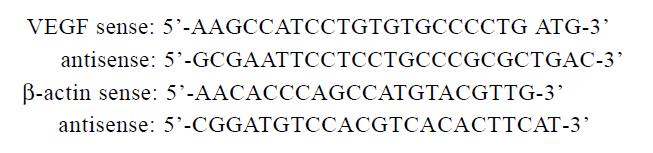Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.153
Revised: September 11, 2001
Accepted: September 27, 2001
Published online: February 15, 2002
AIM: To evaluate the effect of reactive oxygen species such as hydrogen peroxide on the progression of human colon cancer.
METHODS: Human colon carcinoma cell lines, LS174T and HCT8, were treated respectively with 10-5, 10-7 or 10-9 mol·L-1 hydrogen peroxide for 24 h, and co-cultured with human endothelial cell line ECV-304. The migration of ECV-304 induced by cancer cells was calculated and the expression level of vascular endothelial growth factor in cancer cells was determined by RT-PCR analysis and ELISA. Dactinomycin of 1.5 mg·L-1 which could block transcription of cancer cells was applied to observing the effects of H2O2 on transcriptional activity and the relative half-life of VEGF mRNA. Finally, to evaluate the effect of H2O2 on NF-κB activity in colon cancer cells, NF-κB in cytoplasm and nucleus of the cells were detected with FITC-tagged antibody and its presence in the nucleus(Fn) vs cytoplasm(Fc) was monitored by measuring the green fluorescence integrated over the nucleus by laser scanning cytometry(LSC)
RESULTS: Exogenouse hydrogen peroxide of low concentration increased the migration of endothelial cell induced by colon cancer cells. When cancer cells were treated with 10-5 mol·L-1 H2O2, the migration number of endothelial cells induced by LS174T cells was 203 ± 70, and the number induced by HCT8 cells was 145 ± 65. The two values were significantly higher than those treated with other concentrations of H2O2 (P < 0.01).The expression of vascular endothelial growth factor in cancer cells, which could be blocked by dactinomycin, were increased to a certain degree, while the relative half-life of VEGF mRNA was not prolonged after treatment with hydrogen peroxide. The activity of NF-κB in colon cells rose after the cells were exposed to hydrogen peroxide for 24 h.The Fn values in HCT8 cells were 91 ± 13 (0 mol·L-1 H2O2) and 149 ± 40 (10-5 mol·L-1 H2O2) (P < 0.05),in LS174T cells were 127 ± 35 (0 mol·L-1 H2O2) and 192 ± 11 (10-5 mol·L-1 H2O2) (P < 0.05). It is similar to the case of VEGF expression in cancer cells.
CONCLUSION: Hydrogen peroxide increases vascular endothelial growth factor expression in colon cancer cells, and it is likely that reactive oxygen species such as hydrogen peroxide facilitates the development of colon cancer.
- Citation: Zhu JW, Yu BM, Ji YB, Zheng MH, Li DH. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J Gastroenterol 2002; 8(1): 153-157
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.153
Reactive oxygen species (ROS) can be easily produced in intracolonic cavity,due to large amounts of bacteria and dietary metabolites in it. Several reports on the relationship between ROS and cancer suggested that ROS such as oxygen radicals, hydroxyl radicals and hydrogen peroxide (H2O2), were involved in the pathogenesis of colon tumors[1-14]. H2O2, a special intermediate in redox reaction, is able to cross cell membranes in a free manner and modify protein and nucleic acid after being changed into radicals, and now it is thought to be a kind of signal molecular which plays an important role in the growth of tumor cells. Evidences have been given in some reports that H2O2 could promote cell growth and related gene expression in human tumors such as prostate cancer and breast cancer. Considering the special environment in colon and rectum we think it is necessary to evaluate the effects of ROS, especially H2O2, on the progression of colorectal cancer[15-27]. As angiogenesis, which induced by several factors such as vascular endothelial growth factor(VEGF), is often demonstrated in solid tumors, and thought to be an essential requirement for the development of malignant tumors[28-33], we investigated the effects of H2O2 on VEGF expression in colon cancers in this study to find evidences that ROS such as H2O2 plays a role in the progression of the tumor.
Human colon cancer cell lines, LS174T and HCT8, and human umbilical vein endothelial cell line ECV-304 were purchased from American Type Culture Collection. These cell lines were cultured and maintained in RPMI1640 supplemented with 100 mL·L-1 fetal bovine serum at 37 °C in 50 mL·L-1 carbon dioxide and 950 mL·L-1 air.
To determinate the effects of H2O2 on the growth of cancer cells, LS174T and HCT8 cells were grown (103 per well )on 96-well plates and treated with the culture media containing H2O2 (ten concentrations from 10-10 mol·L-1to 10-1 mol·L-1) (300 mL·L-1 H2O2 solution was purchased from Sigma). After 48 h, MTT assay showed that H2O2 inhibited the growth of cancer cells when its concentration was > 10-5 mol·L-1, while had no effect on cell growth when its concentration was ≤ 10-5 mol·L-1. Therefore, those concentrations of H2O2, 10-5,10-7 and 10-9 mol·L-1, were used in subsequent studies.
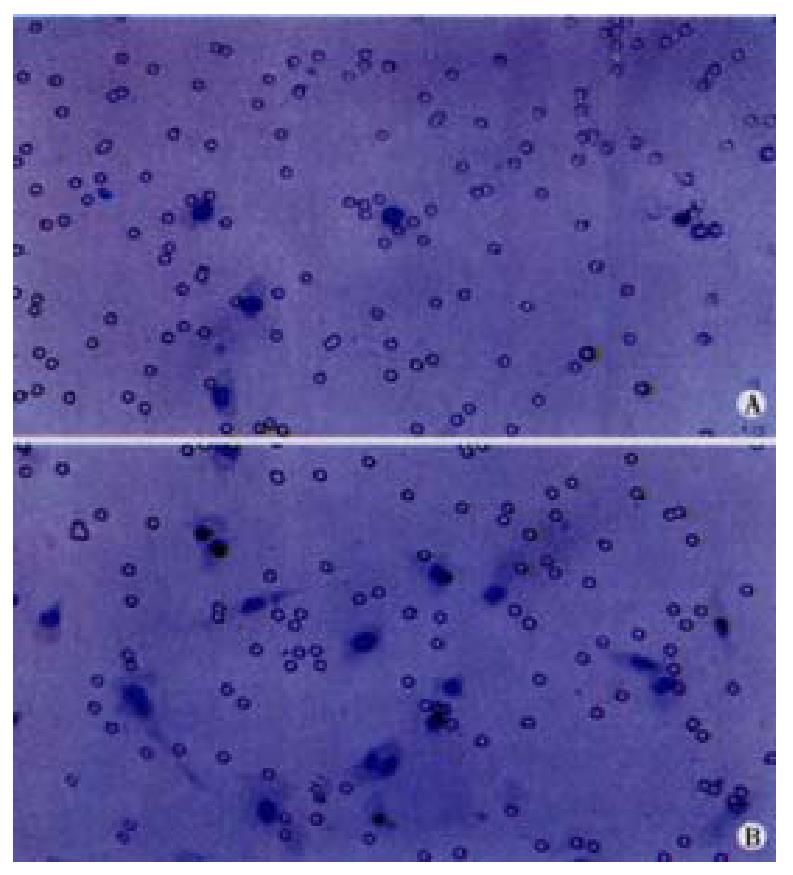
To clarify the effects of H2O2 on the migration of vascular endothelial cells, which could be promoted by VEGF, colon cancer cells were co-cultured with endothelial cells. LS174T and HCT8 cells were plated on 12-well plates (Falcon) at a density of 4 × 105 per well. Four hours later, the cells were washed with serum-free medium, and exposed to 10-5 ,10-7 or 10-9 mol·L-1H2O2 with the H2O2-containing complete media for 24 h. Cell culture inserts with polyethylene terephthalate membranes(PET) and 8 ìm pore size (Becton Dickinson, USA) were then placed into the 12-well plates, and endothelial cells ECV-304 were seeded into the inserts with a density of 1 × 104. Six hours later, the cells on the upper surface of the PET membranes were wiped off completely, and the inserts were fixed and stained. The migration capacity of endothelial cells was estimated by counting the number of the cells beneath the PET membranes. The controls were the groups without cancer cells in the wells or without treating cancer cells with H2O2.
To determine the effects of H2O2 on expression of VEGF, LS174T and HCT8 were grown to 90% confluence to avoid the effects of cell density and incubated in complete media in the presence of H2O2 (10-5,10-7 and 10-9 mol·L-1) for 24 h. Total RNA was extracted and resuspended in sterile RNase-free water for storage at -70 °C. Access RT-PCR system (Promega ) with the sensitive feature was used to determine the relative VEGF mRNA expression. All primers were synthesized by Life Technology, Hongkong.
Math 1
The 50 μL mixture for reverse transcription and PCR amplification were added in one-tube including AMV reverse transcriptase 5U, Tf1 DNA polymerase 5U, MgSO4, 20 μL dNTP mixture, reaction buffer and 20 pmol of each primer and 0.1 μg total RNA sample. The condition for RT-PCR included a 48 °C reverse transcription, a 94 °C AMV inactivation and denaturation, a 60 °C annealing and a 72 °C extension. PCR amplification was subjected to 40 cycles. A volume of 10 μL RT-PCR products was added in 20 g·L-1 agarose gel containing 0.5 mg·L-1 EB. After electrophoresis, the density and area of each band were measured using Fluro-sTM image software (Bio-Rad, USA). The relative RNA level of VEGF in tumor cells was calculated using the house-keeping gene β-actin as an internal control. The experiments were repeated at least four times.
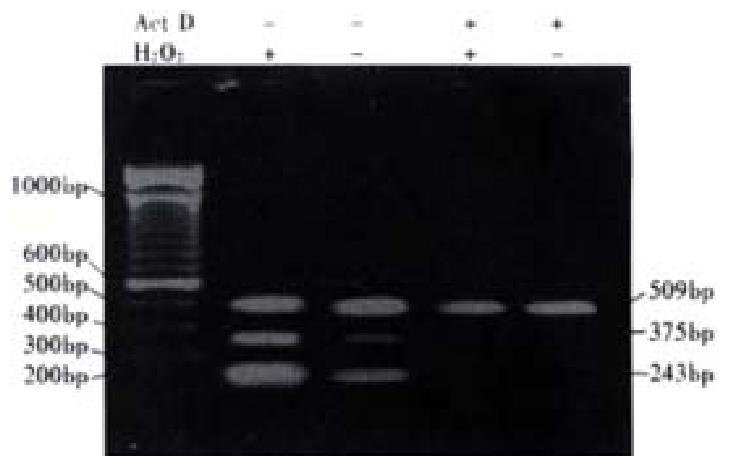
To confirm that the effect of H2O2 on expression of VEGF in colon cancer cells was due to an increase in transcription, transcription activity of cancer cells was blocked by Dactinomycin (ActD, purchased from Sigma ). LS174T cells were incubated in the presence of ActD(1.5 mg·L-1) for 4 h before their exposure to 10-5 mol/L H2O2 in serum-free medium. Total RNA was extracted from cells after 24 h, and RT-PCR analysis was made. Control cells were treated with ActD without H2O2.
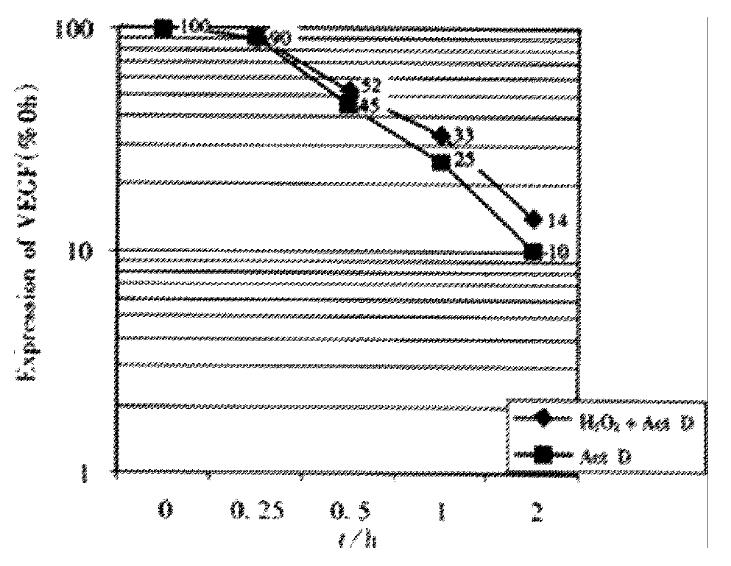
To determine the effect of H2O2 on VEGF mRNA stability, LS174T cells were incubated in the presense or absence of 10-5 mol·L-1 H2O2 for 24 h. Further transcription in cells was then blocked by addition of 1.5 mg·L-1 ActD. Total RNA was extracted from cells at 0, 0.25, 0.5, 1, 2 and 4 h. RT-PCR analysis was made and the relative level of VEGF mRNA expression at each point was compared with the control value (total RNA extracted from cells before ActD treatment was defined as 100% ). The relative half-life of VEGF mRNA was determined by plotting relative VEGF mRNA expression levels on a semilogarithmic axis versus time.
The VEGF protein levels in the supernantant were determined with an enzyme-linked immunosorbent assay (ELISA) kit. Examinations were repeated three times.
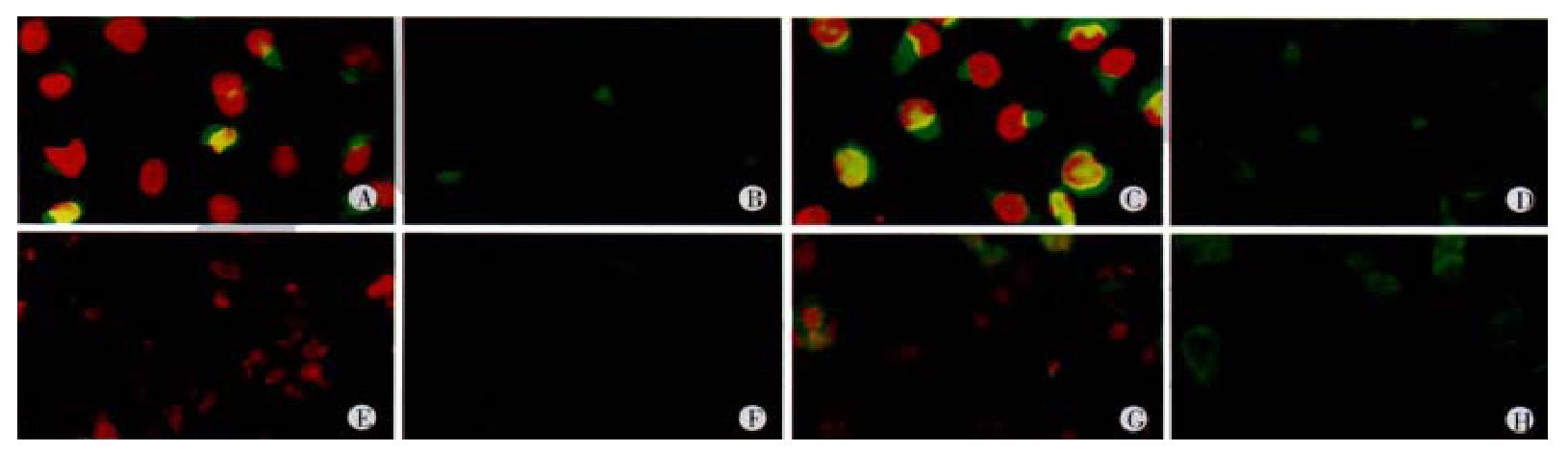
To evaluate the effects H2O2 on NF-κB activity in colon cancer cells, NF-κ B in cytoplasm and nucleus of the cells was detected with FITC-tagged antibody and its presence in the nucleus vs cytoplasm was monitored by measuring the green fluorescence integrated over the nucleus by laser scaning cytometry(LSC) according to the Deptala’s report. Briefly, the cells were first attached to the mocroscope slides, and exposed to 10-5 mol·L-1 H2O2 for 0.5, 1, 3, 6, 12 and 24 h. The cells on slides were fixed and incubated with NF-κB P65 antibody (Santa Cruz) and FITC-tagged goated-antirabbit Ig ( Santa Cruz )at room temperature. Cellular DNA was then counterstained by addition of a solution containing propidium iodide and RNase (Sigma). The cells were placed on microscope slides mounted under coverslips and analyzed by LSC. At least 103 cells were analyzed by LSC per slide. Fluorescence intensity in nucleus (Fn) and in cytoplasm (Fc) were detected and the activity of NF-κB was detected by estimating the value of Fn or Fn/Fc.
When appropriate, data were expressed as -x±s. Analysis of variance and t test were applied to assess the significance of differences. Statistical significance was accepted at P < 0.05.
The migration of endothelial cells induced by cancer cells was promoted to a certain degree when LS174T and HCT8 cells were exposed to 10-5, 10-7 or 10-9 mol·L-1 H2O2 for 24 h. When cancer cells were treated with 10-5 mol·L-1H2O2, the migration number of endothelial cells induced by LS174T cells was 203 ± 70, and the number induced by HCT8 cells was 145 ± 65. The two values were significantly higher than those treated with other concentrations of H2O2(Table 1, Figure 1). When there was no cancer cell in the co-culture system, the number of random motility of endothelial cells was about ten cells.
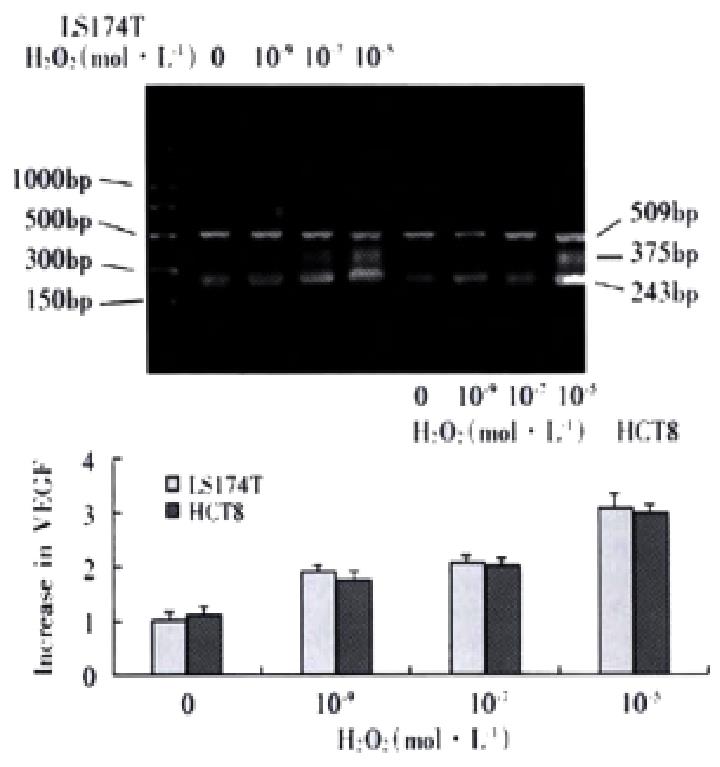
Electrophoresis of RT-PCR products showed the three positive bands, 243, 375 and 509 bp, representing VEGF121,VEGF165 andâ-actin respectively. The internal control demonstrated a stable expression in colon cells treated with each dose of H2O2 The analysis of electrophoresis showed that expression levels of VEGF were elevated in LS174T and HCT8 cells with H2O2 exposure, especially with 10-5 mol·L-1 H2O2 (Figure 2). After inhibition of transcriptional activity by ActD before addition of H2O2, induction of VEGF mRNA expression was completely inhibited in LS174T cells(Figure 3), indicating that H2O2 - induced VEGF expression possibly occurred at the transcriptional level. The relative half-life of VEGF mRNA in LS174T cells treated with H2O2 was similar to that of the cells without H2O2 exposure, demonstrating that the stability of VEGF mRNA was not affected by the treatment with H2O2 (Figure 4). To determine whether secretion of VEGF is increased by H2O2 in colon cancer cells, the supernatant was assayed and the results showed that H2O2 also promoted the VEGF protein expression. The levels of VEGF protein peaked when LS174T and HCT8 cells were incubated in the media containing 10-5 mol·L-1 H2O2. This situation is similar to the increase of VEGF expression.
LS174T and HCT8 cells were incubated in the presence of H2O2 (10-5 mol·L-1) for 0.5, 1, 3, 6, 12 and 24 h in complete medium. NF-κB activity peaked after exposure to H2O2 for 24 h. We investigated the changes of NF-κB activity in LS174T and HCT8 cells treated with 10-5 , 10-7 and 10-9 mol·L-1 H2O2 in media. Compared with the cells without H2O2 treatment, administration of 10-5 mol·L-1 H2O2 for 24 h led to a more remarkable increase in green fluorescence intensity measured over nuclear area (Fn) (Table 2, Figure 5), indicating the increase of NF-κB activity in LS174T and HCT8 cells.
The reactive oxygen species(ROS), which are ubiquitous and occur naturally in all aerobic species, may be divided into two categories :free oxygen radicals (·OH,·NO and O2·-) and nonradical ROS such as H2O2. For decades, H2O2 has been one of the ROS that has been well investigated in flammatory response and oxidant-induced stress. Recently, numerous evidence has been presented to show that H2O2 can act as a signaling molecule involved in many cellular function such as apoptosis and proliferation[1-10].And the regulation of series of genes involved in carcinogenesis and progression is associated with the function of H2O2[3,5,6,9,11-14].Several reports have suggested that ROS such as H2O2 plays a role in the pathogenesis of tumor in colon, where there are a great deal of bacteria and dietary metabolites[15-27]. Diet rich in fat increased the formation of ROS in feces, which then possibly damaged the stem cells in the colon[20,22,26]. However, up to date, little information has been available about the role of H2O2 the special reactive oxygen intermediate, in the biological behaviors of colon cancer cells.
VEGF is a potent and unique angiogenic protein that stimulates capillary formation and has specific mitogenic and chemotactic activity for vascular endothelial cells[28].In colon cancer, VEGF levels are elevated and correlated with a poor clinical outcome[29-33]. VEGF expression is regulated by some pathological processes such as hypoxia[34-36]and by numerous cytokines and growth factors including interleukin1β, interleukin 6, platelet-derived growth factor, transforming growth factorβ, epidermal growth factor, hepatocyte growth factor, insulin-like growth factor, angiotensinII, hypoxia -inducible factor I and EIF4E etc.[37-52] Recently,oncogene p53 is also found to be a regulator of VEGF gene in colon cancer cells[53,54].In the present study, we found, to our knowledge, for the first time, that exogenous H2O2 could up-regulate the expression of VEGF in human colon cancer cells and the migration of endothelial cells induced by the cancer cells after we reviewed those results from RT-PCR assay, ELISA and migration experiment of endothelial cells. Considering the important role of VEGF in neovascurization in solid tumors, we believe that hydrogen peroxide may have the promoting effects on the progression of colon cancer. Related studies also found that hydrogen peroxide could not only increase the expression of VEGF in cultured human vascular smooth muscle cell, human retinal pigment epithelial, melanoma cells and glioblastoma cells, but also promote the growth of prostate and breast cancer cells[55-58].
NF-κB activation, as expressed by its translocation from the cytoplasm to nucleus, can be conveniently assayed by LSC by measuring the intensity of NF-κB-associated immunofluorescence over the area of cell nucleus and comparing it with the intensity over cytoplasm[59]. NF-κB, as a transcriptional factor controlling a variety of target genes such as adhesion molecular and apoptosis, is closely related to the pathogenesis and progression of tumors. NF-κB activity in cells like leukocyte, could be increased by hydrogen peroxide and NF-κB activation was an essential step before VEGF expression level was increased by hydrogen peroxide in murine osteoblastic cells[60,61].It is noteworthy in the present experiment that the increase of NF-κB activity was accompanied by the promotion of expression of VEGF in colon cancer cells exposed to hydrogen peroxide. Thereby, we estimate that the NF-κB activation may be the prerequisite of the effect of hydrogen peroxide on VEGF expression in colon cancer cells.
In view that such reactive oxygen species as hydrogen peroxide are likely to promote the development of colon cancer, it would be helpful in releasing oxidative stress by antioxidants in the colon cancer therapy.
Edited by Ma JY
| 1. | Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999;31:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 502] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Gaté L, Paul J, Ba GN, Tew KD, Tapiero H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacother. 1999;53:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Schwieger A, Bauer L, Hanusch J, Sers C, Schäfer R, Bauer G. ras oncogene expression determines sensitivity for intercellular induction of apoptosis. Carcinogenesis. 2001;22:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437-6444. [PubMed] |
| 5. | Del Bello B, Valentini MA, Zunino F, Comporti M, Maellaro E. Cleavage of Bcl-2 in oxidant- and cisplatin-induced apoptosis of human melanoma cells. Oncogene. 2001;20:4591-4595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Chung YM, Yoo YD, Park JK, Kim YT, Kim HJ. Increased expression of peroxiredoxin II confers resistance to cisplatin. Anticancer Res. 2001;21:1129-1133. [PubMed] |
| 7. | Daré E, Li W, Zhivotovsky B, Yuan X, Ceccatelli S. Methylmercury and H(2)O(2) provoke lysosomal damage in human astrocytoma D384 cells followed by apoptosis. Free Radic Biol Med. 2001;30:1347-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365-4370. [PubMed] |
| 9. | Joseph P, Muchnok TK, Klishis ML, Roberts JR, Antonini JM, Whong WZ, Ong T. Cadmium-induced cell transformation and tumorigenesis are associated with transcriptional activation of c-fos, c-jun, and c-myc proto-oncogenes: role of cellular calcium and reactive oxygen species. Toxicol Sci. 2001;61:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Huang RP, Peng A, Golard A, Hossain MZ, Huang R, Liu YG, Boynton AL. Hydrogen peroxide promotes transformation of rat liver non-neoplastic epithelial cells through activation of epidermal growth factor receptor. Mol Carcinog. 2001;30:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Chen YR, Shrivastava A, Tan TH. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Zmijewski JW, Song L, Harkins L, Cobbs CS, Jope RS. Oxidative stress and heat shock stimulate RGS2 expression in 1321N1 astrocytoma cells. Arch Biochem Biophys. 2001;392:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mendoza L, Carrascal T, De Luca M, Fuentes AM, Salado C, Blanco J, Vidal-Vanaclocha F. Hydrogen peroxide mediates vascular cell adhesion molecule-1 expression from interleukin-18-activated hepatic sinusoidal endothelium: implications for circulating cancer cell arrest in the murine liver. Hepatology. 2001;34:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hardman RA, Afshari CA, Barrett JC. Involvement of mammalian MLH1 in the apoptotic response to peroxide-induced oxidative stress. Cancer Res. 2001;61:1392-1397. [PubMed] |
| 15. | Risau W. Development and differentiation of endothelium. Kidney Int Suppl. 1998;67:S3-S6. [PubMed] |
| 16. | Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, Crucitti F, Barone C. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Ishigami SI, Arii S, Furutani M, Niwano M, Harada T, Mizumoto M, Mori A, Onodera H, Imamura M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer. 1998;78:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Nanashima A, Ito M, Sekine I, Naito S, Yamaguchi H, Nakagoe T, Ayabe H. Significance of angiogenic factors in liver metastatic tumors originating from colorectal cancers. Dig Dis Sci. 1998;43:2634-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP. Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer. 1999;81:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Dachs GU, Chaplin DJ. Microenvironmental control of gene expression: implications for tumor angiogenesis, progression, and metastasis. Semin Radiat Oncol. 1998;8:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ortéga N, L'Faqihi FE, Plouët J. Control of vascular endothelial growth factor angiogenic activity by the extracellular matrix. Biol Cell. 1998;90:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothe-lial growth factor and its receptors. FASEB J. 1998;90:381-390. |
| 24. | Luft FC, Mervaala E, Müller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R. Hypertension-induced end-organ damage : A new transgenic approach to an old problem. Hypertension. 1999;33:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Semenza GL, Agani F, Iyer N, Kotch L, Laughner E, Leung S, Yu A. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann N Y Acad Sci. 1999;874:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Nathan CO, Franklin S, Abreo FW, Nassar R, de Benedetti A, Williams J, Stucker FJ. Expression of eIF4E during head and neck tumorigenesis: possible role in angiogenesis. Laryngoscope. 1999;109:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Arii S, Mori A, Uchida S, Fujimoto K, Shimada Y, Imamura M. Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell. 1999;12:25-30. [PubMed] |
| 28. | Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 535] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 29. | Lamszus K, Laterra J, Westphal M, Rosen EM. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int J Dev Neurosci. 1999;17:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122-6129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Akagi Y, Liu W, Zebrowski B, Xie K, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008-4014. [PubMed] |
| 32. | Akagi Y, Liu W, Xie K, Zebrowski B, Shaheen RM, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon cancer by interleukin-1beta. Br J Cancer. 1999;80:1506-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Bouvet M, Ellis LM, Nishizaki M, Fujiwara T, Liu W, Bucana CD, Fang B, Lee JJ, Roth JA. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58:2288-2292. [PubMed] |
| 34. | Kondo Y, Arii S, Furutani M, Isigami S, Mori A, Onodera H, Chiba T, Imamura M. Implication of vascular endothelial growth factor and p53 status for angiogenesis in noninvasive colorectal carcinoma. Cancer. 2000;88:1820-1827. [PubMed] |
| 35. | Pool-Zobel BL, Abrahamse SL, Collins AR, Kark W, Gugler R, Oberreuther D, Siegel EG, Treptow-van Lishaut S, Rechkemmer G. Analysis of DNA strand breaks, oxidized bases, and glutathione S-transferase P1 in human colon cells from biopsies. Cancer Epidemiol Biomarkers Prev. 1999;8:609-614. [PubMed] |
| 36. | Kondo S, Toyokuni S, Iwasa Y, Tanaka T, Onodera H, Hiai H, Imamura M. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radic Biol Med. 1999;27:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Brás A, Sanches R, Cristóvão L, Fidalgo P, Chagas C, Mexia J, Leitão N, Rueff J. Oxidative stress in familial adenomatous polyposis. Eur J Cancer Prev. 1999;8:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer. 2000;36:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 426] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 39. | van Rossen ME, Sluiter W, Bonthuis F, Jeekel H, Marquet RL, van Eijck CH. Scavenging of reactive oxygen species leads to diminished peritoneal tumor recurrence. Cancer Res. 2000;60:5625-5629. [PubMed] |
| 40. | Owen RW, Spiegelhalder B, Bartsch H. Generation of reactive oxygen species by the faecal matrix. Gut. 2000;46:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Edmiston KH, Shoji Y, Mizoi T, Ford R, Nachman A, Jessup JM. Role of nitric oxide and superoxide anion in elimination of low metastatic human colorectal carcinomas by unstimulated hepatic sinusoidal endothelial cells. Cancer Res. 1998;58:1524-1531. [PubMed] |
| 42. | Giardina C, Inan MS. Nonsteroidal anti-inflammatory drugs, short-chain fatty acids, and reactive oxygen metabolism in human colorectal cancer cells. Biochim Biophys Acta. 1998;1401:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 618] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 44. | Jessup JM, Battle P, Waller H, Edmiston KH, Stolz DB, Watkins SC, Locker J, Skena K. Reactive nitrogen and oxygen radicals formed during hepatic ischemia-reperfusion kill weakly metastatic colorectal cancer cells. Cancer Res. 1999;59:1825-1829. [PubMed] |
| 45. | Liegibel UM, Abrahamse SL, Pool-Zobel BL, Rechkemmer G. Application of confocal laser scanning microscopy to detect oxidative stress in human colon cells. Free Radic Res. 2000;32:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Zheng ZH, Zhang H, Pan YX, Gao Y, Yang JZ. Prevention of postop-erative abdominal adhesions by an antibody to VEGF in mice. Shijie Huaren Xiaohua Zazhi. 1999;7:227-229. |
| 47. | Pan X, Ke CW, Pan W£¬ He X, Cao GW, Qi ZT. Killing effect of DT/ VEGF system on gastric carcinoma cell. Shijie Huaren Xiaohua Zazhi. 2000;8:393-396. |
| 48. | Mao H, Yuan AL, Zhao MF, Lai ZS, Zhang YL, Zhou DY. Effect of p38MAPK signal pathway on ultrastructural change of liver cancer cells induced by VEGF. Shijie Huaren Xiaohua Zazhi. 2000;8:536-538. |
| 49. | Pan X, Pan W, Ni CR, Ke CW, Cao GW, Qi ZT. Killing effect of tetracycline controlled expression of DT/VEGF system on liver cell cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:867-873. |
| 50. | Pan X, Pan W, Ke CW, Zhang B, Cao GW, Qi ZT. Tetracycline controlled DT/VEGF system gene therapy mediated by adenovirus vector. Shijie Huaren Xiaohua Zazhi. 2000;8:1121-1126. |
| 51. | Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296-300. [PubMed] |
| 52. | Xue JT, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF(121) in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6:281-283. [PubMed] |
| 53. | Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271-1279. [PubMed] |
| 54. | Bianchi NO, Bianchi MS, Richard SM. Mitochondrial genome instability in human cancers. Mutat Res. 2001;488:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med. 2000;28:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Castilla MA, Caramelo C, Gazapo RM, Martín O, González-Pacheco FR, Tejedor A, Bragado R, Arroyo MV. Role of vascular endothelial growth factor (VEGF) in endothelial cell protection against cytotoxic agents. Life Sci. 2000;67:1003-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Haklar G, Sayin-Ozveri E, Yüksel M, Aktan AO, Yalçin AS. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Wartenberg M, Diedershagen H, Hescheler J, Sauer H. Growth stimulation versus induction of cell quiescence by hydrogen peroxide in prostate tumor spheroids is encoded by the duration of the Ca(2+) response. J Biol Chem. 1999;274:27759-27767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Deptala A, Bedner E, Gorczyca W, Darzynkiewicz Z. Activation of nuclear factor kappa B (NF-kappaB) assayed by laser scanning cytometry (LSC). Cytometry. 1998;33:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Kaul N, Choi J, Forman HJ. Transmembrane redox signaling activates NF-kappaB in macrophages. Free Radic Biol Med. 1998;24:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Chua CC, Hamdy RC, Chua BH. Mechanism of transforming growth factor-beta1-induced expression of vascular endothelial growth factor in murine osteoblastic MC3T3-E1 cells. Biochim Biophys Acta. 2000;1497:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |