EG, Schuppan D. [Liver toxicity of drugs of plant origin]. Z Gastroenterol. 2001;39:225-32, 234-7. [PubMed] |
| 7. | Adam SE, Al-Yahya MA, Al-Farhan AH. Combined toxicity of Cassia senna and Citrullus colocynthis in rats. Vet Hum Toxicol. 2001;43:70-72. [PubMed] |
| 8. | Tasaka AC, Weg R, Calore EE, Sinhorini IL, Dagli ML, Haraguchi M, Górniak SL. Toxicity testing of Senna occidentalis seed in rabbits. Vet Res Commun. 2000;24:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Calore EE, Weg R, Haraguchi M, Calore NM, Cavaliere MJ, Sesso A. Mitochondrial metabolism impairment in muscle fibres of rats chronically intoxicated with Senna occidentalis seeds. Exp Toxicol Pathol. 2000;52:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Mascolo N, Mereto E, Borrelli F, Orsi P, Sini D, Izzo AA, Massa B, Boggio M, Capasso F. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon. Dig Dis Sci. 1999;44:2226-2230. [PubMed] |
| 11. | Mengs U, Grimminger W, Krumbiegel G, Schuler D, Silber W, Völkner W. No clastogenic activity of a senna extract in the mouse micronucleus assay. Mutat Res. 1999;444:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Mukhopadhyay MJ, Saha A, Dutta A, De B, Mukherjee A. Genotoxicity of sennosides on the bone marrow cells of mice. Food Chem Toxicol. 1998;36:937-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Haraguchi M, Calore EE, Dagli ML, Cavaliere MJ, Calore NM, Weg R, Raspantini PC, Górniak SL. Muscle atrophy induced in broiler chicks by parts of Senna occidentalis seeds.. Vet Res Commun. 1998;22:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Calore EE, Cavaliere MJ, Haraguchi M, Górniak SL, Dagli ML, Raspantini PC, Calore NM, Weg R. Toxic peripheral neuropathy of chicks fed Senna occidentalis seeds.. Ecotoxicol Environ Saf. 1998;39:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Cavaliere MJ, Calore EE, Haraguchi M, Górniak SL, Dagli ML, Raspantini PC, Calore NM, Weg R. Mitochondrial myopathy in Senna occidentalis-seed-fed chicken. Ecotoxicol Environ Saf. 1997;37:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Calore EE, Cavaliere MJ, Haraguchi M, Górniak SL, Dagli ML, Raspantini PC, Perez Calore NM. Experimental mitochondrial myopathy induced by chronic intoxication by Senna occidentalis seeds. J Neurol Sci. 1997;146:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Brusick D, Mengs U. Assessment of the genotoxic risk from laxative senna products. Environ Mol Mutagen. 1997;29:1-9. [PubMed] [DOI] [Full Text] |
| 18. | Zhang HX, Ren P, Huang X, Li Yuan. Regulation of the traditional Chinese medicine on gastrointestinal hormone and motility. Shijie Huaren Xiaohua Zazhi. 2000;8. |
| 19. | Zhu JZ, Yang GH, Leng EF, Chen DF. Effects of the traditional Chinese midicine on gastrointestinal motility. Shijie Huaren Xiaohua Zazhi. 1999;7:689-690. |
| 20. | Pang L, Zhou DR. Regulation of the traditional Chinese medicine on gastrointestinal motility. Huaren Xiaohua Zazhi. 1998;6:535. |
| 21. | Qin XM, Li HF, Wang LD. Effects of metoclopramide on gastrointesti-nal myoelectric activity in rats. Chin Natl J New Gastroenterol. 1997;5:169. |
| 22. | Zheng TZ, Li W, Qu SY, Ma YM, Ding YH, Wei YL. Effects of Dangshen on isolated gastric muscle strips in rats. World J Gastroenterol. 1998;4:354-356. [PubMed] |
| 23. | Lin J, Cai G, Xu JY. A comparison between Zhishi Xiaopiwan and cisapride in treatment of functional dyspepsia. World J Gastroenterol. 1998;4:544-547. [PubMed] |
| 24. | Zhang ZQ, Zhang HP, Ha CL, Li XZ, Lu GQ, Chen GL, Zhang GH. Clinical analysis of therapeutic effect of traditional Chinese medicine on peptic ulcer. World J Gastroenterol. 1998;4:88-89. |
| 25. | Li W, Zheng TZ, Qu SY. Effect of cholecystokinin and secretin on contractile activity of isolated gastric muscle strips in guinea pigs. World J Gastroenterol. 2000;6:93-95. [PubMed] |
| 26. | Lin XZ, Ma DL, Cui ZQ, Kang Y. Effects of rhubarb and the active ingredients of rhubarb on the cytoplasmic free calcium in INT-MNC of rabbits. World J Gastroenterol. 2000;6:301-303. [PubMed] |
| 27. | Tian XL, Mourelle M, Li YL, Guarner F, Malagelada JR. The role of Chinese herbal medicines in a rat model of chronic colitis. World J Gastroenterol. 2000;6:40. |
| 28. | Ma XS, Fan XP, Chen Z, Li CW, Xing YS. Effects of rhizoma atractylodis macrocephalae on the contraction of isolated ileum of guinea pig. Xin Xiaohuabingxue Zazhi. 1996;4:603-604. |
| 29. | Wang ZH, Lu LS. Effect of Weichangtong on gastrointestinal motility disorders. Xin Xiaohuabingxue Zazhi. 1997;5:448-449. |
| 30. | Li Y, Sun SY, Zou Z, Chen SN, Wang XY. Influences of 6 fomula compositions combined of 8 kinds of Chinese medicinals on gastro intestinal motility in mice. Huaren Xiaohua Zazhi. 1998;6:208-209. |
| 31. | Pang L, Zhou DR. Regulative action of Chinese traditional medicine in gastrointestinal motility. Huaren Xiaohua Zazhi. 1998;6:535-536. |
| 32. | Gao F, Zhang SB, Zhang LY, Liu F, Tong WD, Li FZ. An experiment study of the smallintestinal transit injuried by contact laxatives. Shijie Huaren Xiaohua Zazhi. 1999;7:659-660. |
| 33. | Zhu JZ, Yang GH, Leng ER, Chen DF. Gastrointestinal motility promoting action oftraditional Chinese medicine. Shijie Huaren Xiaohua Zazhi. 1999;7:689-690. |
| 34. | Wang J, Hou JY. Effect of granulae Li Wei on gastrointestinal activity. Shijie Huaren Xiaohua Zazhi. 2000;8:377-381. |
| 35. | Hao Q, Li Y, Yin HT. Comparative effect of different varieties of Bupleurum and Citrus on gastrointestinal motility in mice. Huaren Xiaohua Zazhi. 1998;6:205-207. |
| 36. | Li SZ, Tan XH. Effect of Astragalus membranaceus on intestinal blood flow and motility in dogs. Xin Xiaohuabingxue Zazhi. 1997;5:659-660. |
| 37. | Wang X, Zhang ZY, Shi YQ, Lan M, Han QL, Wu HP, Jin JP, Fan DM. Prelimihary study on protein differential expression of small bowel in BALB/c mice induced by croton oil. Chin J Gastroenterol Hepatol. 2000;9:103-106. |
| 38. | Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszódi A, Andersson KE. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 409] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 39. | Wang X, Wang BL, Zhang ZY, Lan M, Wang JC, Yao LB, Chen NC, Jin JP, Fan DM. Improvement and application of two-dimensional gel elec-trophoresis in proteome analysis. J Cell Mol Immunol. 2001;17:191-192. |
| 40. | Radloff M, Delling M, Marti T, Gercken G. Hsp27 phosphorylation is induced in alveolar macrophages exposed to CdO-coated silica particles. Biochem Biophys Res Commun. 1998;248:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Staudenmann W, Hatt PD, Hoving S, Lehmann A, Kertesz M, James P. Sample handling for proteome analysis. Electrophoresis. 1998;19:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Arnott D, O'Connell KL, King KL, Stults JT. An integrated approach to proteome analysis: identification of proteins associated with cardiac hypertrophy. Anal Biochem. 1998;258:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940-17953. [PubMed] |
| 44. | Yagi T, Miyawaki Y, Nishikawa A, Yamauchi K, Kuwano S. Suppression of the purgative action of rhein anthrone, the active metabolite of sennosides A and B, by indomethacin in rats. J Pharm Pharmacol. 1991;43:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Yagi T, Yamauchi K, Kuwano S. The synergistic purgative action of aloe-emodin anthrone and rhein anthrone in mice: synergism in large intestinal propulsion and water secretion. J Pharm Pharmacol. 1997;49:22-25. [PubMed] |
| 46. | Nijs G, de Witte P, Geboes K, Lemli J. Influence of rhein anthrone and rhein on small intestine transit rate in rats: evidence of prostaglandin mediation. Eur J Pharmacol. 1992;218:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Lan M, Wang X, Liu Na, Fan DM. Effect of senna extract on contrac-tion of colonic smooth muscle cells in guinea pig. Di-si Junyi Daxue Xuebao. 2001;(in press). |
| 48. | van Gorkom BA, Karrenbeld A, van Der Sluis T, Koudstaal J, de Vries EG, Kleibeuker JH. Influence of a highly purified senna extract on colonic epithelium. Digestion. 2000;61:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Izzo AA, Mascolo N, Capasso F. Nitric oxide as a modulator of intestinal water and electrolyte transport. Dig Dis Sci. 1998;43:1605-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 50. | Izzo AA, Sautebin L, Rombolà L, Capasso F. The role of constitutive and inducible nitric oxide synthase in senna- and cascara-induced diarrhoea in the rat. Eur J Pharmacol. 1997;323:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Wang X, Zhang ZY, Shi YQ, Lan M, Ma Z, Jin JP, Fan DM. Differen-tial expression of colonic proteins induced by extract of senna in mice. Di-si Junyi Daxue Xuebao. 2001;22:16-19. |
| 52. | Wang X, Shi YQ, Zhao YQ, Wang JC, Yao LB, Zhang ZY, Lan M, Jin JP, Fan DM. Differential display of Vincristine-resistance-related proteins in gastric cancer SGC7901 cell line. Zhonghua Zhongliu Zazhi. 2001;23:281-284. |