Published online Aug 15, 2001. doi: 10.3748/wjg.v7.i4.532
Revised: February 8, 2001
Accepted: February 12, 2001
Published online: August 15, 2001
AIM: To investigate the correlation between lymphogenous metastasis and matrix metalloproteinases (MMPs) activity and the expression of Fas ligand of tumor cells in lymph nodes.
METHODS: Fifty-six inbred 615-mice were equally divided into 2 groups and inoculated with Hca-F and Hca-P cells. Their lymph node metastatic rates were examined. Growth fraction of lymphocytes in host lymph nodes was detected by flow cytometry. The Hca-F and Hca-P cells were cultured with extract of lymph node, liver or spleen. The quantity of MMPs in these supernatants was examined by zymographic analysis. The expression of Fas ligand, PCNA, Bcl-2 protein of Hca-F and Hca-P cells in the mice were examined by immunohistochemistry. The apoptosis signals of macrophages in lymph nodes were observed with in situ DNA fragmentation.
RESULTS: On the 28th day post-inoculation, the lymph node metastatic rate of Hca-F was 80% (16/20), whereas that of Hca-P was 25% (5/20). The growth fraction of lymphocytes was as follows: in the Hca-F cells, the proliferating peak of lymphocytes appeared on the 14th day post-inoculation and then decreased rapidly, while in Hca-P cells, the peak appeared on the 7th day post inoculation and then kept at a high level. With the extract of lymph node, the quantity of the MMP-9 activity increased (P < 0.01) and active MMP-9 and MMP-2 were produced by both Hca-F and Hca-P tumor cells, which did not produce MMPs without the extract of lymph node or with the extracts of the liver and spleen. The expression of Fas Ligand of Hca-F cells was stronger than that of Hca-P cells (P < 0.01). The expressions of PCNA and Bcl-2 protein of Hca-F cells in the tumors of inoculated area were the same as that of Hca-P cells. In situ DNA fragmentation showed that the positive signals of macrophages were around Hca-F cells.
CONCLUSION: Secretion of MMPs which was associated with metastatic ability of Hca-F and Hca-P tumor cells depends on the environment of lymph nodes. The increased expression of Fas ligand protein of Hca-F tumor cells with high lymphogenous metastatic potential in lymph nodes may help tumor cells escape from being killed by host lymphocytes.
- Citation: Hou L, Li Y, Jia YH, Wang B, Xin Y, Ling MY, Lü S. Molecular mechanism about lymphogenous metastasis of hepatocarcinoma cells in mice. World J Gastroenterol 2001; 7(4): 532-536
- URL: https://www.wjgnet.com/1007-9327/full/v7/i4/532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i4.532
Metastasis is the most lethal attribute of a cancer[1,2]. Metastasis is a complex process which is made up of several steps[3]. Lymph nodes are often the first organ to develop metastasis[4,5]. Whether lymph nodes or other sites first develop metastases remain poorly understood. Lymph node metastases form a bridgehead for further metastatic spread. But its molecular mechanism is still unclear because of its complicated course. Anchorage on lymph node and escape from being killed by host immune cells in lymph node were two important steps. A mouse hepatocarcinoma cell line (Hca-F) with high lymphogenous metastatic potential and its syngeneic cell line (Hca-P) with low one were separated from hepatocarcinoma (HCC)[6] in mice. Matrix metalloproteinases (MMPs)[7] are a class of proteinases with variable substrate such as collagen, fibronectin and are related to the invasion-metastasis of hepatocellular cancer (HCC)[8]. Therefore, MMPs can be used as a marker of tumor cell infiltration in lymph node. The potential of tumor cells to induce apoptosis of host immune cells is to escape from being killed by immune cells. In this study, we detected the differences in the MMPs productions of Hca-F from Hca-P cells under various conditions, and different potentials of the carcinoma cells with different lymphogenous potentials to inhibit host immune reaction.
Fifty-six inbred 615-mice maintained in our laboratory were equally divided into two groups. The Hca-F and Hca-P tumor cell lines preserved in our laboratory were inoculated at 2 × 106 in 28 mice subcutaneously in each group. On the 7th, 14th, 21st, and 28th day post-inoculation, two mice from each group were killed, and their lymphocytes were collected and detected for growth fraction with flow cytometry. The process of flow cytometry is as follows[9]: the lymph nodes were minced and centrifuged at 3000 × g, and the supernatant was discarded. After repeated washing, cells were suspended in PBS. The lymphocytes at 105/100 μL suspension were stained for 30 minutes by Propidum Iodide. Flow cytometry was performed on a FACScan cytometer with LYSYII software. The fluorescence of 104 cells was analyzed for each sample. The other 40 mice were terminated on the 28th day post-inoculation, and their lymph nodes were H.E. stained and examined under microscope by paraffin sections. Therefore, the lymph node metastatic rates of Hca-F and Hca-P tumor cells were calculated.
The Hca-F and Hca-P cells cultured were put into different wells at 5 × 105, and then added 50 mg extract of lymph node, liver or spleen respectively. The RPMI 1640 medium without fetal calf serum was placed into each well up to 1 mL. The wells containing only Hca-F or Hca-P cells, and RPMI 1640 medium added only extracts of lymph node, liver or spleen up to 1 mL served as controls. These cells were cultured at 37 °C for 24 h. The supernatant of cultured cells was collected by centrifugation at 3000 × g. MMP-2 and MMP-9 and their active type, and MMP-8 contained in supernatants of Hca-F and Hca-P with or without extracts of lymph node, liver or spleen were detected by zymographic analysis, according to the method described by Fridman et al[10]. The density and area of each band were measured using QuantiScan Software (Biosoft, USA).
The expressions of Fas-L (Santa Cruz, USA), proliferating cell nuclear antigen (Santa Cruz, USA, PCNA) and Bcl-2 (Santa Cruz, USA) protein in the tumor cells of inoculated area of Hca-F and Hca-P tumor cells, and metastatic tumor cells of lymph nodes of Hca-F were detected by standard immunohistochemistry[11]. The semiquantitative estimation of cancer cells stained was classified into 4 categories by assessing the percentage of stained tumor cells: 0, < 2%; 1, 2%-25%; 2, 26%-50%; 3, 51%-75%; and 4, > 75% cells.
We examined DNA fragmentation of the tumor cells of inoculated area of Hca-F and Hca-P cells, and metastatic tumor cells of Hca-F cells in lymph node, by the method of Zhu et al[12]. After desparaffin, these slides were pre-treated with 20 mg·L-1 proteinase K for 30 minutes, and then incubated with terminal deoxynucleotidy transferase and fluorescein labeled dUTP containing nucleotide mixture (TUNEL reaction mixture, in situ Cell Death Detection Kit/POD, Boehringer Mannheim, Germany) in a humid atmosphere at 37 °C for 30 minutes. Each experiment set up by TUNEL reaction mixture without terminal transferase served as negative control.
On the 28th day post-inoculation, the lymph node metastatic rate of Hca-F was 80% (16/20), whereas that of Hca-P was 25% (5/20). The growth fraction of lymphocytes from lymph nodes of mice transplanted with Hca-F and Hca-P tumor cells was examined using flow cytometry (Figure 1). For the Hca-F cells, the proliferating peak of lymphocytes appeared on the 14th day post-inoculation and then decreased rapidly, and for the Hca-P cells, the peak appeared on the 7th day post-inoculation and then kept at the high level.
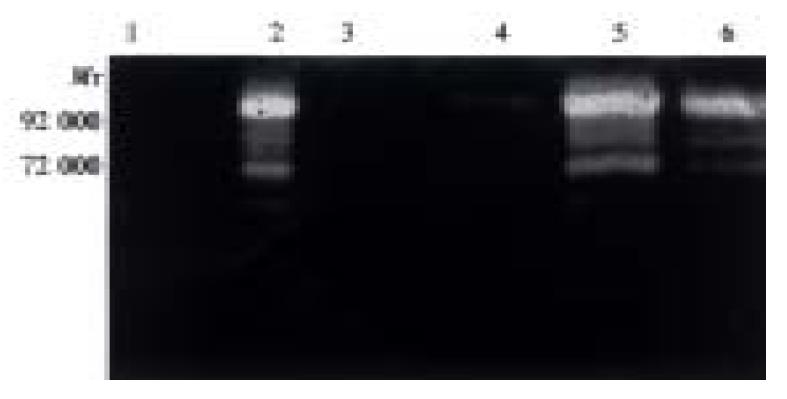
Both Hca-F and Hca-P cells produced a small account of MMP-9, but did not produce MMP-2 and MMP-8 (Table 1, Figure 2). Both Hca-F and Hca-P cells with extract of lymph node produced higher amounts of MMP-9 than Hca-F and Hca-P cells without extract of lymph node (P < 0.01), and produced active MMP-9 and MMP-2. However, the Hca-F cells produce much greater quantities of activity of MMP-9, active MMP-9 and MMP-2 than Hca-P cells (P < 0.05). The extract of lymph node did not contain any MMPs (Table 1, Figure 2). There was activity of MMP-8 in both Hca-F and Hca-P cells with extract of liver (Hca-F: 1767, Hca-P: 1564). The extract of liver contained activity of MMP-8 (1837). There was activity of MMP-8 in both Hca-F and Hca-P cells with extract of spleen (Hca-F: 2036, Hca-P: 1993). The extract of spleen contained the same quantity of activity of MMP-8 (1784). Therefore, we think that both Hca-F and Hca-P cells in the environment of liver and spleen did not produce activity of MMP-8 (Figure 3, Figure 4).
| Condition | MMP-2 | Active MMP-2 | MMP-9 | Active MMP-9 |
| Hca-F | ||||
| RPMI1640 medium | 0 | 0 | 1256 ± 157 | 0 |
| Medium with lymph node extract | 7364 ± 2001 | 2009 ± 901 | 12403 ± 894 | 7297 ± 1657 |
| Hca-P | ||||
| RPMI1640 medium | 0 | 0 | 2642 ± 385 | 0 |
| Medium with lymph node extract | 2997 ± 1990 | 1237 ± 905 | 9086 ± 686 | 3914 ± 1253 |
| Lmyph node extract | 0 | 0 | 0 | 0 |
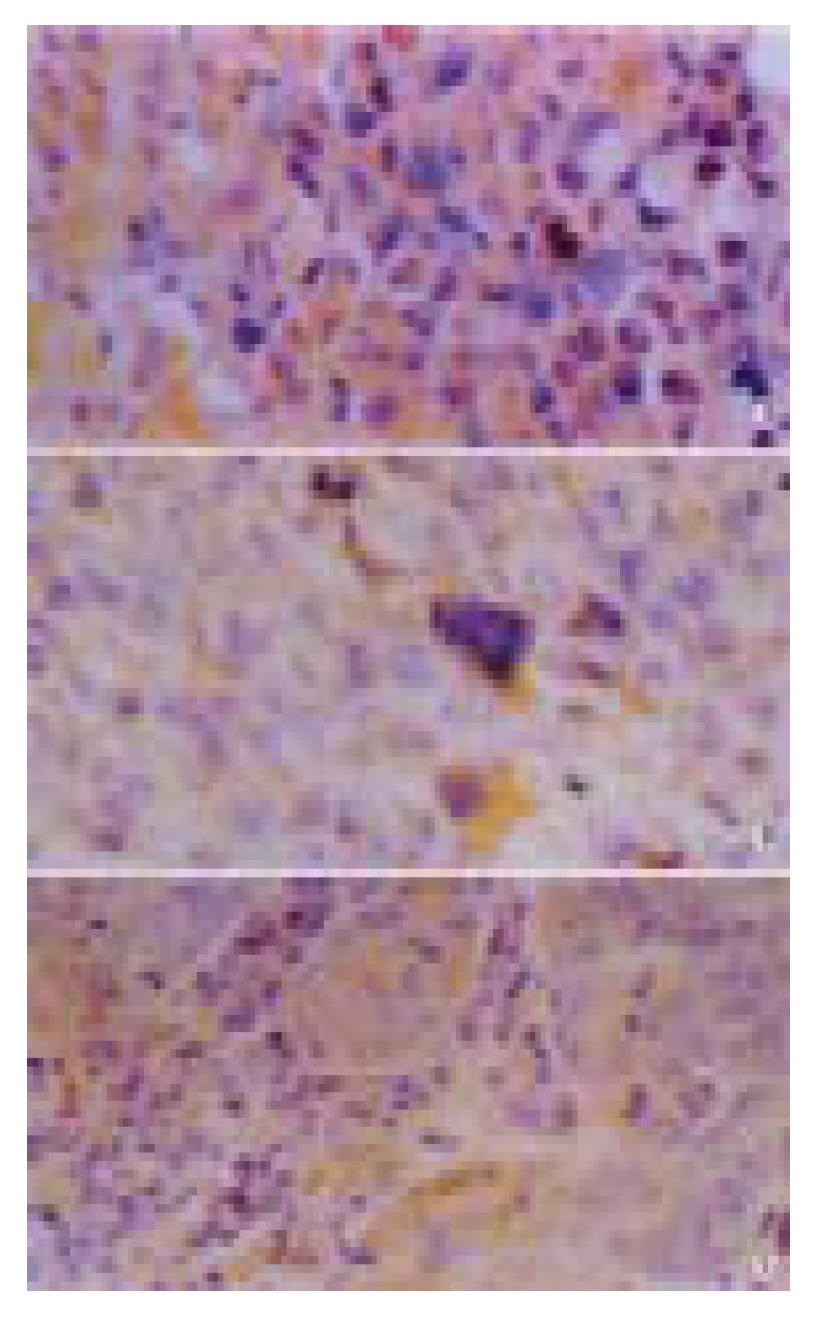
The expression of Fas ligand protein of Hca-F cells was significantly higher than that of Hca-P cells (P < 0.01, Table 2, Figure 5). The expressions of PCNA and Bcl-2 protein of Hca-F cells were as strong as those of Hca-P cells.
| Tumor | Grade | ||||
| 0 | 1 | 2 | 3 | 4 | |
| Primary tumor of Hca-F | 0 | 0 | 0 | 8 | 12 |
| Primary tumor of Hca-P | 2 | 10 | 8 | 0 | 0 |
| Metastatic tumor of Hca-F | 0 | 5 | 9 | 6 | 0 |
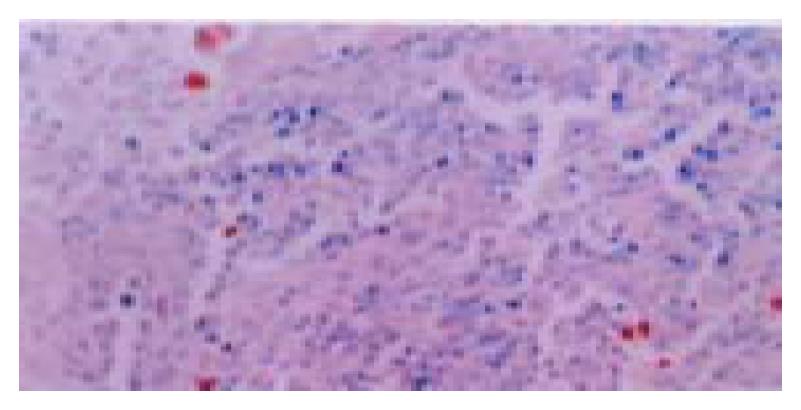
Few positive Hca-F and Hca-P cells were observed. Positive signals appeared in the macrophages around Hca-F cells (Figure 6).
As early as a hundred years ago, the fact that tumor cells had organ-specific metastasis had attracted scientists’ attention. Recent major discoveries concerning invasion and metastasis are identification of certain molecular mechanisms leading to organ-selective metastatization[13]. Cancer cell gene expression is regulated by interactions of tumor cells with host microenvironment, both in primary and secondary lesions[14,15]. Whether the anchorage of carcinoma cells in lymph nodes is influenced by the specific environment of the lymph nodes remains unclear. Hca-F and Hca-P tumor cells have the potentials of specific lymphogenous metastasis. The matrix metalloproteinases[16] (MMPs) are a family of proteolytic enzymes, and the importance of MMPs in the processes of tumor invasion is now widely acknowledged in gastrointestinal cancer[17], breast cancer[18], colorectal cancer[19], and melanoma[20]. Inhibit the activity of MMPs can reduce the metastatic potential of cancer cells[21]. MMps digest collagen-containing structural barriers that cancer cells must pass in the step of cancer cell’s anchoring to lymph node[22]. In this paper when the extract of lymph node was added, the quantity of the MMP-9 activity increased and active MMP-9 and MMP-2 were produced by both Hca-F and Hca-P tumor cells, which did not produce MMPs without the extract of lymph node or with extracts of liver and spleen. These results indicated that the secretion of MMPs of these tumor cells depend on the lymph node environment. In the environment with extract of lymph node, Hca-F cells with high lymphogenous metastatic potential produced much more MMPs than Hca-P cells with low one. So we can conjecture that Hca-F cells with high lymphogenous metastatic potential can easily receive the signal from lymph node and then start to infiltrate in lymph node to form metastatic focus.
The macrometastases were more proliferative than dormant micrometastases[23]. PCNA[24] functions as a cofactor of DNA-polymerase and is an important mark for evaluating the proliferation of colon cancer[25,26], gastric adenocarcinoma[27], lung cancer[28], ovarian cancer[29], thyroid carcinoma[30], and large intestine polyps[31]. We can use PCNA as an index of cellular proliferative status[32]. Bcl-2 proteins can extend cell survival by suppressing apoptosis[33] and are up-regulated in squamous cell carcinoma[34], breast cancer[35], lung cancer[36]. Bcl-2 proteins may promote metastasis in breast cancer[37] and melanoma[38]. The expressions of PCNA and Bcl-2 proteins could reflect accurately the status of cancer cells’s growth. The expressions of PCNA and Bcl-2 protein of Hca-F cells in the tumors of inoculated area are the same as those of Hca-P cells. Therefore, the proliferating ability of Hca-F was equal to Hca-P, although their lymph node metastatic potentials were different.
A metastatic tumor in lymph node may form as long as the tumor cells escape the killing of lymphocytes. Fas/Apo-1, together with its protein-binding partner (Fas ligand), is a key regulator of programmed cell death and induces apoptosis when it binds FasL[39-42]. In this study, we found that growth fraction of lymphocytes in host lymph nodes was lower with Hca-F cells stimulation than with Hca-P cells. The result suggests that Hca-F cells may inhibit the growth and function of lymphocytes in lymph nodes. Tumor cell survives only by evasion of the immune system[43]. The Fas/FasL system is involved in the induction of apoptosis and mediates T-cell cytotoxicyty[44]. The expression of Fas ligand in many cancers plays an important role in establishing immunologically privileged environments that allow tumors to escape the host’s immune surveillance, such as in esophageal carcinomas[45,46], lung cancer[47], melanoma[48], gastric carcinoma[49], intrahepatic cholangiocellular carcinoma[50] and promotes these cancers’ metastasis. The expression of Fas ligand protein of Hca-F cells was stronger than that of Hca-P cells. Hca-F cells also produced Fos ligand in lymph node. Macrophages in lymph node are one of important antigen-presenting cells, and Fas ligand in tumor cells can combine with Fas in the membrane of macrophages to induce apoptosis and decrease their function. Because lymphocytes can not receive the signals from the macrophages, tumor cells in lymph nodes can escape the suppression of lymphocytes, then accomplish the metastatic process in the lymph node.
In a word, secretion of MMPs, which was associated with metastatic ability, of Hca-F and Hca-P tumor cells depend on the environment of lymph nodes. The increased expression of Fas ligand protein of Hca-F tumor cells with a high lymphogenous metastatic potential in lymph nodes may help tumor cells escape from the killing of host lymphocytes and shape up metastatic focus in lymph nodes.
Edited by Ma JY
| 1. | Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Metastasis-suppressor genes: a review and perspective on an emerging field. J Natl Cancer Inst. 2000;92:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Rinker-Schaeffer CW, Welch DR, Sokoloff M. Defining the biologic role of genes that regulate prostate cancer metastasis. Curr Opin Urol. 2000;10:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Wittekind C. Diagnosis and staging of lymph node metastasis. Recent Results Cancer Res. 2000;157:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Ji Y, Ling MY, Li Y, Xie H. Effect of cell fusion on metastatic ability of mouse hepatocarcinoma cell lines. World J Gastroenterol. 1999;5:22-24. [PubMed] |
| 7. | Liu HL, Li XH, Wang DY, Yang SP. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 expression in fibrotic rat liver. World J Gastroenterol. 2000;6:881-884. [PubMed] |
| 8. | Hong ZY, Yu JL, Zhang YS, Gao Y. Relationship between the expression of matrix metalloproteinase-9, CD34 and the inva-sion-metastasis of hepatocellular cancer (HCC). Shijie Huaren Xiaohua Zazhi. 2001;9:170-174. |
| 9. | Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664-670. [PubMed] |
| 10. | Fridman R, Fuerst TR, Bird RE, Hoyhtya M, Oelkuct M, Kraus S, Komarek D, Liotta LA, Berman ML, Stetler-Stevenson WG. Domain structure of human 72-kDa gelatinase/type IV collagenase. Characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J Biol Chem. 1992;267:15398-15405. [PubMed] |
| 11. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 12. | Zhu HZ, Ruan YB, Wu ZB, Zhang CM. Kupffer cell and apoptosis in experimental HCC. World J Gastroenterol. 2000;6:405-407. [PubMed] |
| 13. | Tímár J. [Problems of tumor progression: doubts or hopes at the turn of the Millennium?]. Orv Hetil. 2000;141:891-899. [PubMed] |
| 14. | Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 266] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Ellenrieder V, Adler G, Gress TM. Invasion and metastasis in pancreatic cancer. Ann Oncol. 1999;10 Suppl 4:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 412] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Matsuoka T, Yashiro M, Sawada T, Ishikawa T, Ohira M, Chung KH. Inhibition of invasion and lymph node metastasis of gastrointestinal cancer cells by R-94138, a matrix metalloproteinase inhibitor. Anticancer Res. 2000;20:4331-4338. [PubMed] |
| 18. | Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol. 1999;189:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Garbett EA, Reed MW, Brown NJ. Proteolysis in human breast and colorectal cancer. Br J Cancer. 1999;81:287-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Nischt R, Wallich M, Reibetanz M, Baumann P, Krieg T, Mauch C. BM-40 and MMP-2 expression are not coregulated in human melanoma cell lines. Cancer Lett. 2001;162:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lokeshwar BL. MMP inhibition in prostate cancer. Ann N Y Acad Sci. 1999;878:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61-65. [PubMed] |
| 23. | Barnhill RL. The biology of melanoma micrometastases. Recent Results Cancer Res. 2001;158:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Wu WY, Xu Q, Shi LC, Zhang WB. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol. 2000;6:216-219. [PubMed] |
| 25. | Iwatani Y, Shimada Y, Seima Y, Yamazaki T, Miyazaki N. The biological and clinicopathological characteristics of right-sided colon cancer. Oncol Rep. 2000;7:991-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Kanazawa Y, Onda M, Tanaka N, Seya T. Proliferating cell nuclear antigen and p53 protein expression in submucosal invasive colorectal carcinoma. J Nippon Med Sch. 2000;67:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Elpek GO, Gelen T, Aksoy NH, Karpuzoglu T, Keles N. Microvessel count, proliferating cell nuclear antigen and Ki-67 indices in gastric adenocarcinoma. Pathol Oncol Res. 2000;6:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Expression of proliferating cell nuclear antigen and CD44 variant isoforms in the primary and metastatic sites of nonsmall cell lung carcinoma with intrapulmonary metastases. Cancer. 1999;86:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Wu X, Zhang Z, Cai S. [Proliferating cell nuclear antigen (PCNA) in ovarian carcinoma and its relation to lymph node metastasis and prognosis]. Zhonghua Zhongliu Zazhi. 1998;20:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Moreira Leite KR, de Araujo VC, Rezende Meirelles MI, Lopes Costa AD, Camara-Lopes LH. No relationship between prolif-erative activity and the MACIS prognostic scoring system in papillary thyroid carcinoma. Head & Neck. 1999;21:602-605. [DOI] [Full Text] |
| 31. | Luo YQ, Ma LS, Zhao YL, Wu KC, Pan BR, Zhang XY. Expression of proliferating cell nuclear antigen in polyps from large intestine. World J Gastroenterol. 1999;5:160-164. [PubMed] |
| 32. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] |
| 33. | Xu HY, Yang YL, Guan XL, Song G, Jiang AM, Shi LJ. Expression of regulating apoptosis gene and apoptosis index in primary liver cancer. World J Gastroenterol. 2000;6:721-724. [PubMed] |
| 34. | Hantschmann P, Kürzl R. Regulation of apoptosis in squamous cell carcinoma of the vulva. J Reprod Med. 2000;45:633-642. [PubMed] |
| 35. | Sierra A, Castellsagué X, Escobedo A, Lloveras B, García-Ramirez M, Moreno A, Fabra A. Bcl-2 with loss of apoptosis allows accumulation of genetic alterations: a pathway to metastatic progression in human breast cancer. Int J Cancer. 2000;89:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Zhou Q, Liu J, Zhang W, Jing H, Liu L, Chen J. [Transcriptional expression of apoptosis suppression gene bcl-2 in non-small cell lung carcinoma]. Zhonghua Yiue Yichuanxue Zazhi. 2000;17:262-265. [PubMed] |
| 37. | Sierra A, Castellsague X, Escobedo A, Moreno A, Drudis T, Fabra A. Synergistic cooperation between c-Myc and Bcl-2 in lymph node progression of T1 human breast carcinomas. Breast Cancer Res Treat. 1999;54:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Takaoka A, Adachi M, Okuda H, Sato S, Yawata A, Hinoda Y, Takayama S, Reed JC, Imai K. Anti-cell death activity promotes pulmonary metastasis of melanoma cells. Oncogene. 1997;14:2971-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Mouawad R, Khayat D, Soubrane C. Plasma Fas ligand, an inducer of apoptosis, and plasma soluble Fas, an inhibitor of apoptosis, in advanced melanoma. Melanoma Res. 2000;10:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Müschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. J Mol Med (Berl). 2000;78:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Ueno T, Toi M, Tominaga T. Circulating soluble Fas concentration in breast cancer patients. Clin Cancer Res. 1999;5:3529-3533. [PubMed] |
| 42. | Shimoyama M, Kanda T, Liu L, Koyama Y, Suda T, Sakai Y, Hatakeyama K. Expression of Fas ligand is an early event in colorectal carcinogenesis. J Surg Oncol. 2001;76:63-68; discussion 69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Wullich B. [Molecular genetic principles of progression of malignant diseases]. Urologe A. 2000;39:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 44. | Kume T, Oshima K, Yamashita Y, Shirakusa T, Kikuchi M. Relationship between Fas-ligand expression on carcinoma cell and cytotoxic T-lymphocyte response in lymphoepithelioma-like cancer of the stomach. Int J Cancer. 1999;84:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Younes M, Schwartz MR, Ertan A, Finnie D, Younes A. Fas ligand expression in esophageal carcinomas and their lymph node metastases. Cancer. 2000;88:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Tan LJ, Jiang W, Zhang N, Zhang XR, Qiu DH. Fas/FasL ex-pression of esophageal squamous cell carcinoma, dysplasia tissues and normal mucosa. Shijie Huaren Xiaohua Zazhi. 2001;9:15-19. |
| 47. | Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57:1007-1012. [PubMed] |
| 48. | Ekmekcioglu S, Okcu MF, Colome-Grimmer MI, Owen-Schaub L, Buzaid AC, Grimm EA. Differential increase of Fas ligand expres-sion on metastatic and thin or thick primary melanoma cells com-pared with interleukin-10. Melanoma Res. 1999;9:261-272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Tsutsumi S, Kuwano H, Shimura T, Morinaga N, Mochiki E, Asao T. Circulating soluble Fas ligand in patients with gastric carcinoma. Cancer. 2000;89:2560-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, Imaoka S, Tsujimoto M, Matsuura N. Expression of Fas and Fas ligand reflects the biological characteristics but not the status of apoptosis of intrahepatic cholangiocellular carcinoma. Int J Mol Med. 2000;6:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |