Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.238
Revised: October 21, 2000
Accepted: October 29, 2000
Published online: April 15, 2001
AIM: To detect immunohistochemically the presence of oval cells in chronic viral hepatitis with antibody against c-kit.
METHODS: We detected oval cells in paraffin embedded liver sections of 3 normal controls and 26 liver samples from patients with chronic viral hepatitis, using immunohistochemistry with antibodies against c-kit, π class glutathione S-transferase (π-GST) and cytokeratins 19 (CK19).
RESULTS: Oval cells were not observed in normal livers. In chronic viral hepatitis, hepatic oval cells were located predominantly in the periportal region and fibrosis septa, characterized by an ovoid nucleus, small size, and scant cytoplasm. Antibody against stem cell factor receptor, c-kit, had higher sensitivity and specificity than π-GST and CK19. About 50%-70% of c-kit positive oval cells were stained positively for either π-GST or CK19.
CONCLUSION: Oval cells are frequently detected in human livers with chronic viral hepatitis, suggesting that oval cell proliferation is asso ciated with the liver regeneration in this condition.
- Citation: Ma X, Qiu DK, Peng YS. Immunohistochemical study of hepatic oval cells in human chronic viral hepatitis. World J Gastroenterol 2001; 7(2): 238-242
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.238
Although essentially a quiescent organ, the normal adult liver can fully regenerate following surgical resection or injury. Liver regeneration is usually achieved by the entry of normally proliferatively quiescent, differentiated hepatocytes into the cell cycle, but, when hepatocyte regeneration is defective, oval cells can migrate outward from the portal tracts and then differentiate into hepatocytes[1-3]. The term oval cells described as small cells with oval nuclei that arise in the periphery of the portal tracts in rat models of hepatocarcin ogenesis and injury[4-7]. These cells are thought to have both clonogenic and bipotential capacity, i.e., the ability to proliferate and differentiate into cells of either hepatocyte or biliary epithelial cells[8]. There is also evidence that under certain conditions, oval cells can be induced to differentiate into non-hepatic lineages including intestinal and pancreatic epithelium[9]. The origin of oval cells and their precise location within the liver have remained enigmatic[10]. The aim of this study is to detect immunohistochemically the presence of oval cells in chronic viral hepatitis with antibody against c-kit.
Formalin-fixed, paraffin-embedded liver biopsy specimens from 26 patients with chronic liver diseases were obtained from the Department of Histopathology at Shanghai Institute of Digestive Diseases (Renji Hospital). Patient age ranged from 23 to 71 years, with mean age of 54 years. Twenty-six patients had been diagnosed having chronic viral hepatitis (twenty-one chronic hepatitis B; five chronic hepatitis C) with various degree fibrosis. There were mild fibrosis (n = 5), moderate fibrosis (n = 8), severe fibrosis (n = 6), and hepatic cirrhosis (n = 7). Three specimens of grossly normal liver tissues from the area surrounding benign angiomas were used as references. Specimens were fixed immediately in 10% neutral formalin and embedded in paraffin.
To highlight the presence of oval cells, three primary antibodies were used. The antibody against stem cell factor receptor, c - kit, was purchased from Oncogene Research Products. c-kit (Ab-1) is a purified rabbit polyclonal antibody raised against the peptide (GSTASSSQPLLVHDDV), a sequence found at the Carboxyterminus corresponding to residues 961-976. Antibodies against π-class glutathione S-transferase (π-GST, clone 353-10) and cytokeratins 19 (CK19, clone BA17.1) were purchased from Dako Co, Denmark.
Immunohistochemical staining was performed on serial sections at room temperature, using the alkaline phosphatase method. The sections were deparaffinized in xylene and rehydrated through graded alcohol. The sections were boiled in 6M urea at 95 °C for 10 min for c-kit staining. Endogenous peroxidases were inactivated by immersing the sections in hydrogen peroxide for 10 minutes, then were incubated for 10 min with normal swine serum in Tris-buffered saline to block non-specific binding. The sections were subsequently incubated overnight at 4 °C with relevant antibodies (1:100 dilution respectively). The following day, the sections were incubated with biotinylated anti-mouse or anti-rabbit IgG (1:50 dilation, Maxim Biotech Inc., USA) for 45 min, followed by peroxidase-conjugated streptavidin (1:50 dilation, Maxim Biotech Inc.). The chromogenic reaction was developed with diaminobenzidine for 10 minutes, and all sections were counterstained with hematoxylin. Controls consisted of omission of the primary antibody.
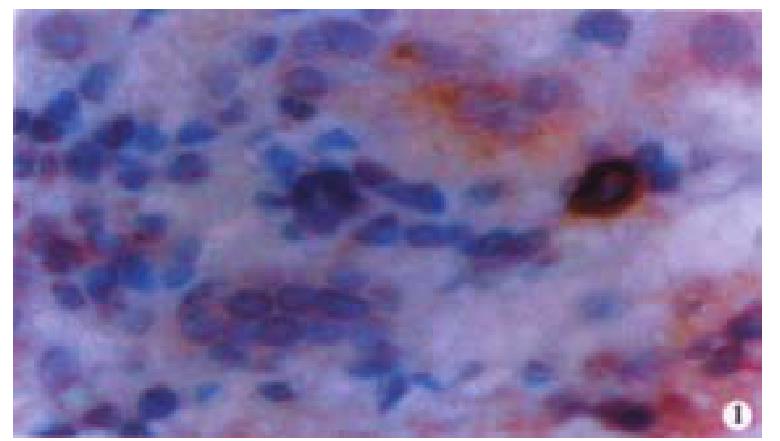
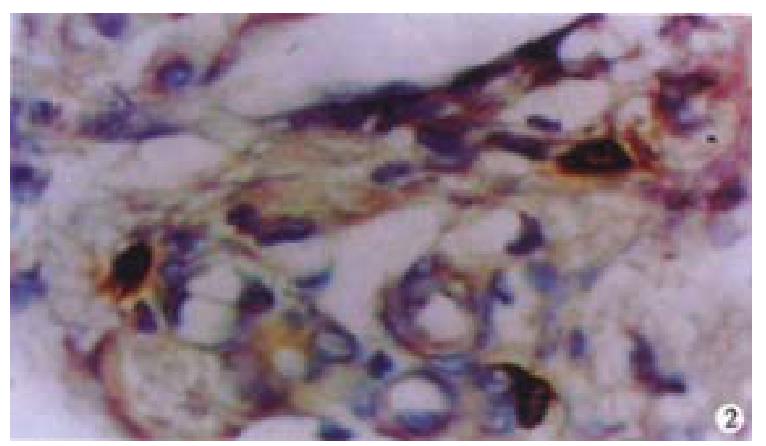
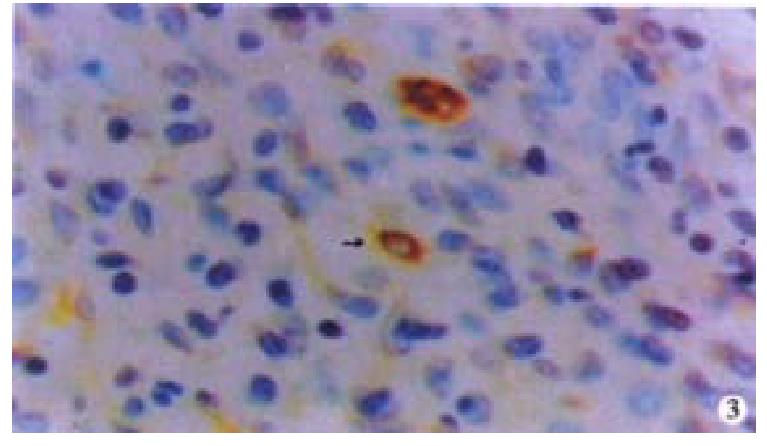
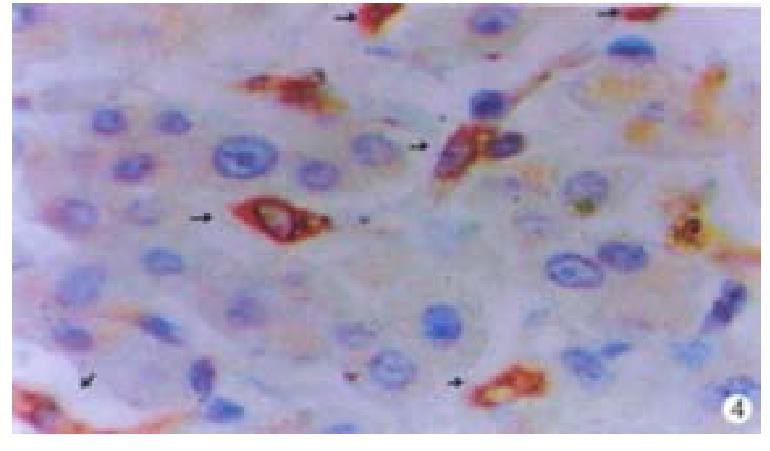
Oval cells were not detected in normal liver tissue, but were detected in most liver tissues from patients with chronic viral hepatitis. Oval cells were characterized by ovoid nuclei from 7 μm × 9 μm to 12 μm × 17 μm, small size, and scant cytoplasm (Figure 1). They were located predominantly in the periportal region (Figure 2) in hepatic cirrhosis, and were often found in close association with inflammatory cells in chronic active hepatitis (Figure 3). There were “transitional cells” in the parenchyma with size and structure between those of human oval cells and mature hepatocytes. They were moderately stained by CK19 antibody, had round nuclei, more cytoplasm, and were smaller in size than mature hepatocytes (Figure 4). c-kit antibody had higher sensitivity and specificity than π-GST and CK19. About 50%-70% of C-kit positive oval cells were stained positively for either π-GST or CK19. Some mature hepatocytes also expressed π-GST. Most mature bile ducts also expressed CK19.
Hepatic oval cells proliferate under certain conditions, mainly when hepatocytes are prevented from proliferating in response to liver damage, and may be stem cells of hepatocytes and bile duct cells or the intermediate progeny of a hepatic stem cell[2]. Oval cells in animals are activated following administration of a variety of toxins and carcinogens alone or combined with other surgical or dietery regimens[11-19]. One of the models studied most is acetylaminofluorene treatment followed by partial hepatectomy, and an array of cytokines and growth factors have been shown to be an up-regulatory mechanism, is being delineated, for example interferon γ is implicated in orchestrating the process[20]. The oval cell itself however, probably represents the activated progeny of a dormant stem cell compartment although oval cells are readily identified in injury liver, one area of great controversy is the question of where these putative stem cells reside in the normal liver[3]. One suggestion is that they are present in the canals of Hering, that is the region where cells are transitional between the periportal hepatocytes and the biliary cells lining the smallest terminal bile duct[21]. Others suggest that there are cells which are located in the portal tracts, in the periductular region, or even that periportal hepatocytes have stem cell or metaplastic properties[2].
Oval cells are of great clinical interest since they may be the progenitor cells of both hepatocellular carcinomas and cholangio-carcinomas. Furthermore, they could be useful vehicles for ex vivo gene therapy for the correction of metabolic liver diseases[2]. However, identifying stem cells or their progeny in human liver has been a challenge. Oval cells, similar in morphology and antigenic profile to those seen in rodents, may be associated with many liver diseases in humans. These include submassive necrosis, focal nodular hyperplasia, primary biliary cirrhosis and primary sclerosing cholangitis, alcoholic hepatitis and cirrhosis, hepatoblastoma and HBV-associated hepatocellular carcinoma, genetic hemochro-matosis, and hepatitis C[22-30]. We detected the presence of hepatic oval cell in patients with chronic viral hepatitis. The human oval cell has a distinct morphology, with oval-shaped nuclei, small size and scant cytoplasm, and thus can be distinguished from the mature hepatocytes and inflammatory cells[1]. Oval cells express a wide variety of antigens that can be detected immunocytochemically. Although by no means specific for oval cells, many antibodies are very highly expressed, and can be used to highlight the presence of oval cells in histological sections[2].
Stem cell factor (SCF), a cytokine with structural similarity to the colony stimulating factors, was first isolated from conditioned medium of Buffalo rat liver cells[31]. Since then, it has become clear that SCF is critically important for early epithelial stem cell differentiation in hematopoiesis[32] or gamaetogenesis[33]. The corresponding receptor for SCF is encoded by the proto-oncogene c-kit, and exhibits a tyrosine kinase activity facilitated by its intracellular domain[34]. Target cells for SCF, which express c-kit, include mast cells[35], hematopoitic progenitor cells[32], and germ cells[33]. Recent studies suggest that SCF and c-kit may be involved in early growth and development of hepatic progenitor cells. Up-regulation of SCF and c-kit has been described in AAF-treated, partially hepatectomized rats, in which the carcinogen treatment inhibits the replication of hepatocytes and the induction of oval cells may be observed[19,36]. A similar increase of SCF and c-kit, accompanied by oval cell proliferation, has also been reported in bile duct - ligated rat[37]. Baumann U et al[38] suggests that c-kit-positive cells may represent a hepatic progenitor cell population in normal and diseased pediatric liver. In our study, we observed that some c-kit-positive cells with an oval-like morphology were present in human livers with chronic viral hepatitis, and that antibody against c-kit is useful in detecting the oval cells. We detected π-GST as a fetal GST in the majority of oval cells, supporting the view that oval cells display characteristics which resemble fetalhepatocytes or liver stem cells[29,39]. CK19n positive staining suggests that oval cells have characteristics of biliary epithelium[40]. The combined use of these antibodies and the knowledge of morphology allows us to reliably identify oval cells.
As hepatitis develops, hepatocyte necrosis is followed by an attempted secondary proliferation response of mature hepatocytes, but this proliferation response is often impaired in chronic liver diseases. Thus, oval cells may have a chance to proliferate and differentiate into hepatocytes or biliary epithelium cells[41]. We observed that oval cells were located predominantly in the periportal region, and were found in close association with fibrosis septa and inflammatory infiltrates. This location suggests that cytokines or other factors associated with the development of inflammation and fibrosis may be required to stimulate oval cell proliferation, differentiation and migration[42]. The matrix in fibrotic and cirrhotic livers is the binding site for both epidermal growth factor[43,44] and hepatocyte growth factor[45], whose receptors have been shown to be involved in proliferation of oval cells[46]. Additionally, the presence of “transitional cells” may be evidence that oval cells are progenitors of hepatocytes.
While the debate on the source and location of hepatic stem cells is ongoing, two recent papers add a new dimension and offer a challenging alternative hypothesis to explain the origin of oval cells. By transplanting rat bone marrow into lethally irradiated recipients and following the fate of syngeneic cells using various markers, Petersen BE et al[47] reported striking changes in the livers of animals induced to regenerate following 2-AAF and CCl4 treatment. Male donor marrow cells were visualized in female recipients. In a second model, marrow from dipeptidyl peptidase IV positive animals was transplanted into dipeptidyl peptidase IV deficients . In both cases, evidence was presented to suggest that the donor cells migrated into the livers of recipient animals and subsequently underwent differentiation to become hepatocytes, although it was less clear whether ductular cells of biliary phenotype developed[47]. A second recently published study describes a similar approach comprising a mouse marrow transplant model which, interestingly, did not include a liver injury step[30]. This new report provides confirmatory evidence that bone marrown derived haematopoietic stem cells can indeed give rise to hepatocytes[48]. The ability to identify and exploit a human hepatic clonal stem cell could have important clinical implications, since generating large numbers of differentiated and therefore fully functional human hepatocytes has enormous potential[49,50].
In conclusion, the presence of oval cells in human livers with chronic viral hepatitis indicates that oval cell proliferation may be one of the mechanisms in liver regeneration in this condition. The origin, growth, and differentiation of this cell is worth investigating further.
Edited by Jason Carr
| 1. | Sell S. Is there a liver stem cell. Cancer Res. 1990;50:3811-3815. [PubMed] |
| 3. | Strain AJ, Crosby HA. Hepatic stem cells. Gut. 2000;46:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Rosenberg D, Ilic Z, Yin L, Sell S. Proliferation of hepatic lineage cells of normal C57BL and interleukin-6 knockout mice after cocaine-induced periportal injury. Hepatology. 2000;31:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273-1280. [PubMed] |
| 6. | Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Alison M, Sarraf C. Hepatic stem cells. J Hepatol. 1998;29:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Dabeva MD, Shafritz DA. Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993;143:1606-1620. [PubMed] |
| 9. | Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Shiojiri N, Lemire JM, Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611-2620. [PubMed] |
| 11. | Factor VM, Radaeva SA, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994;145:409-422. [PubMed] |
| 12. | Braun KM, Sandgren EP. Cellular origin of regenerating parenchyma in a mouse model of severe hepatic injury. Am J Pathol. 2000;157:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Bustos M, Sangro B, Alzuguren P, Gil AG, Ruiz J, Beraza N, Qian C, Garcia-Pardo A, Prieto J. Liver damage using suicide genes. A model for oval cell activation. Am J Pathol. 2000;157:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Hixson DC, Brown J, McBride AC, Affigne S. Differentiation status of rat ductal cells and ethionine-induced hepatic carcinomas defined with surface-reactive monoclonal antibodies. Exp Mol Pathol. 2000;68:152-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Mazurek S, Eigenbrodt E, Failing K, Steinberg P. Alterations in the glycolytic and glutaminolytic pathways after malignant transformation of rat liver oval cells. J Cell Physiol. 1999;181:136-146. [PubMed] |
| 17. | Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology. 1997;26:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Matsusaka S, Toyosaka A, Nakasho K, Tsujimura T, Sugihara A, Takanashi T, Uematsu K, Terada N, Okamoto E. The role of oval cells in rat hepatocyte transplantation. Transplantation. 2000;70:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Matsusaka S, Tsujimura T, Toyosaka A, Nakasho K, Sugihara A, Okamoto E, Uematsu K, Terada N. Role of c-kit receptor tyrosine kinase in development of oval cells in the rat 2-acetylaminofluorene/partial hepatectomy model. Hepatology. 1999;29:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Bisgaard HC, Müller S, Nagy P, Rasmussen LJ, Thorgeirsson SS. Modulation of the gene network connected to interferon-gamma in liver regeneration from oval cells. Am J Pathol. 1999;155:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Fausto N, Lemire JM, Shiojiri N. Cell lineages in hepatic development and the identification of progenitor cells in normal and injured liver. Proc Soc Exp Biol Med. 1993;204:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Roskams T, De Vos R, Desmet V. 'Undifferentiated progenitor cells' in focal nodular hyperplasia of the liver. Histopathology. 1996;28:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Ruck P, Xiao JC, Pietsch T, Von Schweinitz D, Kaiserling E. Hepatic stem-like cells in hepatoblastoma: expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6. Histopathology. 1997;31:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER, Thorgeirsson SS. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Crosby HA, Hubscher SG, Joplin RE, Kelly DA, Strain AJ. Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology. 1998;28:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | De Vos R, Desmet V. Ultrastructural characteristics of novel epithelial cell types identified in human pathologic liver specimens with chronic ductular reaction. Am J Pathol. 1992;140:1441-1450. [PubMed] |
| 27. | Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Xiao JC, Ruck P, Kaiserling E. Small epithelial cells in extrahepatic biliary atresia: electron microscopic and immunoelectron microscopic findings suggest a close relationship to liver progenitor cells. Histopathology. 1999;35:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 336] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Libbrecht L, Desmet V, Van Damme B, Roskams T. Deep intralobular extension of human hepatic 'progenitor cells' correlates with parenchymal inflammation in chronic viral hepatitis: can 'progenitor cells' migrate. J Pathol. 2000;192:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Zsebo KM, Wypych J, McNiece IK, Lu HS, Smith KA, Karkare SB, Sachdev RK, Yuschenkoff VN, Birkett NC, Williams LR. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990;63:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 511] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 32. | Hassan HT, Zander A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol. 1996;95:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Sandlow JI, Feng HL, Cohen MB, Sandra A. Expression of c-KIT and its ligand, stem cell factor, in normal and subfertile human testicular tissue. J Androl. 1996;17:403-408. [PubMed] |
| 34. | Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341-3351. [PubMed] |
| 35. | Baghestanian M, Hofbauer R, Kiener HP, Bankl HC, Wimazal F, Willheim M, Scheiner O, Füreder W, Müller MR, Bevec D. The c-kit ligand stem cell factor and anti-IgE promote expression of monocyte chemoattractant protein-1 in human lung mast cells. Blood. 1997;90:4438-4449. [PubMed] |
| 36. | Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511-516. [PubMed] |
| 37. | Omori M, Evarts RP, Omori N, Hu Z, Marsden ER, Thorgeirsson SS. Expression of alpha-fetoprotein and stem cell factor/c-kit system in bile duct ligated young rats. Hepatology. 1997;25:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell. Hepatology. 1999;30:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Tee LB, Smith PG, Yeoh GC. Expression of alpha, mu and pi class glutathione S-transferases in oval and ductal cells in liver of rats placed on a choline-deficient, ethionine-supplemented diet. Carcinogenesis. 1992;13:1879-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Mitaka T, Norioka K, Nakamura T, Mochizuki Y. Effects of mitogens and co-mitogens on the formation of small-cell colonies in primary cultures of rat hepatocytes. J Cell Physiol. 1993;157:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology. 1997;25:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | St Hilaire RJ, Hradek GT, Jones AL. Hepatic sequestration and biliary secretion of epidermal growth factor: evidence for a high-capacity uptake system. Proc Natl Acad Sci USA. 1983;80:3797-3801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Burwen SJ, Barker ME, Goldman IS, Hradek GT, Raper SE, Jones AL. Transport of epidermal growth factor by rat liver: evidence for a nonlysosomal pathway. J Cell Biol. 1984;99:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144:129-140. [PubMed] |
| 46. | Evarts RP, Hu Z, Fujio K, Marsden ER, Thorgeirsson SS. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993;4:555-561. [PubMed] |
| 47. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 48. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 49. | Thorgeirsson SS. Hepatic stem cells. Am J Pathol. 1993;142:1331-1333. [PubMed] |
| 50. | Lázaro CA, Rhim JA, Yamada Y, Fausto N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998;58:5514-5522. [PubMed] |