Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.124
Revised: September 1, 2001
Accepted: September 5, 2001
Published online: February 15, 2002
AIM: To observe synthesis of CD14 protein and expression of CD14 mRNA in hepatic tissue and hepatocytes of rats during endotoxemia.
METHODS: The endotoxemia model of Wistar rat was established by injection of a dose of lipopolysaccharide (LPS) (5 mg·kg-1, Escherichia coli O111:B4) via the tail vein, and then the rats were sacrificed after 3, 6, 12 and 24 h in batches. Hepatocytes were isolated from normal and LPS-injected rats by in situ collagenase perfusion technique and were collected to measure the expression of CD14 mRNA and synthesis of CD14 protein by reverse transcript-polymerase chain reaction (RT-PCR) or Western blot analysis. The binding of fluorescein isothiocyanate (FITC)-CD14 polyclonal antibody to isolated hepatocytes was also assessed by flow cytometric analysis (FCM).
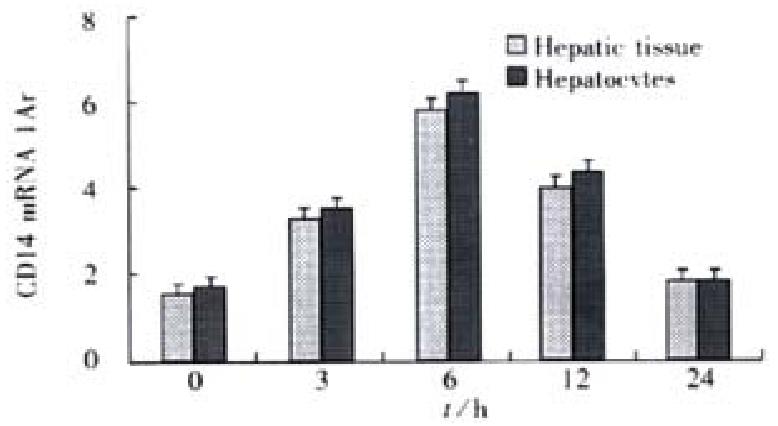
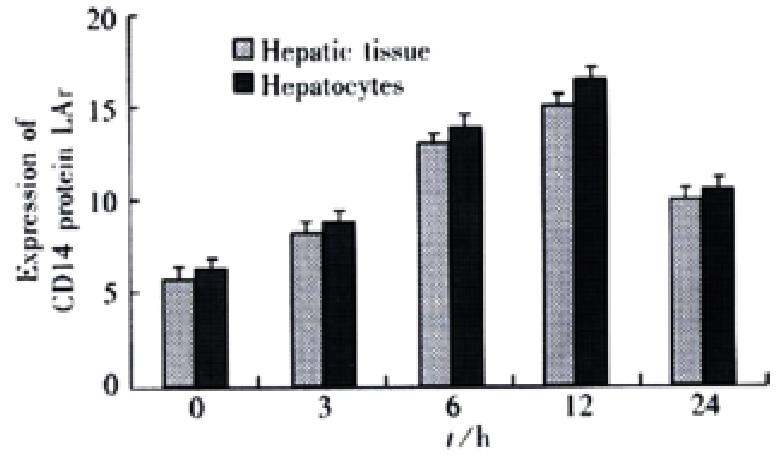
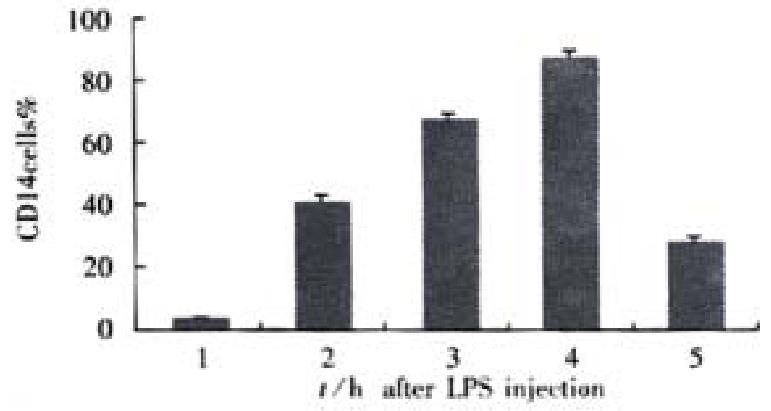
RESULTS: In the rats with endotoxemia, the expressions of CD14 mRNA in hepatic tissue and isolated hepatocytes were stronger at 3, 6, and 12 h than that in control rats (3.48 ± 0.15, 5.89 ± 0.62, 4.33 ± 0.18, vs 1.35 ± 0.14 in hepatic tissue, P < 0.01; 4.12 ± 0.17, 6.24 ± 0.64, 4.35 ± 0.18, vs 1.87 ± 0.15 in hepatocytoes, P < 0.01).The synthesis of CD14 protein in hepatic tissue and isolated hepatocytes increases also obviously in 6 and 12 h when compared to that in control rats (13.27 ± 1.27, 17.32 ± 1.35, 11.42 ± 1.20,vs 7.34 ± 0.72 in hepatic tissue, P < 0.01; 14.68 ± 1.30, 17.95 ± 1.34, 11.65 ± 1.19, vs 7.91 ± 0.70 in hepatocytes, P < 0.01). FCM showed that mean fluorescence intensity (MFI) and numbers of FITC-CD14 positive cells in the rats with endotoxemia increased obviously at 3, 6, 12 and 24 h when compared with normal control group (43.4%, 70.2%, 91.4%, 32.6% vs 4.5%, P < 0.01).
CONCLUSION: LPS can markedly promote the synthesis of CD14 protein and up-regulate the expression of CD14 mRNA in isolated hepatocytes and hepatic tissue. Liver might be a main source for soluble CD14 production during endotoxemia.
- Citation: Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol 2002; 8(1): 124-127
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/124.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.124
CD14 is a glycosylphosphatidylinositol-anchored lipopolysaccharide (LPS) receptor, and first reported to be a differentiation marker expressed on the surface of macrophages, neutrophils and other myeloid lineage cells[1,2]. Recent works have shown that the CD14 antigen is expressed in many types of cell and tissues[3-8]. But it is not yet clear whether hepatocytes can express CD14 protein and gene. Hepatocytes are the major source of most acute-phase proteins. If in fact soluble CD14 (sCD14) is an acute-phase protein, then hepatocytes might be expected to express CD14, which is upregulated during endotoxemia[9-11]. Furthermore, hepatocytes isolated from endotoxemic animals exhibit markedly enhanced responses to LPS, raising the possibility that these cells may express CD14[3,4,12-14]. To determine whether hepatocytes express CD14, our experiments were to observe the synthesis of CD14 protein and expression of CD14 mRNA in hepatocytes and hepatic tissue of rats during endotoxemia and to verify hepatocytes as a main source for soluble CD14 (sCD14) production.
LPS (Escherichia coli O111:B4) and type IV of collagenase were purchased from Sigma Chemical Company (St. Louis, Mo.). An anti-CD14 polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). SP Reagent boxes and fluorescein isothiocyanate (FITC)-IgG were purchased from Zhongshan Biotechnology Company (Beijing, China).
Male Wistar rats, which were pathogen-free and weighed approximately 250 g each, were purchased from the Animal Center of Chongqing University of Medical Science. The rats were exposed each day 12 h of light and darkness. Rodent chow and water were provided ad libitum. Experimental protocols were approved by the Institutional Care and Use Committee of the Chongqing university of Medical Science.
The acute endotoxemia model of Wistar rat was established as described by Li SW, et al[4]. In brief, animals were injected with a dose of LPS (5 mg·kg-1, Escherichia coli O111:B4) via the tail vein, and then the rats were sacrificed at 3, 6, 12 and 24 h respectively. There were six rats at each time point, and other six animals were used as controls (0 h).
Hepatocytes were isolated from normal and LPS-injected rats by an in situ collagenase perfusion technique, modified as described previously[15,16]. In brief, livers were removed after a portal vein perfusion with Hanks’ balanced salt solution (HBSS) and the homogenate was digested in a solution of 0.5 g·L-1 collagenase. Hepatocytes were separated from the nonparenchymal cells by two cycles of differential centrifugation (50 g for 2 min) and further purified over a 30% Percoll gradient. Hepatocyte purity exceeded 90% as assessed by light microscopy, and viability was typically greater than 95% as determined by trypan blue exclusion assay.
Total RNA was isolated from rat liver tissue and hepatocytes by using the TRIZOL Reagent (Life Technologies, USA). The quality of RNA was controlled by the intactness of ribosomal RNA bands. A total of 0.5 mg of each intact total RNA samples was reverse-transcribed to complementary DNA (cDNA) by using the reverse transcription-polymerase chain reaction (RT-PCR) kit (Roche, USA). cDNA was stored at -70 °C until polymerase chain reaction(PCR) analysis.
The PCR primers used were CD14 : sense (5’-CTCAACCTAGAGCCGTTTCT-3’), anti-sense (5’-CAGGATTGTCAGACAGGTCT-3’); β-actin: sense (5’-ACCACAG-CTGAGAGGGAAATCG-3’), anti-sense(5’-AGAGGTCTTTACGGATGTCAA-’). The sizes of the amplified PCR products were 267 bp for CD14 and 281 bp for β-actin. The conditions for amplification were as follows: denaturation at 93 °C for 1 min, annealing at 57 °C for 1 min, and extension at 70 °C for 2 min for 30 cycles. The PCR products were electrophoresed in 20 g·L-1 agarose gels, and the gels were ethidium bromide stained and video photographed on an ultraviolet transilluminator, and the results were showed with the relative absorbance (Ar: relative optical density, ROD).
Cultured hepatocytes were washed twice with phosphate-buffered saline (PBS), pelleted by centrifugation. Cell pellets were resuspended in 50 μL of lysis buffer containing 20 mmol·L-1 HEPES (pH 7.9), 25% glycerol, 0.42 mmol·L-1 NaCl, 15 mmol·L-1 MgCl2, 0.2 mmol·L-1 EDTA, 0.5 mmol·L-1 phenylmethylsulfonyl fluoride (PMSF) and 0.5 mmol·L-1 dithiothreitol (DTT). After three freeze-thaw cycles, cell lysates were centrifuged at 12000 g for 30 min, and the supernatant was saved. The liver tissue was homogenized with homogenizer before the freeze-thaw lysis, as described above for cultured hepatocytes. For Western blot analysis, samples (20 μg per lane) were separated on an SDS-100 g·L-1 polyacrylamide gel and transferred to nitrocellulose membrane. The membrane was sequentially blocked in PBS-Tween (1 g·L-1) containing 50 mL·L-1 milk and then incubated with 5 ìg of anti-rat CD14 polyclonal antibody per mL, washed three times, and further incubated with a goat anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibody. Blocking and antibody incubations each lasted 1 h at room temperature. After several washes, the membrane was developed with DAB reagent, and the results were showed with relative absorbance Ar (relative optical density, ROD).
Expression of CD14 protein in hepatocytes was examined by FCM. In brief, hepatocytes were incubated with the anti-CD14 polyclonal antibody (0.1 mg·L-1) after washing, cells were incubated with goat anti-rabbit immunoglobulin G labled with FITC, after being washed three times, and 10000 cells were analyzed by flow cytometry (Coulter, USA).
All results were expressed as -x±s. Statistical difference between means were determined by using Student’s t test. A P value of < 0.01 was considered significant.
We postulated that hepatocytes and hepatic tissue could express CD14 gene which could be upregulated during endotoxemia. Rats were injected with LPS and total RNA was extracted from freshly isolated and purified hepatocytes and hepatic tissue at different time points indicated. RT-PCR analysis showed that hepatocytes and liver tissue from controls had low but detectable levels of CD14 mRNA. LPS treatment showed steady-state CD14 mRNA levels in hepatocytes, inducing a threefold elevation by as early as 3 h after LPS treatment. The levels increased with times, reaching a maximum of six-fold by 6 h after treatment, and subsequently declined to near baseline levels by 24 h. We also examined the CD14 mRNA levels in RNA isolated from hepatic tissue during endotoxemia and found that the pattern of CD14 mRNA induction by LPS was similar to that of the isolated hepatocytes, indicating that the upregulation of CD14 mRNA was not likely to be simply a consequence of the hepatocyte isolation procedure (Figure 1).
To determine if the upregulation of CD14 expression could also be appreciated in protein levels, Western blot analysis was performed on both hepatocytes and hepatic tissue sample from LPS-treated animals or control animals. In hepatocytes extracts, increases of CD14 protein were seen 6 h after LPS treatment, peaked at 12 h, and declined thereafter. A similar increase of CD14 protein in hepatic tissue was observed. there were significantly different when compared with control animals (P < 0.01, Figure 2).
To confirm the expression of CD14 on hepatocytes, we also examined the binding of FITC to the cells. FITC-CD14 positive cells were 4.5% in rats of normal group. But in rats with endotoxemia, the mean fluorescence intensity (MFI) increased, the numbers of FITC-CD14 positive cells were 43.4%, 70.2%, 91.4%, and 32.6%, respectively in 3,6, 12 and 24 h after stimulation of LPS. There was significant difference when compared to normal group animals (P < 0.01, Figure 3).
CD14 was first described as a myeloid differentiation antigen in 1980’. It is a 55-kDa glycoprotein with multiple leucine-rich repeats and is encoded on chromosome (5q) together with growth factors, such as granulocyte macrophage colony stimulating factor. CD14 has been identified as a receptor for complexes of LPS and LPS-binding protein but it also binds other bacterial products[17-22]. The LPS-binding region within the CD14 molecule is remarkably conserved across species with a high degree of gene sequence homology, and it has therefore been suggested that CD14 is a pattern recognition receptor[17,23-25]. CD14 as a key LPS signaling molecule was reported to be expressed mainly in the monocyte-macrophages system[1,26-29]. Recent works have shown that the CD14 antigen is expressed in many types of cells and tissues[5-8,12]. But it is not yet clear whether hepatocytes express CD14. Although shedding from leukocytes has been proposed as the major source of sCD14 in blood, it is likely that other sources exist[3,5,30-32]. Some reports suggested that sCD14 behaves like other acute-phase proteins[9-11,33-36]. Hepatocytes are the major source of most acute-phase proteins. So we think if sCD14 is an acute-phase protein, then hepatocytes might be expected to express CD14 gene and synthesize CD14 protein, which is upregulated during endotoxemia[37-40].
To determine whether hepatocytes synthesize CD14 protein and express CD14 gene, we measured steady-state CD14 protein and its mRNA both in vivo and in vitro. We found that: ⑴ isolated hepatocytes and liver tissue could synthesize basal levels of CD14 protein and express basal levels of CD14 gene and that synthesis and expression of CD14 were markedly upregulated by LPS during endotoxicmia;these results are in agreement with previous report[41,42]; ⑵ synthesis of CD14 protein and expression of CD14 mRNA in both Hepatocytes and liver tissue indicated that such synthesis and expression were not likely to be simple a consequence of hepatocyte isolation procedure; ⑶ in liver, besides hepatocytes, nonparenchymal cells such as Kupffer cells, endothelial cells, neutrophils and other cells can also express CD14 gene and synthesize CD14 protein[4,43-47], but the fact that both isolated hepatocytes and hepatic tissue expressed CD14 protein and its mRNA indicated that the nonparenchymal cells could hardly have any effect on such expression in liver tissue.
Although we do not provide direct evidence here that sCD14 in plasma originates from hepatocytes during endotoxemia, our results showed that there was the possibility that the liver is an important source for sCD14 during endotoxemia. Pan et al[5] found that the liver is one of the major organs for the production of soluble CD14. Liu et al[41,42] reported also that CD14 transcription rates were signficantly increased in hepatocytes from LPS-treated rat, indicating that the upregulation in CD14 mRNA levels observed in rats hepatocytes after LPS treatment was dependent, in part, on increased transcription, and their observations supportted the idea that sCD14 would be an acute-phase protein and hepatocytes might be a source for circulating sCD14 production[3,4,8,48-52]. Our data indicate that hepatocytes from LPS-stimulated rats express higher amounts of CD14 gene and CD14 protein, and may release more sCD14. Further investigation of the expression of CD14 in hepatocytes is actively ongoing in our laboratory.
In summary, our in vitro and in vivo data indicate that hepatocytes can synthesize CD14 protein and express CD14 mRNA and their synthesis and expression are upregulated effectively by LPS during endotoxemia. Liver is a main source for sCD14 production during sepsis or endotoxemia.
Edited by Lu HM
| 1. | Lichtman SN, Wang J, Lemasters JJ. LPS receptor CD14 participates in release of TNF-alpha in RAW 264.7 and peritoneal cells but not in kupffer cells. Am J Physiol. 1998;275:G39-G46. [PubMed] |
| 2. | Fearns C, Ulevitch RJ. Effect of recombinant interleukin-1beta on murine CD14 gene expression in vivo. Shock. 1998;9:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Su GL, Dorko K, Strom SC, Nüssler AK, Wang SC. CD14 expression and production by human hepatocytes. J Hepatol. 1999;31:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Li S, Wu C, Shi Y, Liu C. [Lipopolysaccharide upregulates expression of CD14 gene and CD14 proteins of hepatocytes in rats]. Zhonghua Ganzangbing Zazhi. 2001;9:103-104. [PubMed] |
| 5. | Pan Z, Zhou L, Hetherington CJ, Zhang DE. Hepatocytes contribute to soluble CD14 production, and CD14 expression is differentially regulated in hepatocytes and monocytes. J Biol Chem. 2000;275:36430-36435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Ahmed AF, Nio M, Ohtani H, Nagura H, Ohi R. In situ CD14 expression in biliary atresia: comparison between early and late stages. J Pediatr Surg. 2001;36:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Furusako S, Takahashi T, Mori S, Takahashi Y, Tsuda T, Namba M, Mochizuki H. Protection of mice from LPS-induced shock by CD14 antisense oligonucleotide. Acta Med Okayama. 2001;55:105-115. [PubMed] |
| 8. | Jersmann HP, Hii CS, Hodge GL, Ferrante A. Synthesis and surface expression of CD14 by human endothelial cells. Infect Immun. 2001;69:479-485. [PubMed] |
| 9. | Patiño R, Ibarra J, Rodriguez A, Yagüe MR, Pintor E, Fernandez-Cruz A, Figueredo A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol. 2000;85:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Choi YH, Lee WH, Lee Y, Kim JK, Lee SY, Park JE. Correlation between monocyte and T-lymphocyte activation markers in patients with acute coronary syndrome. Jpn Heart J. 2000;41:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Glück T, Silver J, Epstein M, Cao P, Farber B, Goyert SM. Parameters influencing membrane CD14 expression and soluble CD14 levels in sepsis. Eur J Med Res. 2001;6:351-358. [PubMed] |
| 12. | Nanbo A, Nishimura H, Muta T, Nagasawa S. Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. Eur J Biochem. 1999;260:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Fang W, Yao Y, Shi Z. [The significance of the expressions of lipopolysaccharide binding protein mRNA and lipopolysaccharide receptor CD14 mRNA in the liver of burned rat]. Zhonghua Shaoshang Zazhi. 2000;16:157-160. [PubMed] |
| 14. | Jiang JX, Xie GQ, Chen YH, Liu DW, Zhou JH, Zhu PF, Wang ZG, Zhang HJ. Expression of scavenger recepor and CD14 on Kupffer cells and its relationship with endotoxin-induced hepatic injury. Zhonghua Chuangshang Zazhi. 2000;16:478-481. |
| 15. | Gong JP, Xu MQ, LiK . Expression of CD14 in Kupff's cells induced by Lipopolysaccharide. Di-San Junyi Daxue Xuebao. 2001;23:425-428. |
| 16. | Gong JP, Hun BL. Isolation, culture and identification of live cells. Shijie Huaren Xiaohua Zazhi. 1999;7:417-419. |
| 17. | Bernardo J, Billingslea AM, Blumenthal RL, Seetoo KF, Simons ER, Fenton MJ. Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor. Infect Immun. 1998;66:28-35. [PubMed] |
| 18. | Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748-6755. [PubMed] |
| 19. | Hetherington CJ, Kingsley PD, Crocicchio F, Zhang P, Rabin MS, Palis J, Zhang DE. Characterization of human endotoxin lipopolysaccharide receptor CD14 expression in transgenic mice. J Immunol. 1999;162:503-509. [PubMed] |
| 20. | Fang W, Yao Y, Shi Z, Yu Y, Wu Y, Lu L, Chang G, Sheng Z. [Gene expression of lipopolysaccharide receptor CD14 and tumor necrosis factor-alpha in rats after thermal injury]. Zhonghua Waike Zazhi. 1999;37:271-273. [PubMed] |
| 21. | Ben D, Huang J, Yang Y. [Increased expression of peritoneal macrophage CD14 in severely burned mice]. Zhonghua Shaoshang Zazhi. 2000;16:96-99. [PubMed] |
| 22. | Peng Z, Zhang ZX, Xu YJ. Expressions of CD11c,CD14 and TGF-β 1 mRNA in alveolar macrophages in chronic bronchitis. Zhonghua Weishengwuxue He Mianyixue Zazhi. 2000;20:408-410. |
| 23. | Gong JP, Hum BL. Role of CD14 in activation of Kuppfer cell induced by lipopolysaccharide. Shijie Huaren Xiaohua Zazhi. 1999;7:875-877. |
| 24. | Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841-849. [PubMed] |
| 25. | Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser MP, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect Immun. 2001;69:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Peppelenbosch MP, DeSmedt M, ten Hove T, van Deventer SJ, Grooten J. Lipopolysaccharide regulates macrophage fluid phase pinocytosis via CD14-dependent and CD14-independent pathways. Blood. 1999;93:4011-4018. [PubMed] |
| 28. | Liu S, Morris SM, Nie S, Shapiro RA, Billiar TR. cAMP induces CD14 expression in murine macrophages via increased transcription. J Leukoc Biol. 2000;67:894-901. [PubMed] |
| 29. | Stelter F, Witt S, Fürll B, Jack RS, Hartung T, Schütt C. Different efficacy of soluble CD14 treatment in high- and low-dose LPS models. Eur J Clin Invest. 1998;28:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Hiki N, Berger D, Prigl C, Boelke E, Wiedeck H, Seidelmann M, Staib L, Kaminishi M, Oohara T, Beger HG. Endotoxin binding and elimination by monocytes: secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun. 1998;66:1135-1141. [PubMed] |
| 31. | Hiki N, Berger D, Dentener MA, Mimura Y, Buurman WA, Prigl C, Seidelmann M, Tsuji E, Kaminishi M, Beger HG. Changes in endotoxin-binding proteins during major elective surgery: important role for soluble CD14 in regulation of biological activity of systemic endotoxin. Clin Diagn Lab Immunol. 1999;6:844-850. [PubMed] |
| 32. | Bessler H, Komlos L, Punsky I, Ntambi JA, Bergman M, Straussberg R, Sirota L. CD14 receptor expression and lipopolysaccharide-induced cytokine production in preterm and term neonates. Biol Neonate. 2001;80:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Wang SC, Klein RD, Wahl WL, Alarcon WH, Garg RJ, Remick DG, Su GL. Tissue coexpression of LBP and CD14 mRNA in a mouse model of sepsis. J Surg Res. 1998;76:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Imai K, Takeshita A, Hanazawa S. Transforming growth factor-beta inhibits lipopolysaccharide-stimulated expression of inflammatory cytokines in mouse macrophages through downregulation of activation protein 1 and CD14 receptor expression. Infect Immun. 2000;68:2418-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Liu S, Salyapongse AN, Geller DA, Vodovotz Y, Billiar TR. Hepatocyte toll-like receptor 2 expression in vivo and in vitro: role of cytokines in induction of rat TLR2 gene expression by lipopolysaccharide. Shock. 2000;14:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Hiki N, Berger D, Mimura Y, Frick J, Dentener MA, Buurman WA, Seidelmann M, Kaminishi M, Beger HG. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J Surg. 2000;24:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | van Oosten M, van de Bilt E, van Berkel TJ, Kuiper J. New scavenger receptor-like receptors for the binding of lipopolysaccharide to liver endothelial and Kupffer cells. Infect Immun. 1998;66:5107-5112. [PubMed] |
| 38. | Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652-G661. [PubMed] |
| 39. | Jiang S, Naito M, Kaizu C, Kuwata K, Hasegawa G, Mukaida N, Shultz LD. Lipopolysaccharide-induced cytokine and receptor expression and neutrophil infiltration in the liver of osteopetrosis (op/op) mutant mice. Liver. 2000;20:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Jiang J, Xie G, Chen Y, Liu D, Qiu J, Zhou J, Zhu P, Wang Z. Intra-hepatic expression of scavenger receptor and CD14 and their relationship with local inflammatory responses in endotoxemia in mice. Shock. 2001;16:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Liu S, Khemlani LS, Shapiro RA, Johnson ML, Liu K, Geller DA, Watkins SC, Goyert SM, Billiar TR. Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia. Infect Immun. 1998;66:5089-5098. [PubMed] |
| 42. | Liu S, Shapiro RA, Nie S, Zhu D, Vodovotz Y, Billiar TR. Characterization of rat CD14 promoter and its regulation by transcription factors AP1 and Sp family proteins in hepatocytes. Gene. 2000;250:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Netea MG, Kullberg BJ, van der Meer JW. Lipopolysaccharide-induced production of tumour necrosis factor and interleukin-1 is differentially regulated at the receptor level: the role of CD14-dependent and CD14-independent pathways. Immunology. 1998;94:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Toshima K, Mochida S, Ishikawa K, Matsui A, Arai M, Ogata I, Fujiwara K. Contribution of CD14 to endotoxin-induced liver injury may depend on types of macrophage activation in rats. Biochem Biophys Res Commun. 1998;246:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Lukkari TA, Järveläinen HA, Oinonen T, Kettunen E, Lindros KO. Short-term ethanol exposure increases the expression of Kupffer cell CD14 receptor and lipopolysaccharide binding protein in rat liver. Alcohol Alcohol. 1999;34:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Yin M, Ikejima K, Wheeler MD, Bradford BU, Seabra V, Forman DT, Sato N, Thurman RG. Estrogen is involved in early alcohol-induced liver injury in a rat enteral feeding model. Hepatology. 2000;31:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Song PI, Abraham TA, Park Y, Zivony AS, Harten B, Edelhauser HF, Ward SL, Armstrong CA, Ansel JC. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867-2877. [PubMed] |
| 48. | Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841-849. [PubMed] |
| 49. | Fang W, Yao Y, Shi Z. [The effect of bactericidal/permeability-increasing protein on lipopolysaccharide-binding protein and lipopolysaccharide receptor CD14 mRNA expression in rats after thermal injury]. Zhonghua Yixue Zazhi. 1999;79:289-291. [PubMed] |
| 50. | Wang F, Wang LY, Wright D, Parmely MJ. Redox imbalance differentially inhibits lipopolysaccharide-induced macrophage activation in the mouse liver. Infect Immun. 1999;67:5409-5416. [PubMed] |
| 51. | Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261-268. [PubMed] |