Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.108
Revised: September 21, 2001
Accepted: October 16, 2001
Published online: February 15, 2002
AIM: To confirm the existence of CD226 ligand and its distribution, which is a novel molecule that was cloned in 1996.
METHODS: The mRNA was extracted from TPA activated Jurkat cells and used as a template for reverse-transcription. After PCR amplification, the fragment including CD226 extracellular region and the splice donor sequence “ACTTACCTGT” was obtained and cloned into fusion expression vector pIG. The recombinant vector pCD226/Ig was transfected in COS-7 cells by DEAE-Dextran method, the secreting fusion protein was identified by Sandwich ELISA, and was purified by anti-CD226 affinity chromatography. This fusion protein was used as a probe in the investigation of CD226 ligand by immunohistochemistry. Existence of CD226 ligand was further identified by adhesion experiment.
RESULTS: Expression of a secreting fusion protein was identified by sandwich ELISA, indicating that both CD226 extracellular domain and IgGFc domain could be recognized respectively by anti-CD226 and anti-hIgFc mAb. About 130 μg CD226/Ig fusion protein could be obtained from 100 mL COS-7 culture supernatants by anti-CD226 affinity chromatography purification. SDS-PAGE showed that this fusion protein has a molecular mass of 83 ku. It was confirmed by immunohistochemistry that CD226 ligand expressed on the Colo205 cells, but not on Jurkat cell, U937 cell and mixed lymphocyte culture cells. In adhesive assay, resting Jurkat cells did not have significant adhesion to Colo205 cells. In contrast, activated Jurkat cells could bind to colon carcinoma Colo205 cells and this adhesive reaction could be blocked by CD226/Ig fusion protein or anti-CD226 mAb. Immunochemical experiment showed that Colo205 cells could be specifically stained by CD226/Ig, indicating that CD226 ligand exists on the surface of Colo205 cells.
CONCLUSION: Existence of CD226 ligand on the surface of Colo205 cells was identified by immunohistochemistry and adhesion blocking experiment. In addition, the secreting CD226/Ig fusion protein prepared in this study will be a potential tool for further investigation of CD226 ligand.
- Citation: Sun K, Jin BQ, Feng Q, Zhu Y, Yang K, Liu XS, Dong BQ. Identification of CD226 ligand on colo205 cell surface. World J Gastroenterol 2002; 8(1): 108-113
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.108
CD226 was initially reported by Burns in 1985 with a name TLiSA1 (T lineage specific activation antigen 1, TLiSA1)[1], and was renamed PTA1 (platelet and T cell antigen 1, PTA1) in 1989 because of its expression on platelet as well[2]. Since 1985, a lot of investigations have been done on CD226 expression, functions and its relationship with diseases[2-6]. CD226 was mainly expressed on the activated T cells, NK cells, monocytes, platelets and megakaryocytes lineage[4-8], taking part in signal transduction of T cell activation and differentiation as well as platelet activation and aggregation. CD226 mAb was found to stimulate the activation and aggregation of platelets[2,3], inhibit differentiation of CTL[2,9] and T and NK cell mediated cytotoxicity[7]. In 1997, PTA1 cDNA was cloned from cDNA library of TPA activated Jurkat cells[10]. It belongs to an immunolglobulin super family (IgSF) with a 232aa extracellular region, 25aa transmembrane region and 61aa intracellular region. Interestingly, PTA1 cDNA was almost the same as that of DNAM-1 (DNAX associated molecule-1), a novel molecule cloned by Shibuya in 1996[7]. In 2000, PTA1 (DNAM-1) was designated as CD226 on the 7th Workshop and Conference on the Human Leucocyte Differentiation Antigen in Harrogate. CD226 is the only IgSF member whose extracellular region consists of two IgV-like domains.
Almost all the important Ig-superfamily members are highly conserved during molecular evolution[11-13], such as CD2, CD4/ CD8 and CD28. Homological analysis showed that CD226 molecules are highly conserved (93%-95%) among human, ape and monkey, suggesting that CD226 may have very important functions[14]. It seems that CD226 takes part in the mechanism of platelet function disorders, autoimmune diseases, transplantation, virus infection diseases and tumors[3,15-20], suggesting that CD226 plays an important role in immune system and may have a potential application to clinical diagnosis and treatment. Up to, little is known about CD226 ligand. So, the aim of this study is to make a preliminary research on CD226 ligand.
pIG vector was a gift kindly presented by Dr. Xu. COS-7, U937, Jurkat, Colo205 cell line and CD226 hybridoma (secreting mAb Leo-A1 against the extracellular domain of CD226) were provided by Prof. Jin. Goat anti-human Ig-SABC immunohistochemistry kit was purchased from Boster Co. Vectors and restrict enzymes were purchased from Huamei Co; Trizol reagent kit was purchased from Gibco Co, and CNBr-Sepharose 4B is a product of Pharmacia Co.
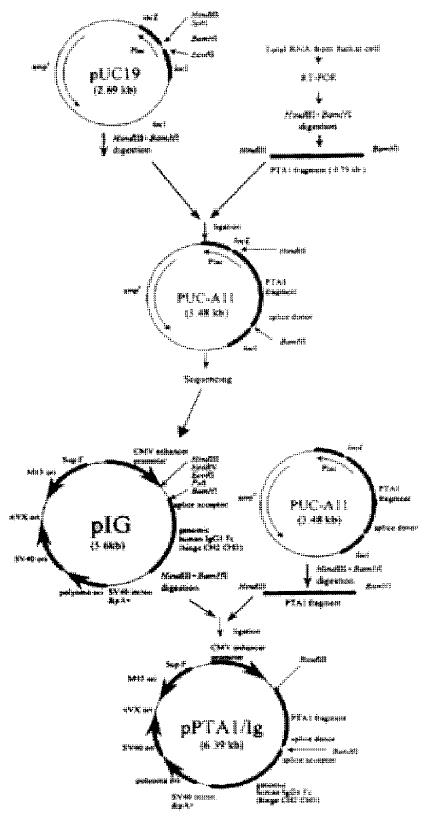
Primers were designed according to the sequence of CD226 cDNA: primer 1, 5’-GCAAGCTTCAGAGATGGATTATCCTACT-3’ (forward, containing HindIII enzyme site at 5’); primer 2, 5’-GCGGATCCACTTACCTGTAGCCACAAAGAGGGTATATTGG-3’ (reverse, containing BamHIenzyme site and donor splice region “ACTTACCTGT” at 5’); primer 3, 5’-ACTCTAGTCTTTGGTCCTGC-3’. Primer1 and Primer 3 were used to amplify the whole length CD226 cDNA, and primer 1 and primer 2 were used to amplify cDNA encoding CD226 extracellular region (Figure 1).
Jurkat cells were cultured at a concentration of 1 × 10 9·L-1 with TPA (50 μg·L-1) for 18-30 h at 37 °C, and the total RNA was isolated by Trizol reagent kit. RNA was primed and reverse transcribed into cDNA in a 20 μL reaction volume containing 10U reverse transcriptase, 10 μg RNA, 10 × Buffer 2 μL, 10 mmol·L-1 dNTP complex 4 μL, and primer 3. After incubation at 42 °C for 1 h, the reverse transcription product was PCR amplified by Primer 1 and Primer 3, and then by Primer 1 and Primer 2. The PCR parameters were 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 80 s for 35 cycles.
Expression vector for CD226 extracellular region was prepared as previously described[21]. The corresponding PCR fragment was cloned into pUC-19 and M13mp18/19 vector for DNA sequencing. After DNA sequencing, the 793 bp fragment encoding CD226 extracellular region and the splice donor sequence “ACTTACCTGT” was subcloned into pIG, a mammalian fusion protein expression vector containing the cytomegalovirus (CMV) promoter, splice acceptor, genomic human IgG1, and SV40 polyadenylation signal. The resulting expression vector pCD226/ Ig was identified by PCR and restrict enzyme digestion.
COS-7 cell transfection was performed by a modified method[22]. Briefly, COS-7 cells at 50%-75% confluence were transfected in 7 mL glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 100 mL·L-1 fetal calf serum (FCS), 400 μg DEAE - dextran/mL, 100 μmol·L- 1 chloroquine diphosphate, and 40 μg purified DNA. After 3 h at 37 °C, the transfection mixture was removed and the cells were treated with 100 g·L-1 dimethyl sulfoxide in PBS for 2 min. Cells were then returned to DMEM supplemented with 10 mL·L-1 fetal calf serum (FCS) for 4 d to allow CD226/Ig expression.
Leo-A1 affinity chromatography column was prepared as previously described[23]. The pCD226/Ig transfected COS-7 supernatant was spined out cell debris for 5 min at 10000 r·min-1, filtered through a 0.45 μm filter and then purified by Leo-A1 affinity chromatography. The expression of a secreting fusion protein was identified by sandwich ELISA (anti-CD226 mAb and HRP-conjugated anti-hIg mAb, anti-IL-8 antibody was used as control). SDS-PAGE and Western-blot (polyclonal anti-hIgG antibody) were performed by routine methods.
Immunohistochemical experiment was performed by SABC methods. For light microscopy, cytospin preparations of Colo205 cells were incubated with 3 g·L-1 methanol-hydrogen peroxide solution at 37 °C for 30 min to inactivate endogenous peroxidases. The slides were then blocked in goat serum blocking solution at 37 °C for 30 min. Without washing, the slides were incubated with CD226/Ig fusion protein (10 mg·L-1) at 4 °C. After overnight incubation, the slides were washed three times with PBS, and incubated with biotin labeled goat anti human IgG for 4 h at room temperature. Finally, the slides were washed, allowed to sit for 2 min, and then reacted with SABC complex and DAB peroxidase substrate solution. Human IgG1 was used instead of CD226/Ig in the control experiment.
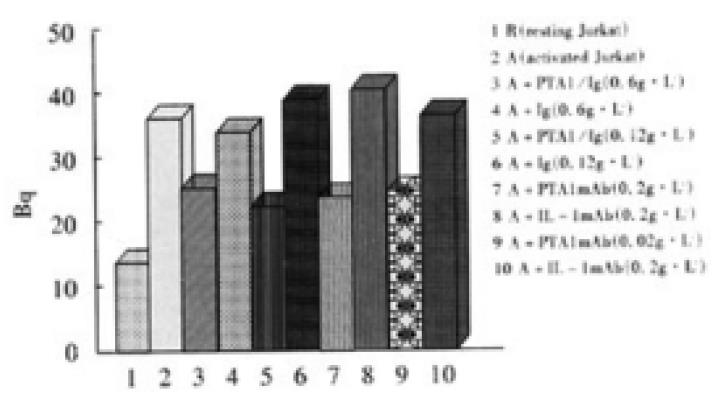
In 96 well plate, Colo205 cells with the density of 0.5 × 105/well were cultured for one day to let cells confluence, and 3.7 MBq 51Cr labeled resting or activated Jurkat cells were added with 1 × 105/well and cultured for 4 h, and non-adhered cells were moved by gently washing, the adhered cells were lysed by 20 g·L-1 Triton X-100, and Bq was measured. Different concentrations of CD226/Ig fusion protein and anti-CD226 mAb Leo-A1 were used in the adhesion blocking experiment
A very low level of CD226 molecules was expressed on resting Jurkat cells, and the expression level could be enhanced by TPA stimulation for 24 h. The CD226 mRNA from Jurkat cells was enriched by TPA stimulating. After retro-transcription, semi-nests PCR amplification, a single band about 800 bp has been obtained. This fragment was then ligated into pUC19 and subcloned into M13mp18/19 vector for sequencing. The sequencing data showed that the fragment was 793 bp long in total, a HindIII enzyme site could be found at the upstream and the “ACTTACCTGT”donor splicing sequence and a BamHI site could be found at the downstream. The 793 bp fragment was ligated between HindIII and BamHI sites of pIG vector. The recombinant vector pCD226/ Ig was identified by restrict enzyme digestion assay and PCR identification (Figure 2).
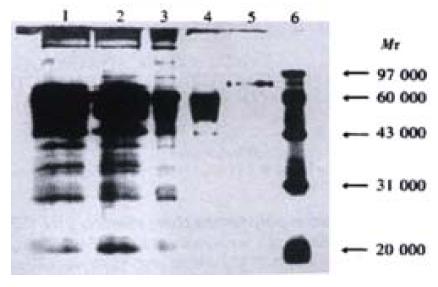
The pCD226/Ig vector was transiently transfected into COS-7 cells. The optimal conditions was checked before large-scale transfection, and it was found that transfected COS-7 cells cultured in medium with 10 mL·L-1 serum for 96 h could produce CD226/Ig most effectively. The secreted CD226/Ig fusion protein was purified by Leo-A1 affinity chromatography, About 130 μg CD226/Ig fusion protein could be obtained from 100 mL COS-7 culture supernatants by anti-CD226 affinity chromatography purification. SDS-PAGE showed CD226/Ig fusion protein has a molecular mass of 83 ku (Figure 3) and could be recognized by anti-IgG polyclonal antibody in Western-blot assay (Figure 4). In ELISA assay, CD226/Ig expression could be identified in pCD226/Ig transfected COS-7 supernatant, but a negative result was obtained in pIG vector transfected COS-7 supernatant (Table 1). Both CD226 extracellular domain and Fc domain of the CD226/Ig protein could be recognized respectively by anti-CD226 and anti-hIg Fc mAb, suggesting that CD226/Ig could mimic the nature CD226 molecule and be a potential tool in the research of CD226 ligand and its functions.
| Coated antibody | Samples tested | Conjugated secondary antibody | Results |
| Anti-CD226 | pCD226/Ig transfected COS supernatant | HRP-Gα hIgG | +++ |
| Anti-CD226 | pIG transfected COS supernatant | HRP-Gα hIgG | - |
| Anti-IL-8 | pCD226/Ig transfected COS supernatant | HRP-Gα hIgG | - |
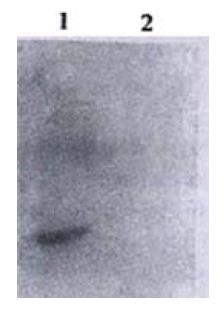
Immunohistochemical experiment showed that Colo205 cells could be specifically stained by CD226/Ig but not by control protein (hIgG), indicating that CD226 ligand exists on the surface of Colo205 cells (Figure 5). Resting Jurkat cells had a low-level expression of CD226 on cell surface, whereas activated Jurkat cells expressed high level of CD226 after TPA treatment for 24 h. In adhesive assay, resting Jurkat cells have a very low level adhesion to Colo205 cells. In contrast, activated Jurkat cells could bind to colon carcinoma Colo205 cells and this adhesive reaction could be blocked by CD226/Ig fusion protein or Leo-A1 mAb but not control Ig and anti-IL-1 mAb, indicating that CD226 takes part in this specific adhesion (Figure 6).
CD226 is a 65 ku glycoprotein expressed on the surface of activated T cells, NK cells, and platelets. It is a member of the Ig-superfamily containing 2 Ig-like domains of the V-set and is encoded by a gene on human chromosome 18q22.3[24]. Ig-superfamily members play essential roles in many aspects of immune responses by acting as immunoglobulin Fc receptors, cytokine receptors, adhesive molecules and accessory molecules[11,25-27]. The main function of Ig-super family concentrated in cell-to-cell recognition and interaction, such as LFA-1/CD2, CD4/MHC, CD28/B7, and many of these Ig-super family members are involved in the T cell activation[28-32]. Cross-linking CD226 with antibodies cause the activation of T cells and aggregation of platelets. Previous studies also showed the cross-linking of LFA-1 induced tyrosine phosphorylation of CD226, in which the Fyn protein tyrosine kinase may play a role[33,34]. The above results indicate that CD226 mediates cellular adhesion to other cells bearing an unidentified ligand and takes part in signal transduction. Interaction between ligand and receptor is the basis of immune response. So, identification of CD226L is very important for the further investigation of CD226.
Intercellular adhesive molecules play an important role in the immune response, they provide not only intercellular binding, but also participate in signal transduction, and are closely related with allograft rejection, tolerance, cell differentiation, lymphocyte homing and tumor immunity[35-39]. In addition to the specific antigen recognition signal provided by CD3-TCR complex, a lot of other signals are required in the T cells mediated immune response, such as signals provided by CD4/CD8, CD2, LFA-1, CD28[40-42]. Our previous results showed that resting T cells expressed low-level of CD226 molecule. When T cells were activated, they expressed high-level CD226 molecules. This finding suggested that CD226 might be closely related to the function of activated T cells. Differentiation of T cell to CTL could be inhibited when Leo-A1 mAb or Leo-A1 F(ab’)2 were added into mixed lymphocyte culture (MLC), and production of LAK cells was also reduced, this action of Loe-A1 was not dependent on Fc of Leo-A1. The inhibiting effect of Loe-A1 only works in the differentiation phase of CTL but not in the cytotoxicity phase, suggesting that the epitope recognized by CD226 is related with the differentiation of CTL[1,2,7]. Another mAb DX11 against CD226 (DNAM-1) was reported to significantly inhibit the cytotoxicity of CTL to several tumor cell lines, such as Colo205, PA-1, MCF7[7]. Therefore, epitope recognized by DX11 mAb was different from that recognized by Leo-A1 mAb, and these two different epitopes may take part in the differentiation and cytotoxicity of CTL.
Since the mid 1980s, there has been a rapid increase in our knowledge about the specific cell surface molecules mediating cell-cell interaction and adhesion events. This has been largely due to the success of molecular cloning techniques, allowing the isolation of functional cDNA clones that encoding these glycoproteins. Always methods have been successfully used in the cloning of novel cDNA. One of these methods reported by Aruffo and Seed is very effective[43,44]. This method is based on the transient expression of cDNA library in cells and specific mAb recognition (capture and panning). Several molecules, such as CD2, CD22, CD28, CD36 and CD58[43-47], have been cloned by this method. So, once a suitable antibody, ligand or cell line has been identified to recognize a cell surface molecule, the cDNA encoding this molecule could be cloned by this method. CD226 molecule or its homologue to be used in the investigation of CD226 ligand should have the following characteristics: easy to obtain, high in purity and natural in motif. So these molecules could be obtained by purification from platelet, preparations of anti-idiotype mAb or preparations of recombinant CD226 molecule. In this study, we prepared the CD226/Ig fusion protein containing CD226 extracellular region 232aa (including 10 glycosylation sites, 57 ku) and IgG Fc region (CH2, CH3 and H region, 26 ku), the putative molecule weight is consistent with that identified in SDS-PAGE and Western-blot. CD226/Ig fusion protein is expressed in secreting form that was detected by Sandwich-ELISA, its two different domains could be recognized by anti-CD226 mAb and anti-hIg Fc mAb, respectively, but could not be recognized by other mAb, suggesting CD226/Ig can mimic the nature of CD226 molecule and can be used in the research of CD226 ligand. Ig fusion proteins have many advantages such as easy to purify, easy to label and easy to detect, and thus were widely used in ligand identification[48-53], and disease protection[54-56]. These advantages make it more convenient to further clone CD226 ligand.
Immunohistochemical research suggested that CD226 ligand was expressed on Colo205 cells and this result was also supported by adhesion-blocking experiment. It was shown that resting Jurkat cells expressed very low level of CD226 molecules, but activated Jurkat cells expressed high level CD226 molecules after TPA activation for 24 h, and the expression of CD226 was regulated by cytokines, such as TGF-β, TNF-α and IL-2[57]. In the experiment about adhesion, activated Jurkat cells could bind to colon carcinoma Colo205 cells and this adhesion reaction could be blocked by CD226/Ig fusion protein or Leo-A1 mAb but not by control Ig and anti-IL-1 mAb, indicating that CD226 ligand exist in Colo205 cells and takes part in this specific adhesion.
When T cells were activated, they expressed high-level CD226 molecules. Interestingly, CD226 ligand seemed to express on the surface of tumor cells. Activated T cells not only need the specific antigen recognition by TCR, but also the engagement of accessory molecules on T cells by their respective ligans expressed on the target cells[38,39,58,59]. More researches need to be done to reveal their relationship and what signal have CD226 transducted during immune response against tumors. The above results made a solid foundation for further cloning of CD226 ligand and is helpful for thorough investigations on CD226L structure and function.
Dr. Xu and Dr. Lan for their valuable technical advice and assistance.
Edited by Ma JY
| 1. | Burns GF, Triglia T, Werkmeister JA, Begley CG, Boyd AW. TLiSA1, a human T lineage-specific activation antigen involved in the differentiation of cytotoxic T lymphocytes and anomalous killer cells from their precursors. J Exp Med. 1985;161:1063-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Scott JL, Dunn SM, Jin B, Hillam AJ, Walton S, Berndt MC, Murray AW, Krissansen GW, Burns GF. Characterization of a novel membrane glycoprotein involved in platelet activation. J Biol Chem. 1989;264:13475-13482. [PubMed] |
| 3. | Zuzel M, Walton S, Burns GF, Berndt MC, Cawley JC. A monoclonal antibody to a 67 kD cell membrane glycoprotein directly induces persistent platelet aggregation independently of granule secretion. Br J Haematol. 1991;79:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Sun C, Jin B, Liu X. [PTA1 monoclonal antibody induces human platelet aggregation and intra-cytoplasmic Ca2+ elevation]. Zhonghua Xueyexue Zazhi. 1998;19:133-137. [PubMed] |
| 5. | Li Demin, Jin Boquan, Su Li, Jia Wei, Sun Kai, Liu Xuesong, Zhang Tingting. Distribution and function of murine platelets and T cell activation antigen 1. Zhonghua Weishengwu Xuehemian Yixue Zhazhi. 1999;19:232-235. |
| 6. | Sun Kai, Feng Qi, Jin Boquan. Platelet and T cell antigen 1 (PTA1). Zhonghua Weishengwuxue He Mianyixue Zhazhi. 1999;19:261-264. |
| 7. | Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573-581. [PubMed] |
| 8. | Slupsky JR, Cawley JC, Kaplan C, Zuzel M. Analysis of CD9, CD32 and p67 signalling: use of degranulated platelets indicates direct involvement of CD9 and p67 in integrin activation. Br J Haematol. 1997;96:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Chen LK, Bensussan A, Burns GF, Tourvieille B, Soulié A, Sasportes M. Allospecific proliferative human T-cell clones acquire the cytotoxic effector function after three months in culture, in IL-2 conditioned medium. Hum Immunol. 1986;17:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Sherrington PD, Scott JL, Jin B, Simmons D, Dorahy DJ, Lloyd J, Brien JH, Aebersold RH, Adamson J, Zuzel M. TLiSA1 (PTA1) activation antigen implicated in T cell differentiation and platelet activation is a member of the immunoglobulin superfamily exhibiting distinctive regulation of expression. J Biol Chem. 1997;272:21735-21744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Halaby DM, Mornon JP. The immunoglobulin superfamily: an insight on its tissular, species, and functional diversity. J Mol Evol. 1998;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Hawke NA, Yoder JA, Litman GW. Expanding our understanding of immunoglobulin, T-cell antigen receptor, and novel immune-type receptor genes: a subset of the immunoglobulin gene superfamily. Immunogenetics. 1999;50:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ioerger TR, Du C, Linthicum DS. Conservation of cys-cys trp structural triads and their geometry in the protein domains of immunoglobulin superfamily members. Mol Immunol. 1999;36:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Tian F, Li D, Xia H, Liu X, Jia W, Sun C, Sun K, Jin B. Isolation of cDNAs encoding gibbon and monkey platelet and T cell activation antigen 1 (PTA1). DNA Seq. 1999;10:155-161. [PubMed] |
| 15. | Huang Chuanshu, Jin Boquan, Wang Meixian, Li Enshan. Study on the activated antigen expression on the lymphocytes and lympho-blasts from HFRS patients. Shanhai Mianyixue Zhazhi. 1991;11:94-96. |
| 16. | Su Min, Niu Shumiao, Tian Dongping, Li Jian, Li Hengli, Guo Shangwen, Shi Bingyin. The clinical significance of aberrant HLA-DR antigen expression on thyrocytes. Zhonghua Binglixue Zazhi. 1994;23:34-36. |
| 17. | Tabata H, Hara M, Kitani A, Hirose T, Norioka K, Harigai M, Suzuki K, Kawakami M, Kawagoe M, Nakamura H. Expression of TLiSA1 on T cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1989;52:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Miller FW, Love LA, Barbieri SA, Balow JE, Plotz PH. Lymphocyte activation markers in idiopathic myositis: changes with disease activity and differences among clinical and autoantibody subgroups. Clin Exp Immunol. 1990;81:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Huang C, Jin B, Wang M, Li E, Sun C. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J Infect Dis. 1994;169:868-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Loertscher R, Forbes RD, Halabi G, Lavery P, Quinn T. Expression of early and late activation markers on peripheral blood T lymphocytes does not reliably reflect immune events in transplanted hearts. Clin Transplant. 1994;8:230-238. [PubMed] |
| 21. | Sun Kai, Jin Boquan, Sun Chen, Tian Fang, Zhu Yong, Yang Kun, Liu Xuesong. Construction and expression of a novel human plate-let/T cell activation antigen 1(PTA1) fusion protein in COS-7 cells. Xibao Yu Fenzi Mianyixue Zhazhi. 1998;14:1-4. |
| 22. | Serghides L, Crandall I, Hull E, Kain KC. The Plasmodium falciparum-CD36 interaction is modified by a single amino acid substitution in CD36. Blood. 1998;92:1814-1819. [PubMed] |
| 23. | Sun Kai, Jin Boquan, Sun Chen, Zhu Yong, Jia Wei, Yang Kun, Liu Chenggang. Purification of PTA1/Ig fusion protein by affinity chro-matography for PTA1. Xibao Yu Fenzi Mianyixue Zhazhi. 1998;14:113-116. |
| 24. | Callen DF, Baker E, Eyre HJ, Chernos JE, Bell JA, Sutherland GR. Reassessment of two apparent deletions of chromosome 16p to an ins(11; 16) and a t(1; 16) by chromosome painting. Ann Genet. 1990;33:219-221. [PubMed] |
| 25. | Hawke NA, Yoder JA, Litman GW. Expanding our understanding of immunoglobulin, T-cell antigen receptor, and novel immune-type receptor genes: a subset of the immunoglobulin gene superfamily. Immunogenetics. 1999;50:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Linsley PS, Peach R, Gladstone P, Bajorath J. Extending the B7 (CD80) gene family. Protein Sci. 1994;3:1341-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Taheri M, Saragovi U, Fuks A, Makkerh J, Mort J, Stanners CP. Self recognition in the Ig superfamily. Identification of precise subdomains in carcinoembryonic antigen required for intercellular adhesion. J Biol Chem. 2000;275:26935-26943. [PubMed] |
| 28. | Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 210] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Springer TA, Dustin ML, Kishimoto TK, Marlin SD. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 737] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Dustin ML, Sanders ME, Shaw S, Springer TA. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987;165:677-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 207] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Kishimoto TK, O'Connor K, Lee A, Roberts TM, Springer TA. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987;48:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 364] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, Nakauchi H, Shibuya A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J Immunol. 1998;161:1671-1676. [PubMed] |
| 35. | Isobe M, Suzuki J, Yamazaki S, Yazaki Y, Horie S, Okubo Y, Maemura K, Yazaki Y, Sekiguchi M. Regulation by differential development of Th1 and Th2 cells in peripheral tolerance to cardiac allograft induced by blocking ICAM-1/LFA-1 adhesion. Circulation. 1997;96:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Gunji Y, Nakamura M, Hagiwara T, Hayakawa K, Matsushita H, Osawa H, Nagayoshi K, Nakauchi H, Yanagisawa M, Miura Y. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1- cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992;80:429-436. [PubMed] |
| 37. | Hogg N, Landis RC. Adhesion molecules in cell interactions. Curr Opin Immunol. 1993;5:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4705] [Cited by in RCA: 4727] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 39. | Phillips JH, McKinney L, Azuma M, Spits H, Lanier LL. A novel beta 4, alpha 6 integrin-associated epithelial cell antigen involved in natural killer cell and antigen-specific cytotoxic T lymphocyte cytotoxicity. J Exp Med. 1991;174:1571-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Adler B, Ashkar S, Cantor H, Weber GF. Costimulation by extracellular matrix proteins determines the response to TCR ligation. Cell Immunol. 2001;210:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Park WR, Park CS, Tomura M, Ahn HJ, Nakahira Y, Iwasaki M, Gao P, Abe R, Hamaoka T, Fujiwara H. CD28 costimulation is required not only to induce IL-12 receptor but also to render janus kinases/STAT4 responsive to IL-12 stimulation in TCR-triggered T cells. Eur J Immunol. 2001;31:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Clavreul A, Fisson S, D'hellencourt CL, Couez D. Interelationship between CD3 and CD28 pathways in a murine T cell thymoma. Mol Immunol. 2000;37:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573-8577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 585] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365-3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 701] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 45. | Sewell WA, Brown MH, Dunne J, Owen MJ, Crumpton MJ. Molecular cloning of the human T-lymphocyte surface CD2 (T11) antigen. Proc Natl Acad Sci USA. 1986;83:8718-8722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Wilson GL, Fox CH, Fauci AS, Kehrl JH. cDNA cloning of the B cell membrane protein CD22: a mediator of B-B cell interactions. J Exp Med. 1991;173:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Wyler B, Daviet L, Bortkiewicz H, Bordet JC, McGregor JL. Cloning of the cDNA encoding human platelet CD36: comparison to PCR amplified fragments of monocyte, endothelial and HEL cells. Thromb Haemost. 1993;70:500-505. [PubMed] |
| 48. | Ianelli CJ, Edson CM, Thorley-Lawson DA. A ligand for human CD48 on epithelial cells. J Immunol. 1997;159:3910-3920. [PubMed] |
| 49. | Koopman G, Keehnen RM, Lindhout E, Zhou DF, de Groot C, Pals ST. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur J Immunol. 1997;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Piepkorn M, Hovingh P, Bennett KL, Aruffo A, Linker A. Chondroitin sulphate composition and structure in alternatively spliced CD44 fusion proteins. Biochem J. 1997;327:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Lee JW, Gersuk GM, Kiener PA, Beckham C, Ledbetter JA, Deeg HJ. HLA-DR-triggered inhibition of hemopoiesis involves Fas/Fas ligand interactions and is prevented by c-kit ligand. J Immunol. 1997;159:3211-3219. [PubMed] |
| 52. | Zöllner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol. 1997;136:707-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Mannori G, Santoro D, Carter L, Corless C, Nelson RM, Bevilacqua MP. Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol. 1997;151:233-243. [PubMed] |
| 54. | Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience. 1997;78:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789-8794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 726] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 56. | Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S, Koopman WJ, Mohler K. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1091] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 57. | Jin B, Scott JL, Vadas MA, Burns GF. TGF beta down-regulates TLiSA1 expression and inhibits the differentiation of precursor lymphocytes into CTL and LAK cells. Immunology. 1989;66:570-576. [PubMed] |
| 58. | O'Rourke AM, Mescher MF. Cytotoxic T-lymphocyte activation involves a cascade of signalling and adhesion events. Nature. 1992;358:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Rodrigues M, Nussenzweig RS, Romero P, Zavala F. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J Exp Med. 1992;175:895-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |