Published online Aug 15, 2001. doi: 10.3748/wjg.v7.i4.527
Revised: April 15, 2001
Accepted: April 22, 2001
Published online: August 15, 2001
AIM: To extract and purify the transforming growth factor β (TGFβ), and to demonstrate its biological activity in vivo and induction of apoptosis of hepatocytes in vitro.
METHODS: TGFβ was isolated from fresh bovine platelets by acid/ethanol extraction method and purified with ion exchange and gel chromatography. The extracted TGFβ as injected subcutaneously to mice, and its biological activity in vivo was observed 72 hrs post-injection by HE staining. The morphological changes were observed by HE staining and the occurrence of apoptosis was detected by TUNEL method after the human normal hepatic cell line QZG was treated with 8 μg·L-1TGFβ for 12 hrs in vitro.
RESULTS: The molecular mass 25 ku TGFβ protein was successfully extracted. It was able to induce localized granulation tissue formation in vivo. TGFβ-treated hepatocytes showed obvious apoptotic morphological changes, including the pyknosis and dense-stained nuclei and cytoplasm, the fragmentary, annular or crescent nuclei, and the “bubbling” cytoplasm. Moreover, its apoptotic rate was significantly higher than that of the control group (P < 0.05).
CONCLUSION: Biological active TGFβ protein is extracted and purified successfully from bovine platelets, and it is able to induce the apoptosis of hepatocytes.
- Citation: Si XH, Yang LJ. Extraction and purification of TGFβ and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol 2001; 7(4): 527-531
- URL: https://www.wjgnet.com/1007-9327/full/v7/i4/527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i4.527
Liver diseases are very common in China[1-10], and apoptosis is the research focus in recent years[11-20]. Transforming growth factor β (TGFβ) is a kind of polypeptide growth factors that is extensively present in most tissues and cells. A variety of cell types, both nonneoplastic and neoplastic, synthesize TGFβ and most of these cells have specific high-affinity receptors for TGFβ[21-24]. It is a multifunctional molecule which is known to play an important regulatory role in cell growth, migration and differentiation[25], embryogenesis[26,27], tumorigenesis[28], wound healing[29-31], bone formation[32-35] and immunomodulation[36], acting by both autocrine and paracrine mechanisms. It is also suggested that TGFβ may be responsible for some pathological process, such as scarring and fibrosis, renal diseases and immunosuppressant[37-39]. Furthermore, TGFβ is able to induce many kinds of cells, including hepatic and hepatoma cells to undergo apoptosis[40,41]. The molecular mechanisms underlying TGFβ induction of the apoptosis of hepatocytes is still unclear. In this study, TGFβ was extracted from the fresh bovine platelets by acid/ethanol procedure and purified by ion exchange and gel chromatography, then its biological activity was detected in vivo and its induction of apoptosis of cultured hepatocytes was observed in vitro, and to provide the basis for the study of the relationship between TGFβ and the signal transduction of hepatocellular-apoptosis.
The fresh anticoagulant bovine blood was collected in bags containing 0.1 volume of 8.78 g·L-1 NaCl and 22.50 g·L-1 EDTA (pH7.4). The blood was centrifuged at 2000 r·min-1 for 15 min and the supernatant was recentrifuged at 4000 r·min-1 for 15 min. Then the supernatant was discarded and the precipitated platelets was washed twice with PBS (pH7.4) by centrifugation at 5000 r·min-1 for 30 min[42,43]. All the centrifugations were carried out at 0 °C.
TGFβ was extracted by a modified acid/ethanol procedure of Roberts and others[44,45]. The platelets were suspended in acid/ethanol extraction solution containing 375 mL of 950 mL·L-1 ethanol and 7.5 mL of concentrated HCl, plus 33 mg phenylmethylsulfonyl fluoride (PMSF) and 1.9 mg pepstatin A as protease inhibitors. The mixture was sonicated in ice-bath, extracted overnight at 4 °C, and centrifuged at 15000 r·min-1 for 40 min at 0 °C. The supernatant was adjusted to pH3.0 with concentrated ammonium hydroxide. Then 2 volumes of cold anhydrous ethanol (-20 °C) and 4 volumes of cold anhydrous ether (-20 °C) were immediately added. After the mixture stood at -20 °C for 48 hrs, the resulting precipitate was collected by centrifugation at 20000 r·min-1 for 30 min at 0 °C and redissolved in 1 mol·L-1 acetic acid. After extensive dialysis at 4 °C against 0.17 mol·L-1 acetic acid in a dialyzing tube (molecular mass cutoff, 10000 ku), the sample was then subjected to the next purification.
The above crude sample was centrifuged at 20000 r·min-1 for 40 min at 0 °C to remove the small precipitate and the supernatant was then applied to a CM-Sepharose column (1.6 cm × 11 cm, Phamacia Biotech, Uppsala, Sweden) equilibrated with 0.17 mol·L-1 acetic acid. The column was
eluted successively with 0.17 mol·L-1 acetic acid, 41.02 g·L-1 NaOAc, 82.04 g·L-1 NaOAc and 4 g·L-1 NaOH at a flow rate of 90 mL·h-1 at room temperature. The fraction that eluted by 4 g·L-1 NaOH was immediately neutralized by adding 100 mL·L-1 acetic acid. The eluted fractions were collected respectively and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE).
The above fractions containing 25 ku component were collected, concentrated with glycol polyethylene and extensive dialyzed at 4 °C against tridistilled water and then 1 mol·L-1 acetic acid. After being centrifuged at 20000 r·min-1 for 10 min, the supernatant was applied to a Superdex 75 column (1.6 cm × 70 cm, Phamacia Biotech, Uppsala, Sweden) equilibrated with 1 mol·L-1 acetic acid. The elution was carried out at a flow rate of 36 mL·h-1 with 1 mol·L-1 acetic acid containing 11.69 g·L-1 NaCl at room temperature. The eluted fractions were collected and analyzed by SDS-PAGE. The fraction containing the 25 ku component was concentrated using glycol polyethylene. After extensive dialysis at 4 °C against tridistilled water and 0.17 mol·L-1 acetic acid, the samples were stored at -20 °C for the following assays. The protein content was determined by Coomassie brilliant blue G-250 method and bovine serum albumin (BSA) was used as control.
Nine male Balb/c mice (10 d old) were injected subcutaneously each day in the back with the purified TGFβ (0.5 g·L-1). After 72 hrs, the tissues at the injection sites were removed and fixed in 100 mL·L-1 formalin and paraffin sections were then subjected to the routine HE staining. The other 3 mice were injected with equivalent BSA as controls.
Human normal hepatic cell line QZG (purchased from the Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China) were cultured routinely on cover slides. After having grown to logarithmic phase, 8 μg·L-1 of TGFβ was added and then for a further 12 hrs culture. BSA (8 μg·L-1) was added as control. The cells were subjected to HE staining. In addition, the cells were stained by terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labeling (TUNEL) method to detect apoptosis[47]. A random field of cells was chosen with a magnification of × 400, and adjacent non overlapping fields were counted until the minimum 500 limits for each slide was obtained. TUNEL index was expressed as the number of positive cells/the total number of cells.
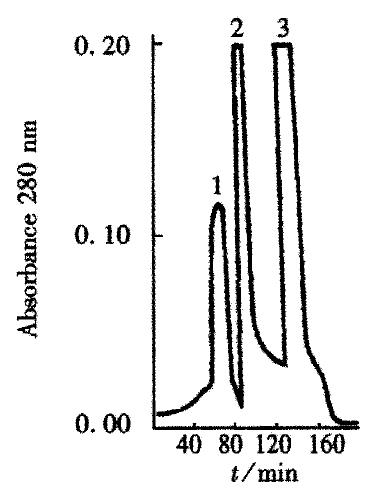
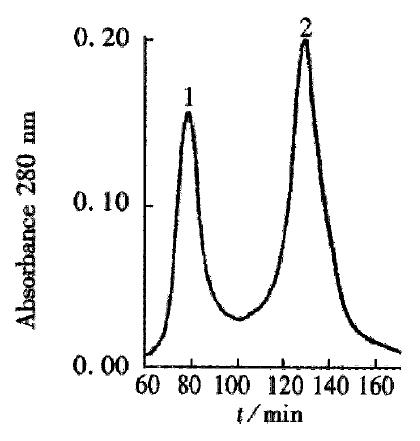
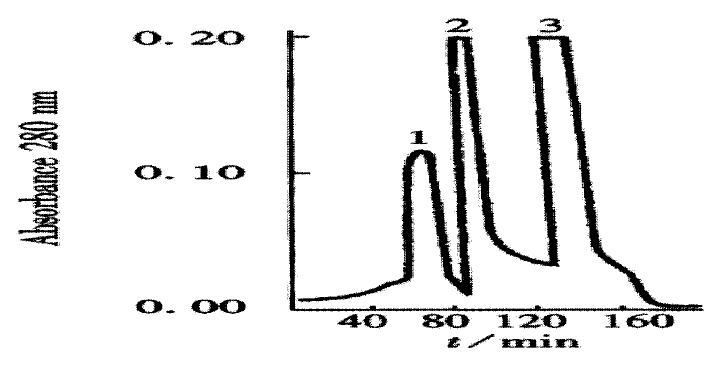
The crude TGFβ was purified by ion exchange chromatography on a CM-Sepharose column, as shown in Figure 1. SDS-PAGE analysis showed that peaks 2 and 3 contained the 25 ku component. The peaks 2 and 3 fractions were collected and then purified by gel chromatography on a Superdex-75 column (Figure 2). SDS PAGE analysis showed that peak 2 predominantly contained 25ku component (Figure 3). The protein content was 1.1 g·L-1.
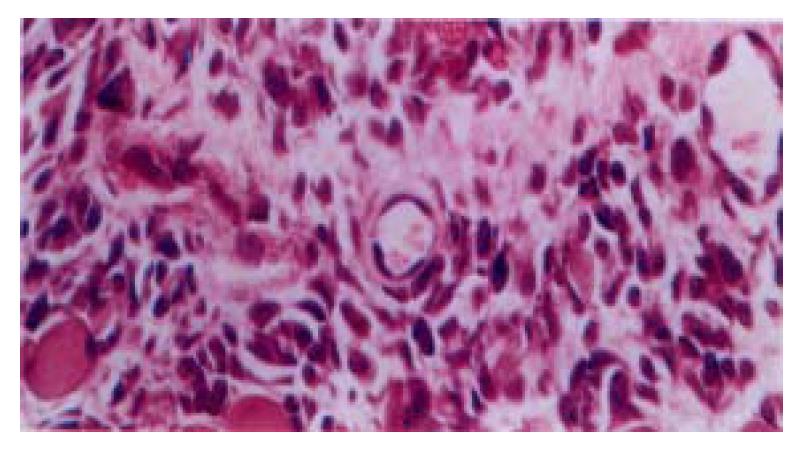
Seventy-two hrs after subcutaneous injection of TGFβ, there was evident proliferation of fibroblasts and a few infiltration of inflammatory cells at the injection sites. The newly formed blood capillaries were also seen at the injection sites (Figure 4). The granulation tissue was confined at the injection sites. No obvious tissue change was observed in the control group.
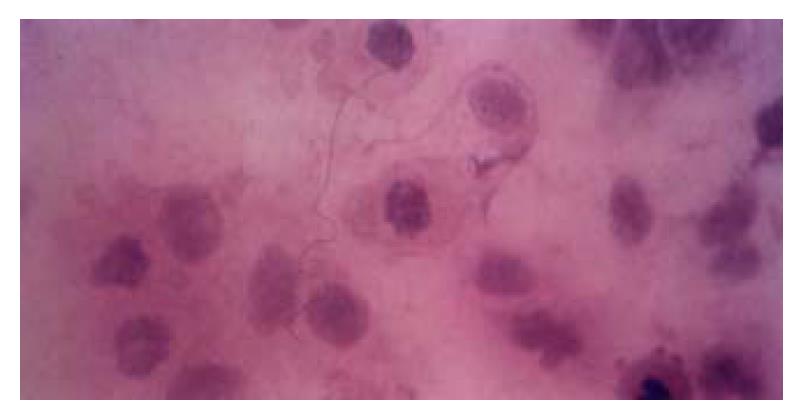
HE staining revealed that apoptotic hepatocytes exhibited the pyknosis and dense-stained nuclei and cytoplasm. Moreover, the nuclei demonstrated fragment, annular or crescent body, and the cytoplasm showed “bubbling” (Figure 5). TUNEL positive signals were olivine or yellow florescence in the nuclei. The TUNEL index of the TGFβ treated group was 0.31 and distinctly higher than that of control group (0.09), (P < 0.05)
TGFβ is a disulfide-linked homodimeric 25 ku protein that consists of two identical 112 amino acid subunits, and only the dimer is biologically active. It is acid and heat stable and the presence of 18 half cystine residues in each dimeric molecular contributes to this stability[48-50]. There are at least five described subtypes of TGFβ, encoded by distinct but closely related genes. TGFβ 1, 2 and 3 have been found in many species, including humans; TGFβ 4 has been found in chickens and TGFβ 5 in amphibians[51] . Bone is the richest source of TGFβ in the body, as it contains more than 200 μg per kg of wet weight, whereas blood platelets represent the most concentrated source of TGFβ (up to 20 mg per kg of wet weight) and is released from β granules of platelets when blood clots[52,53]. Therefore, TGFβ is generally extracted from the fresh platelets. It is suggested that TGFβ is highly stable under acidic condition and can be activated by heating in boiling water for 5 min, or treatment with 1N acetic acid or 6 M urea. The acid/ ethanol procedure is a practical way and previously used to extract biologically active polypeptides such as insulin, insulin like growth factor and platelet-derived growth factor[54,55]. Some other studies verify the effectiveness of this extraction procedure for isolation of TGFβ from many tissues, including platelets, placenta and kidney[53,56,57]. Isolation of TGFβ from platelets includes five steps: collection and washing of platelets, acid/ethanol extraction, ethanol/ether precipitation, ion exchange chromatography and gel chromatography. In the present study, crude TGFβ was isolated from the fresh bovine platelets and then purified by ion exchange and gel chromatography. The SDS-PAGE analysis showed that the molecular weight of its main component was 25 ku,which corresponded to that of standard TGFβ protein.
Wound healing and tissue repair involve a complex series of biological events which include inflammation, cellular migration, fibroblasts proliferation, production of collagen and tissue remodeling. Growth factors have been reported to enhance the repair process in animal models by increasing the degree of cellularity, the rate of angiogenesis, and the amount of collagen accumulated[58,59]. TGFβ has been studied in association with wound healing and the ability of TGFβ to initiate a cascade of events leading to enhanced wound healing has been clearly demonstrated by many reports. Pierce and co-workers[60-65] have reported that TGFβ significantly
accelerate soft tissue repair by attracting fibroblasts into the wound and stimulating rapid synthesis, deposition and maturation of collagen in vitro and in vivo. Other major activities of TGFβ are its abilities to promote the synthesis and deposition of various extracellular matrix (ECM) proteins and increase the expression of integrins and fibronectin, receptors that mediate cellular interactions with ECM proteins[66,67]. Different assay systems have been developed to measure the TGFβ activities, including cell proliferation and inhibition assays, radio receptor assays, immunoassays and matrix formation or cell surface antigens expression assays[68,69]. In the present study, the analysis of activity in vivo demonstrated that there were fibroblast proliferation and blood capillaries formation after subcutaneous injection of the extracted TGFβ. Moreover, the granulation tissue was only located in the injection sites and had no tendency to diffuse. That the action of the extracted TGFβin vivo led to granulation tissue formation suggested the successful extraction of TGFβ and its participation in repair of tissue injury.
TGFβ has been shown to either stimulate or inhibit proliferation in different cell types, and within same cell types, depending upon the stage of cell differentiation, in vitro condition and the presence of other growth factors. TGFβ has a stimulatory effect on the proliferation of cells of mesenchymal origin, such as fibroblasts, osteoblasts and Schwann cells, yet is a growth inhibitor for cells of epithelial or neuroectodermal origin, including epithelial cells, osteoclasts, keratinocytes, T and B lymphocytes, endothelial cells and hepatocytes[70-72].
Apoptosis is a genetically and highly conserved process. Regulation of the balance between cell proliferation and apoptosis is essential for development and maintenance of multicellular organisms[73-77]. Previous studies suggest that TGFβ is able to induce evidently apoptosis of hepatocytes and hepatoma cells in vitro. The animal experiments in vivo also manifest that the hepatocytes undergoing apoptosis have obviously elevated level of TGFβ expression[78,79]. Furthermore, hepatoma cells which have a high apoptotic incidence rate, simultaneously demonstrate a high level of TGFβ expression[80]. These studies suggest an involvement of TGFβ in the initiation of apoptosis of hepatocytes. In our HE staining, normal hepatic cell line QZG showed remarkable morphological changes of apoptosis, including the pyknotic and hyperchromic cytoplasm and nuclei, and the fragmentary, crescent form or annular nuclei, and the “bubbling” cytoplasm, after being treated with exogenous TGFβ. TUNEL staining also showed that the incidence rate of apoptosis was distincly higher in the TGFβ treated group than that of the control group. The present study further supported the apoptotic induction of hepatocytes by TGFβ, and verified the good biological activity of the extracted TGFβ as well. Better understanding of the relationship between TGFβ and the signal transduction of hepatocellular apoptosis requires further investigations.
Edited by Ma JY
| 1. | Tang ZY. Clinical research of hepatocellular carcinoma in the 21st century. China Natl J New Gastroenterol. 1995;1:2-3. |
| 2. | Worman HJ, Feng L, Mamiya N. Molecular biology and the diagnosis and treatment of liver diseases. World J Gastroenterol. 1998;4:185-191. [PubMed] |
| 3. | Pan RM, Shao XD. Clinical characteristics of alcoholic liver disease. World J Gastroenterol. 1998;4:95-96. |
| 4. | Zhang LF, Peng WW, Yao JL, Tang YH. Immunohistochemical detection of HCV infection in patients with hepatocellular carcinoma and other liver diseases. World J Gastroenterol. 1998;4:64-65. [PubMed] |
| 5. | Wu GY, Wu CH. Gene therapy and liver diseases. World J Gastroenterol. 1998;4:18-19. |
| 6. | Wang YJ, Sun ZQ, Quan QZ, Yu JJ. Fat-storing cells and liver fibrosis. China Natl J New Gastroenterol. 1996;2:58-60. |
| 7. | Luo YQ, Teng JB, Pan BR, Zhang XY. Liver disease and Helicobacter. World J Gastroenterol. 1999;5:338-334. [PubMed] |
| 8. | Yang DH, Xiu C, Yang B, Gu JR, Qian LF, Qu SM. Expression of insulin-like growth factor II and its receptor in liver cells of chronic liver diseases. China Natl J New Gastroenterol. 1997;3:117-118. |
| 9. | Wang X, Chen YX, Xu CF, Zhao GN, Huang YX, Wang QL. Relationship between tumor necrosis factor-alphaand liver fibrosis. World J Gastroenterol. 1998;4:18. [PubMed] |
| 10. | An SZ, Yao XX, Cui DL. Experimental and clinical study on interventional therapy with sclerotic complex agents for he-patic cysts. World J Gastroenterol. 1998;4:110. |
| 11. | Zhuang XQ, Yuan SZ, Wang XH, Lai RQ, Luo ZQ. Oncoprotein expression and inhibition of apoptosis during colorectal tumorigenesis. China Natl J New Gastroenterol. 1996;2:3-5. |
| 12. | Wang LD, Zhou Q, Wei JP, Yang WC, Zhao X, Wang LX, Zou JX, Gao SS, Li YX, Yang C. Apoptosis and its relationship with cell proliferation, p53, Waf1p21, bcl-2 and c-myc in esophageal carcinogenesis studied with a high-risk population in northern China. World J Gastroenterol. 1998;4:287-293. [PubMed] |
| 13. | Deng LY, Zhang YH, Xu P, Yang SM, Yuan XB. Expression of IL 1betaconverting enzyme in 5-FU induced apoptosis in esophageal carcinoma cells. World J Gastroenterol. 1999;5:50-52. [PubMed] |
| 14. | Kong XP, Zou QY, Li RB, Zheng PL, Yang LP, Jin SW. Apoptosis of neoplasm cell lines induced by hepatic peptides extracted from sucking porcine hepatocytes. World J Gastroenterol. 1999;5:435-439. [PubMed] |
| 15. | Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210-215. [PubMed] |
| 16. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 17. | Wu P, Li X, Zhou T, Zhang MJ, Chen JL, Wang WM, Chen N, Dong DC. Role of P-selectin and anti-P-selectin monoclonal antibody in apoptosis during hepatic/renal ischemia reperfusion injury. World J Gastroenterol. 2000;6:244-247. [PubMed] |
| 18. | Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263-265. [PubMed] |
| 19. | Huang PL, Zhu SN, Lu SL, Dai ZS, Jin YL. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol. 2000;6:295-297. [PubMed] |
| 20. | Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435-437. [PubMed] |
| 21. | Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;119:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 583] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000;267:6954-6967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 403] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845-G850. [PubMed] |
| 24. | Massagué J. Receptors for the TGF-beta family. Cell. 1992;69:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 491] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987;49:437-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 524] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917-2931. [PubMed] |
| 27. | Dünker N, Krieglstein K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000;267:6982-6988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Huang YX, Zhang GX, Lu MS, Fan GR, Chen NL, Wu GH. Increased expression of transforming growth factor-β 1 in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:150-152. |
| 29. | Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol. 2000;115:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Tyrone JW, Marcus JR, Bonomo SR, Mogford JE, Xia Y, Mustoe TA. Transforming growth factor beta3 promotes fascial wound healing in a new animal model. Arch Surg. 2000;135:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Iwasawa M. Accelerated maturation in prefabricated flaps by transforming growth factor-beta: an experimental study in the rabbit. Ann Plast Surg. 1993;31:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Frenkel SR, Saadeh PB, Mehrara BJ, Chin GS, Steinbrech DS, Brent B, Gittes GK, Longaker MT. Transforming growth factor beta superfamily members: role in cartilage modeling. Plast Reconstr Surg. 2000;105:980-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Tatakis DN, Wikesjo UM, Razi SS, Sigurdsson TJ, Lee MB, Nguyen T, Ongpipattanakul B, Hardwick R. Periodontal re-pair in dogs: effect of transforming growth factor-β 1 on alveo-lar bone and cementum regeneration. J Clin Periodontol. 2000;27:698-704. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Si X, Jin Y, Yang L, Tipoe GL, White FH. Expression of BMP-2 and TGF-beta 1 mRNA during healing of the rabbit mandible. Eur J Oral Sci. 1997;105:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Si X, Jin Y, Yang L. Induction of new bone by ceramic bovine bone with recombinant human bone morphogenetic protein 2 and transforming growth factor beta. Int J Oral Maxillofac Surg. 1998;27:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Sasaki H, Pollard RB, Schmitt D, Suzuki F. Transforming growth factor-beta in the regulation of the immune response. Clin Immunol Immunopathol. 1992;65:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 833] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 38. | Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, Cohen IK, Diegelmann RF. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988;23:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Liu F, Liu JX. Transforming growth factor β 1 in hepatic fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:86-88. |
| 40. | Brown TL, Patil S, Howe PH. Analysis of TGF-beta-inducible apoptosis. Methods Mol Biol. 2000;142:149-167. [PubMed] |
| 41. | Bursch W, Oberhammer F, Jirtle RL, Askari M, Sedivy R, Grasl-Kraupp B, Purchio AF, Schulte-Hermann R. Transforming growth factor-beta 1 as a signal for induction of cell death by apoptosis. Br J Cancer. 1993;67:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976;34:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Nakamura T, Kitazawa T, Ichihara A. Partial purification and characterization of masking protein for beta-type transforming growth factor from rat platelets. Biochem Biophys Res Commun. 1986;141:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA. 1980;77:3494-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 264] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | van den Eijnden-van Raaij AJ, Koornneef I, van Zoelen EJ. A new method for high yield purification of type beta transforming growth factor from human platelets. Biochem Biophys Res Commun. 1988;157:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH. Transforming growth factor β : rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167-4171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1935] [Cited by in RCA: 2022] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 47. | Liang YR, Zheng SY, Shen YQ, Wu XY, Huang ZZ. Relationship between expression of apoptosis-related antigens in hepatocel-lular carcinoma and in situ end labeling. World J Gastroenterol. 1998;4:99. |
| 48. | Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 834] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 49. | Fausto N. Multifunctional roles for transforming growth factor-beta 1. Lab Invest. 1991;65:497-499. [PubMed] |
| 50. | Böttner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Centrella M, McCarthy TL, Canalis E. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am. 1991;73:1418-1428. [PubMed] |
| 52. | Seyedin SM, Thomas TC, Thompson AY, Rosen DM, Piez KA. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci USA. 1985;82:2267-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 328] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155-7160. [PubMed] |
| 54. | Antoniades HN, Scher CD, Stiles CD. Purification of human platelet-derived growth factor. Proc Natl Acad Sci USA. 1979;76:1809-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 366] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | DAVOREN PR. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962;63:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Proper JA, Bjornson CL, Moses HL. Mouse embryos contain polypeptide growth factor(s) capable of inducing a reversible neoplastic phenotype in nontransformed cells in culture. J Cell Physiol. 1982;110:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Roberts AB, Anzano MA, Meyers CA, Wideman J, Blacher R, Pan YC, Stein S, Lehrman SR, Smith JM, Lamb LC. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983;22:5692-5698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 249] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Chen TL, Bates RL, Xu Y, Ammann AJ, Beck LS. Human recombinant transforming growth factor-beta 1 modulation of biochemical and cellular events in healing of ulcer wounds. J Invest Dermatol. 1992;98:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Narayanan AS, Page RC, Swanson J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-beta in the presence of other inflammatory mediators. Biochem J. 1989;260:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Cromack DT, Porras-Reyes B, Purdy JA, Pierce GF, Mustoe TA. Acceleration of tissue repair by transforming growth factor beta 1: identification of in vivo mechanism of action with radiotherapy-induced specific healing deficits. Surgery. 1993;113:36-42. [PubMed] |
| 61. | Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992;140:1375-1388. [PubMed] |
| 62. | Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 690] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 63. | Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 438] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Pierce GF, Vande Berg J, Rudolph R, Tarpley J, Mustoe TA. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991;138:629-646. [PubMed] |
| 65. | Pierce GF, Brown D, Mustoe TA. Quantitative analysis of inflammatory cell influx, procollagen type I synthesis, and collagen cross-linking in incisional wounds: influence of PDGF-BB and TGF-beta 1 therapy. J Lab Clin Med. 1991;117:373-382. [PubMed] |
| 66. | Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7:89-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993;120:253-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 194] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Meager A. Assays for transforming growth factor beta. J Immunol Methods. 1991;141:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Randall LA, Wadhwa M, Thorpe R, Mire-Sluis AR. A novel, sensitive bioassay for transforming growth factor beta. J Immunol Methods. 1993;164:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Moses HL, Yang EY, Pietenpol JA. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 724] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 71. | Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79-87. [PubMed] |
| 72. | Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 947] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 73. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Zhu HZ, Ruan YB, Wu ZB, Zhang CM. Kupffer cell and apoptosis in experimental HCC. World J Gastroenterol. 2000;6:405-407. [PubMed] |
| 75. | Deng LY, Zhang YH, Zhang HX, Ma CL, Chen ZG. Observation of morphological changes and cytoplasmic movement in apoptosis process. World J Gastroenterol. 1998;4:66-67. |
| 76. | Schulte-Hermann R, Bursch W, Kraupp-Grasl B, Oberhammer F, Wagner A, Jirtle R. Cell proliferation and apoptosis in normal liver and preneoplastic foci. Environ Health Perspect. 1993;101 Suppl 5:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Yang LJ, Wang WL. Ethanol-induced apoptosis of HCC-9204 hepatoma cells. J Gastroenterol Hepatol. 2000;15:1142. |
| 78. | Kroll B, Kunz S, Tu N, Schwarz LR. Inhibition of transforming growth factor-beta1 and UV light-induced apoptosis by prostanoids in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 1998;152:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Albright CD, Zeisel SH. Choline deficiency causes increased localization of transforming growth factor-beta1 signaling proteins and apoptosis in the rat liver. Pathobiology. 1997;65:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Shima Y, Nakao K, Nakashima T, Kawakami A, Nakata K, Hamasaki K, Kato Y, Eguchi K, Ishii N. Activation of caspase-8 in transforming growth factor-beta-induced apoptosis of human hepatoma cells. Hepatology. 1999;30:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |