INTRODUCTION
Protein kinases perform important regulatory roles in response to both intra cellular and extracellular signals. Specific protein kinases are thought to control various cellular functions including glycogen metabolism, muscle contraction, and growth. Myosin light chain kinase (MLCK) is an enzyme of the kinase family, which phosphorylates the light chain of smooth muscle, skeletal muscle and no n-muscle myosin in the presence of Ca2+ and CaM, id it requires the Ca2+ and the Ca2+ binding protein CaM for the activity. Phosphorylation of the light chain in skeletal muscle is involved in modulating the tension produced during contraction, whereas in smooth muscle it appears to be required for initiation of contraction. Phosphorylation of myosin regulatory light chain (RLC) by smooth muscle myosin light chain kinase is a key event initiating smooth muscle contraction. However, the roles of myosin phosphorylation in non-muscle function is not well understood. However, present researches show that a variety of non-muscle processes are associated with MLCK, including endothelial cell retraction, fibroblast con traction, mast cell secretion, receptor capping in lymphocytes, and platelet aggregation, contraction, etc. That MLCK controls endothelial Ca2+ entry in endothelial cells not through myosin light chain phosphorylation suggests its role in vasodilation through its action in endothelial cells[1]. The activation of volume-regulated anion channels in macrovascular endothelium is modulated by myosin light chain phosphorylation through the action of MLCK or myosin light chain phosphatase[2].
Ma et al[3] incubated the filter-grown Caco-2 intestinal monolayers in Ca2+ free solution (CFS) and found an increase in tight junction permeability of the monolayer. The CFS-induced modulation of tight junction barrier was related with the activation of MLCK activity and centripetal retraction of peri-junctional actin and myosin filaments. Ueno et al[4] reported that a Ca2+-CaM- dependent protein kinase purified from rabbit liver phosphorylated the regulatory light chain of hepatocyte myosin. The kinase catalyzed the incorporation of phosphate into the 22 ku light chains of hepatocyte myosin, which resulted in a 7-fold activation of the Mg (2+)-ATPase activity by F-actin. MLCK is possibly involved in many Ca2+-dependent activities of monocytes or macrophages. It is found that there are at least two different stress fiber systems in human foreskin fibroblasts including central stress fiber system and peripheral stress fiber system, and the latter system depends on MLCK[5]. MLCK plays an important role in the development of neuron. Preventing calcium influx through blocking of MLCK activity selectively decreased dendritic branching[6]. A novel MLCK cDNA was isolated from a HeLa cell cDNA library. The deduced amino acid sequence was identical to that of a zipper-interacting protein kinase, which mediates apoptosis. With the fragment of the bovine stomach MLCK gene including kinase and calmodulin regulatory domains as a probe. Murata-Hori et al[7] screened a HeLa cell cDNA library. They found that one serine/threonine kinase, HeLa zipper-interacting protein kinase, from non-muscle cells phosphorylated regulatory chain of myosin II. Is there any novel MLCK in hepatocytes How is MLCK involved in cellular functions of hepatocytes In order to investigate the roles of MLCK in the maintenance of liver functions and its association with some liver diseases in the future study, we preliminarily studied the expression of the enzyme in the rabbit liver by reverse transcription polymerase chain re action (RT-PCR) and observed some of its properties preliminarily through assaying its catalytic activity of the phosphorylation of myosin light chain by γ-32P incorporation method. Our preliminary research provides basis for our further investigation of functions of MLCK in the liver and its relation with the pathology of some hepatic diseases.
MATERIALS AND METHODS
Reagents and instruments
CaM, rabbit smooth muscle MLCK positive control and myosin regulatory light chain were the gifts from Dr. Zhi at University of Texas Southwestern Medical Center, USA. EGTA and MOPS were purchased from Sigma Chemical Company, and DTT, phenylmethy sulfonylflouride (PMSF) and proteinase inhibitors from Sino-American Biotechnological Corp, Shanghai. HEPES was produced by MERCK; EDTA was obtained from Life Technology, GibicoBRL. [γ-32P]ATP (radio activity > 185 PBq •mol-1•L¯¹) was purchased from Yahui Biomedical Engineering Corp of Beijing. Other reagents were made in China and they were of analytical purity. Backman LS1701, Liquid Scintillation System was made in USA and DYY-III type-2 electrophoresis and transfer system were made in June 1 Instrument Factory of Beijing and idEA Ideal Sci Co. UV-754 Spectrophometer was made by The Third Factory of Analytical Instruments of Shanghai.
Tissue samples and hepatocyte isolation
Fresh liver tissues were obtained from New Zealand white rabbits and hepatocytes were isolated in reference as described elsewhere[8]. For liver tissues they were rinsed with cold Hanks solution (NaCl 137; KCl 5.0; CaCl2 1.3; MgSO4·7H2O 0.8; Na 2 HPO4 0.6; KH2PO4 0.4; NaHCO3 3.0; glucose 5.6 mmol•L¯¹; pH7. 4). Blood vessels and other tissues were removed carefully. After washing with Hanks solution, the liver was cut into about 1 mm3 slices and homogenized or stored at -80 °C for further processing.
RT-PCR of MLCK fragments[
9,
10]
For RT-PCR, hepatocytes were directly lysed with TRIZOL Reagents (GibcoBRL), and total cellular RNA was isolated according to the manufacturer’s instructions. First-strand cDNA was generated in the presence of 0.5 μg•L¯¹ Oligo (dT)12-18 from 5 μg total RN A with reverse transcriptase (SuperscriptTM Preamplification System, GibcoBRL). The paired primers to detect endogenous MLCK fragments were designed according to the reported MLCK sequence, and they are paired primer one: 5’ > AAGAATTCGATGTCAGCTGA AC < 3’ and 5’ CTTCTCCAGAAGCTTATAGGA < 3’, and paired primer two: 5’ > CCACTGGTGAAGCTTAAAATC < 3’ and 5’ > TGGAATTCCATGGGGGACGTGAA < 3’. The PCR was performed in reference as described elsewhere[9]. The PCR products were examined by 20 g•L¯¹ agarose gel and ethidium bromide staining. The photos were taken for the analysis of the PCR products.
Preparation of MLCK from rabbit liver
Using method described by Ramji et al and Tang et al[11], the liver tissues were obtained. Briefly, the liver slices removed blood vessels and other tissues were put into glass homogenizer with addition of suit amount of tissue buffer for homogenization on the ice block, and the homogenized tissues were thawed for three times (5 min once) after homogenizing. 16000 r•min-1 centrifugation for 30 min at 4 °C, and MLCK was prepared by the method described by Wang et al[8] and Bartelt et al[21]. The purified MLCK was used for activity assay.
Assay of calmodulin- and calcium-dependent MLCK activity
Ca2+/CaM-dependent activity of MLCK was measured by rates of [γ-32P] ATP incorporation into myosin light chain as substrate referring to Wang et al[9] and Blumenthal et al. Briefly, the relation of MLCK activity with time, regulatory light chain and CaM concentrations as well as with MLCK concentrations were analyzed. Maximal activity was determined in the reaction buffer containing 50 mmol•L¯¹ MOPS in mmol•L¯¹ at pH7.0: magnesium acetate 10, dithiothereitol 1, CaCl2 0.3; 1 mmol•L¯¹ [γ-32P] ATP (200-300 cpm·pmol-1); 1.2 μmol•L¯¹ CaM, 25 μmol•L¯¹ regulatory light chain of myosin, and diluted MLCK at room temperature. MLCK was freshly diluted in 10 mmol•L¯¹ MOPS (pH7.0), 1 mmol•L¯¹ dithiothreitol, and 1 g•L¯¹ bovine serum albumin and added to the reaction mixture. Final MLCK concentrations used in kinetic measurements showed linear phosphorylation rates with respect to time and enzyme concentration in a certain range.
Protein contents determination
Lowry’s method was used for the determination of contents of various proteins, in which bovine serum albumin was used as the standard.
Statistical analysis
Data of MLCK activity were collected and processed through analysis of difference of MLCK activity by Student’s t test with SPSS 8.0 Windows software. P < 0.05 or P < 0. 01 was considered as statistically significant in the difference of MLCK activity.
RESULTS
Confirmation of MLCK expression in rabbit hepatocytes by RT-PCR
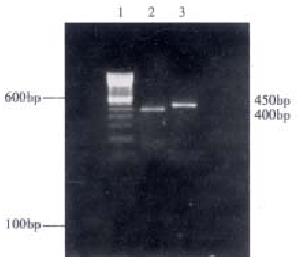
Two pairs of specific primers were used to detect the expression of MLCK in freshly isolated hepatocytes. The total cellular RNA was isolated and first-strand cDNA was generated. The results showed that the RT-PCR products were 400 bp and 450 bp respectively, confirming with the expected molecular size (Figure 1), which suggests that there is expression of MLCK in rabbit liver.
Figure 1 Reverse transcription polymerase chain reaction of cellular total RNA from hepatocytes in New Zealand rabbit.
1.100 bp DNA ladder; 2-3, PCR products: amplified MLCK DNA fragments.
Features of rabbit liver MLCK activity analysis
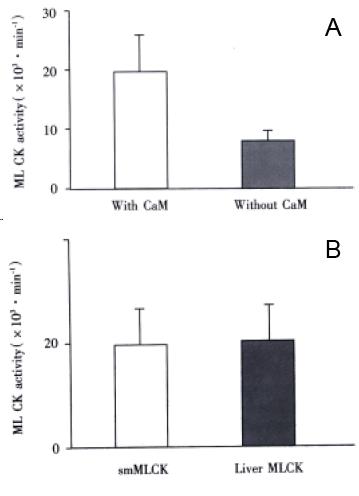
Comparison of activity of rabbit liver MLCK with that of smooth muscle MLCK To observe the activity level of our isolated MLCK in rabbit liver, we assayed the activity of the MLCK and smooth muscle MLCK. It was found that both kinds of MLCK had similar catalytic activity, there is no statistically significant difference between two kinds of enzymes (P < 0.01) by Student’s t test (Figure 2 A).
Figure 2 A: Comparison of enzymatic activity between smMLCK and rabbit liver MLCK (P > 0.
05); B: Effect of CaM on MLCK in rabbit liver activity (P < 0.01).
MLCK from rabbit liver was CaM-dependent To confirm the MLCK isolated from the rabbit liver is dependent on CaM, we assayed the effect of phosphorylation of MLC K on regulatory light chain of myosin. It was found that the light chain was obviously phosphorated when CaM was added into the reaction buffer at the suitable concentration of Ca2+. The MLCK activity increased markedly with CaM added. There is significantly statistical difference when compared with the activity of the MLCK without adding CaM (P < 0.01) through Student’s t test analysis with SPSS 8.0 Windows software. See Figure 2 (B).
Figure 2 A: Comparison of enzymatic activity between smMLCK and rabbit liver MLCK (P > 0.05); B: Effect of CaM on MLCK in rabbit liver activity (P < 0.01)
Action characteristics of MLCK of rabbit liver In the reaction system, enzymatic catalytic action of the MLCK increased gradually at first three min and the extent of phosphorylation of the regulatory light chain of myosin was at the highest level at about three min on the suitable concentrations of substrate, Ca2+ and CaM. In addition, the activity of MLCK of rabbit liver changed with the changes of the substrate concentration or the concentration of light chain of myosin. When the concentration was 0. 6 5 mg•L¯¹, the activity of MLCK was at the highest level, the most amount of light chain was phosphorated at this time. Finally, MLCK concentration influenced the activity of the enzyme itself. On the suitable substrate, CaM and calcium ion concentrations, the activity of MLCK from the rabbit liver was basically at the higher level when MLCK ranged from 15 mg•L¯¹ to 25 mg•L¯¹.
DISCUSSION
MLCK is the key regulator of cell motility and smooth muscle contraction in higher vertebrates. MLCK expression shows a complex pattern. In undifferentiated myoblasts, 220-ku long or non-muscle form of MLCK is expressed during differentiation of skeletal muscle. During myoblast differentiation, expression of the 22 0-ku MLCK declines and expression of this long-form is replaced by 130 ku smooth muscle MLCK and a skeletal muscle-specific MLCK. In fact, 130 ku smooth muscle MLCK is not a smooth muscle-specific protein, it is ubiquitous in all adult tissues[12]. A prerequisite for vertebrate smooth muscle contraction, potentiation of skeletal and cardiac muscle contraction and various non-muscle motile events in response to intracellular Ca2+ signaling is the phosphorylation of myosin II R LC at Ser-19 by MLCK. The phosphorylation of RLC increases the Mg2+-ATP activity of myosin, which catalyses cyclic conversion of ATP chemical energy into mechanical work through the reversible actomyosin interactions. Ca2+-calmodulin -dependent phosphorylation of RLC of myosin by the catalytic COOH-terminal half of MLC K activates myosin II in smooth and non-muscle cells. Three-dimensional reconst ructions showed MLCK density on the extreme periphery of subdomain-1 of each actin monomer forming a bridge to the periphery of subdomain-4 of the azimuthally adjacent actin. There is interaction of MLCK-147 close to the COOH terminus of the first actin and near residues 228-232 of the second. The unique location ensure that MLCK binds to actin without interfering with the binding of any other key actin-binding proteins, including myosin, tropomyosin, caldesmon, and calponin[13]. In addition, the unique sequence of MLCK-210 is involved in its interaction with the microfilaments and contributes to its tighter association with the actin cytoskeleton[14]. Non-muscle cells may use different mechanisms for targeting the long-form MLCK to actomyosin structures during interphase and mitosis. MLCK and myosin II phosphatase act cooperatively to regulate the level of Ser 19 -phosphorylated myosin II during mitosis and initiate cytokinesis through the a ctivation of myosin II motor activity[15].
Ca2+/CaM forms a ternary complex with MLCK, facilitating in activation of the kinase and phosphorylation of RLC. Phosphorylation induces a conformational change, which allows myosin crossbridges to along actin filaments. Some non-muscle processes are also regulated by RLC phosphorylation of the myosin, while smooth muscle MLCK plays important roles in contractile-motile processes of a variety of cells[16]. MLCK has an actin-binding activity in addition to its kinase activity, which assembles actin filaments in to bundles morphologically and biochemically. There are two actin-binding sites on MLCK, including calcium- and calmodulin-sensitive site and insensitive site. The cross-linking between this two sites assembles actin into bundles[17]. Recently, it is found that a novel approximately 60 ku MLCK immunogen contributes to the aberrant contractility associated with preterm labour[18], while Sohn et al[19] reported that calmodulin and MLCK play a role in Ach-induced lower esophageal sphincter contraction, whereas the classical MLCK may not be the major kinase responsible for contraction and phosphorylation of myosin light chain in esophagus. Esophagus contraction is protein kinase C dependent contraction. Various new functions of MLCK have been found recently, activation of myosin II by MLCK produces force for many cellular processes including mitosis, migration, and other cellular shape changes. Inhibition or potentiation of myosin II activation via over-expression of a dominant negative or wild type MLCK can delay or accelerate tumor necrosis factor-α induced apoptotic cell death in cells[20]. MLC K specific ally mediates agonist-induced sarcomere organization during early hypertrophic response[21]. During vascular injury, the expression of MLCK decreased[22]. In rabbit portal vein myocytes, MLCK mediates noradrenaline-evoked non-selective cation current[23]. In the liver, agents that elevate intracellular free Ca2+ concentration increase tight junctional permeability and stimulate bile canalicular contraction. Myosin phosphorylation is probably responsible for the tight junction al permeabilization caused by elevation of intracellular Ca2+ in hepatocytes. Moreover, the integrity of the phosphorylation system of myosin is essential for normal bile flow. In addition, hepatic sinusoidal Ito cells (fat storing cells) play a regulatory role on hepatic sinusoidal blood flow through their contraction, while the integrity of myosin light chain kinase is essential for Ito cell con traction and normal sinusoidal blood flow. However, the roles of myosin phosphorylation by MLCK in non-muscle tissues is not well characterized but correlates with important activities such as cell division, receptor capping, etc. Recently, the study showed the existence of a 208 ku protein, named embryonic MLCK because its expression can be detected in early embryonic tissues, stem cells, and in proliferating cultured cells. In the liver, down-regulation of this 208 ku embryonic MLCK is not so dramatic, and the less dramatic decline in the expression of embryonic MLCK may possibly reflect the high regenerative capacity of liver tiss ue. It is now found that MLCK is associated with non-muscle cells closely[1,2,4-7]. MLCK activation is also a critical step in the cytoskeletal changes causing pseudopod formation during polymorphonuclear leukocyte phagocytosis[24]. MLC K immunoreactivity was found to be colocalized with the insulin granules which suggests that it increases insulin granules in the ready-releasable pool by acting on different steps in the secretory cascade[25]. In 3T3 fibroblasts, M LCK is responsible for phosphorylation of MLC at the cell periphery, showing its unique spatial regulation of myosin RLC[26]. From the gene of vertebrate smooth muscle and non-muscle MLCK there are at least four proteins expressed. Two high molecular weight MLCK splice variants, EC MLCK-1 and EC MLCK-2 (210-214 ku) in human endothelium are identical except for a deleted single exon in MLCK-2 encoding a 69-amino acid stretch that contains potentially important consensus sites for phosphorylation by p60 (Src) kinase, while p60 (Src)-mediated tyrosine phosphorylation represents an important mechanism for splice variant-specific regulation of non-muscle and cascular cell function[27]. MLCK is also associated with the gap fomation and endothelial hyperpermeability of coronary venular endothelial cell monolayers[28]. Our preliminary study revealed that MLCK in the rabbit liver could phosphorate myosin light chain obviously, and was calmodulin-dependent, which may play an important role in maintaining the normal functions of the tissue. Bu t in the liver, what form of MLCK expressed more, long form MLCK (embryonic MLCK) or short form MLCK with the molecular weight about 130-150 ku What are their exact roles in the liver What roles it will play in liver regeneration, in liver injury or in hepatic carcinoma And what are the action mechanisms These remain to be elucidated in our further study.