Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.157
Revised: March 19, 2001
Accepted: March 21, 2001
Published online: April 15, 2001
- Citation: Stanca C, Jung D, Meier PJ, Kullak-Ublick GA. Hepatocellular transport proteins and their role in liver disease. World J Gastroenterol 2001; 7(2): 157-169
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.157
Na+-dependent bile salt uptake Uptake of bile salts into the liver was first characterized in experimental models such as the isolated perfused rat liver[1], isolated hepatocyte cultures and basolateral plasma membrane vesicles[2-4]. These studies indicated that more than 80% of taurocholate uptake but less than 50% of cholate uptake into hepatocytes is sodium-dependent[5-11]. Whereas unconjugated bile salts are uncharged molecules that can traverse membranes by passive nonionic diffusion, conjugation with glycine or taurine decreases their pKa values and necessitates the presence of a specific carrier protein for hepatocellular uptake[12].
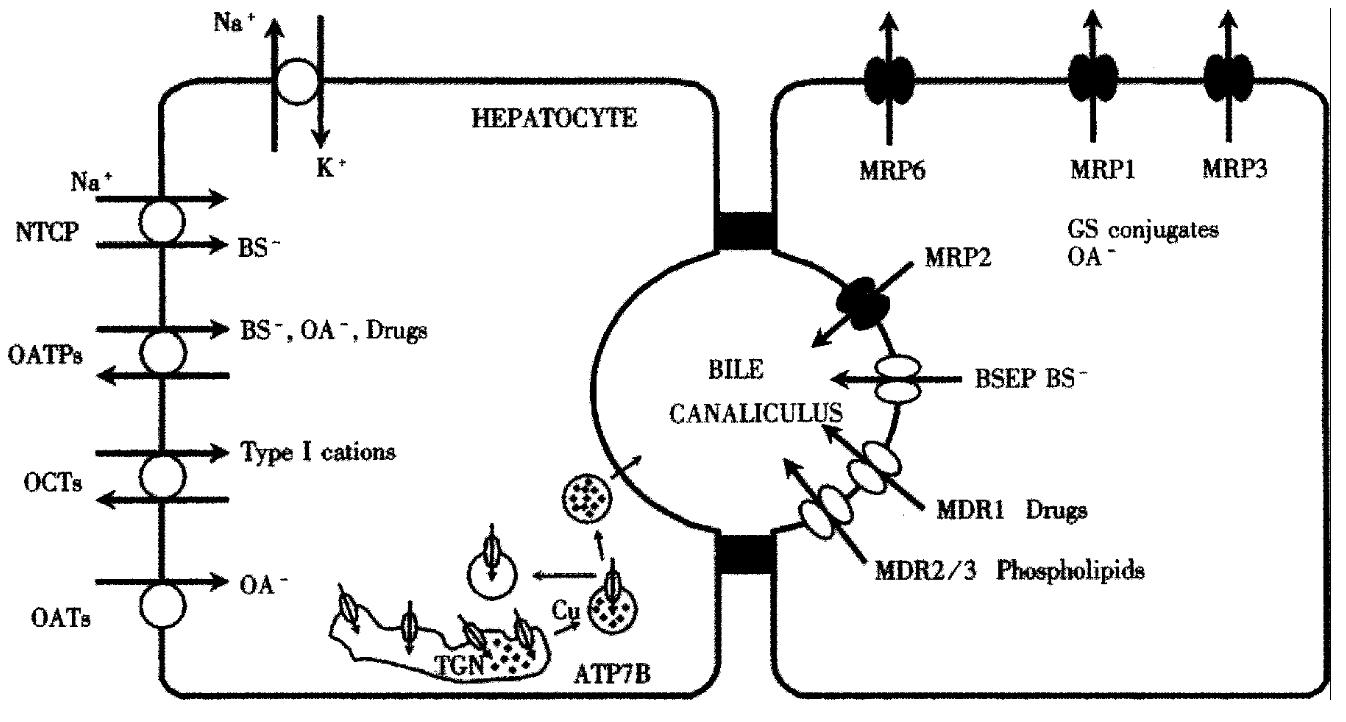
The chief uptake system for conjugated bile salts in mammalian liver was isolated by expression and molecular cloning strategies and has been called the Na+-taurocholate cotransporting polypeptide (gene symbol: SLC10A1)[13-16]. Rat Ntcp consists of 362 amino acids with an apparent molecular mass of 51 kD[17,18] and is expressed exclusively at the basolateral membrane of hepatocytes (Figure 1)[17]. Ntcp mediates sodium-dependent uptake of taurocholate and other bile salts when expressed in stably transfected COS-7, Chinese hamster ovary (CHO) and hepatoblastoma (HepG2) cells or in cRNA injected Xenopus laevis oocytes, with apparent Km values between 17-42 μmol/L[10,13,17,19,20]. The only non-bile salt substrates that are transported by Ntcp are selected sulfated steroid conjugates such as estrone-3-sulfate[21] and dehydroepiandrosterone sulfate (DHEAS) [20]. In human liver, NTCP represents a 349-amino acid protein[14]. NTCP is structurally related to the intestinal bile salt transporter (IBAT), that also mediates the Na+-dependent uptake of bile salts[22]and that is expressed not only in ileum, but also in the kidney[23] and in cholangiocytes[24].
Na+-dependent taurocholate uptake is reduced in experimental models of cholestasis such as bile duct ligation[25], endotoxinemia[26,27] and partial hepatectomy[28], is reduced in primary hepatocyte cultures[29] and is absent in various hepatoma cell lines [30,31]. These changes in hepatic Na+ dependent bile salt uptake correlate with expression levels of Ntcp. Thus, Ntcp mRNA and protein levels are decreased in bile duct ligation[25,32], endotoxinemia[26,33] and ethinyl estradiol induced cholestasis[34]. In patients with a diagnosis of extrahepatic biliary atresia and clinical evidence of cholestasis, NTCP mRNA levels are also decreased[35].
Na+-independent hepatic uptake of amphipathic substrates: the organic anion transporting polypeptide family (OATP) Whereas uptake of conjugated bile salts into the liver is largely a Na+-dependent process mediated by Ntcp, numerous other endogenous and xenobiotic compounds including non-bile salt organic anions and drugs are cleared from sinusoidal blood by carrier-mediated uptake into hepatocytes. Following hepatocellular uptake, many of these compounds are biotransformed in two phases. Phase I is mediated by cytochrome P450 enzymes and prepares the drug for conjugation by creating polar groups. Phase II conjugates drugs with a glucuronate, sulfate, glycine or methyl group and represents a detoxification step. The conjugates can then be excreted into bile or urine.
Na+-independent hepatocellular uptake of bile salts and non-bile salt amphipathic compounds cannot be attributed to the function of a single transport protein, but is mediated by a family of transport proteins called the “organic anion transporting polypeptides” (Oatps) (Figure 1). In rat hepatocytes, at least three members of the Oatp family have been identified, called Oatp1 (Slc21a1)[36], Oatp2 (Slc21a5)[37] and Oatp4 (Slc21a10)[38]. Oatp1 is a 670 amino acid protein with an apparent molecular mass of 80 kDa that is localized at the basolateral membrane of hepatocytes[39-41] and at the apical membranes of kidney proximal tubular cells[39] and choroid plexus epithelial cells[42,43]. Many of the functional characteristics of Oatp1 indicate that it could represent the “multispecific bile acid transporter” identified in previous experimental models[3,44]. Thus, studies in numerous heterologous expression systems have shown that Oatp1 mediates the hepatocellular uptake of bile salts, bromosulphophthalein (BSP), conjugated steroids, thyroid hormones, leukotriene C4, bilirubin monoglucuronide, ouabain, ochratoxin A, the anionic magnetic resonance imnaging agent gadoxetate (Gd-EOB-DTPA), the angiotensin-converting enzyme inhibitors enalapril and temocaprilat, the HMG-CoA reductase inhibitor pravastatin, and even oligopeptides including the thrombin inhibitor CRC-220, the endothelin antagonist BQ - 123 and the opioid receptor agonists [D-penicillamine-2,5] enkephalin (DPDPE) and deltorphin II (for detailed review of the substrate specificities of Oatps/OATPs see reference[45]). The driving force for Oatp mediated substrate transport is not fully understood, although it has been shown that Oatp1 can mediate bidirectional transport of BSP[46] and anion exchange of taurocholate/HCO3-[47]. An important driving force for organic anion uptake via Oatp1 appears to be countertransport of reduced glutathione[48].
Oatp2 is a 661 amino acid protein with an apparent molecular mass of 92 kD at the basolateral plasma membrane of hepatocytes[41]. Oatp2 has also been detected in the retina[49], in endothelial cells of the blood brain barrier[43] and at the basolateral plasma membrane of choroid plexus epithelial cells[43]. Oatp2 is a close homologue of Oatp1 and transports bile salts, the cardiac glycosides ouabain and digoxin, and cyclic peptides[37,41]. An important difference between Oatp1 and Oatp2 is their acinar localization in the liver . Whereas Oatp1 is distributed homogeneously within the liver acinus [41,50], Oatp2 exhibits a heterogeneous lobular distribution with predominant expression in perivenous hepatocytes excluding the innermost 1-2 cell layers surrounding the central vein[41,51]. Interestingly, treatment of rats with phenobarbital, a known inducer of microsomal drug metabolizing P450 enzymes[52] and of hepatocellular ouabain, digoxin and thyroxine uptake[53-57], resulted in a significant increase in Oatp2 expression and in the appearance of positive immunofluorescence signals even in the innermost layer of perivenous hepatocytes[58].
Oatp4 (Slc21a10) can also mediate Na+-independent uptake of bile salts in rat hepatocytes and represents a full-length isoform of the so-called “liver-specific transporter 1” or rlst-1[38,59]. Oatp4 transports numerous organic anions including taurocholate, BSP, conjugated steroids, prostaglandin E-2, leukotriene C4, the thyroid hormones T3 and T4, and gadoxetate[38]. Oatp4 is 43% and 44% identical on the amino acid level with Oatp1 and Oatp2, respectively.
In human liver, at least four OATPs have been identified to date, called OATP-A (SLC21A3), OATP-B (SLC21A9), OATP-C (SLC21A6) and OATP8 (SLC21A8). OATP-C (also called OATP2 and LST-1)[60-62] and OATP8[63] are exclusively expressed at the basolateral membrane of hepatocytes and exhibit 80% mutual identity. The closest homologue expressed in rat liver is Oatp4, which is 64% and 66% identical with OATP-C and OATP8, respectively. Accordingly, the substrate specificities of human OATP-C and OATP8 and rat Oatp4 are very comparable [38,64]. Transport substrates of OATP-C include taurocholate (Km-14-34 µM)[60,61], bilirubin monoglucuronide, DHEAS, estradiol-17β-glucuronide (Km-8 µM)[62], estrone-3-sulfate, prostaglandin E2, thromboxane B 2, leukotriene C4, leukotriene E4, T3 (Km-3 µM), T4 (Km-3 µM)[60], pravastatin (Km-35 µM)[61] and BSP (Km-0.3 µM)[64]. OATP8 exhibits a closely overlapping substrate specificity compared with OATP-C but additionally transports the cardiac glycoside digoxin (similar to rat Oatp2) and is particularly efficient in transporting the oligopeptides BQ-123 (endothelin receptor antagonist), DPDPE (opioid receptor agonist) and cholecystokinin (Km-11 µM)[64,65].
OATP-B (SLC21A9) is also strongly expressed in human liver, with additional expression in spleen, placenta, lung, kidney, heart, ovary, small intestine and brain[64]. OATP-B is a 709 amino acid protein with an apparent molecular mass of 85 kDa that is localized at the basolateral plasma membrane of hepatocytes[64]. Compared to OATP-C and OATP8, OATP-B exhibits a limited substrate specificity for the organic anions BSP (Km-0.7 µM), estrone-3-sulfate (Km-6 µM) and DHEAS.
The fourth OATP known to be expressed in hepatocytes, albeit at relatively low levels, is OATP-A (SLC21A3)[66].Although OATP-A was originally isolated from human liver, it is predominantly expressed in human cerebral endothelial cells[67]. OATP-A is a 670 amino acid protein that transports bile salts, BSP (Km-20 µM), estrone-3-sulfate (Km-59 µM)[68], DHEAS (Km-6.6 µM)[69], the magnetic resonance imaging agent Gd-B 20790[70], the opioid receptor agonists DPDPE (Km-202 µM) and deltorphin II (Km-330 µM)[67], the antihistamine fexofenadine[71], and the amphipathic organic cations APD-ajmalinium, rocuronium, N-methyl-quinine (Km-5 µM) and N-methyl-quinidine (Km-26 µM)[72]. Thus, in contrast to the preference of OATP-B, OATP-C and OATP8 for organic anions, OATP-A additionally transports amphipathic organic cations indicating that it can mediate substrate uptake into hepatocytes charge independently. Overall, the Oatp/OATP family of transporters plays a central role in hepatocellular organic anion and drug clearance.
Na+-independent hepatic uptake of hydrophilic organic anions and organic cations: the organic ion transporter family (OAT/OCT) In addition to NTCP and OATPs, the basolateral hepatocyte membrane possesses a third family of transport proteins mediating substrate uptake, called the organic anion transporter (OAT) family[73]. This family comprises the OAT, the organic cation transporter (OCT)[74,75] and the organic cation transporter novel type (OCTN)/carnitine transporter families[73]. Whereas Oat1 is expressed only in rat kidney[76,77], Oat2 is expressed exclusively [78] and Oat3 predominantly[79] in rat liver. In human liver, only OAT2 (SLC22A7) has been isolated (Figure. 1). Oat2 mediates sodium-independent transport of α-ketoglutarate and salicylates, whereas Oat3 transports para-aminohippurate (PAH), estrone-3-sulfate, and the cationic compound cimetidine.
The first organic cation transporter, called OCT1, was cloned from rat kidney[80] and is expressed at the basolateral membrane of hepatocytes, small intestinal enterocytes and cells of the renal proximal tubule S1 segment[74]. In man, hOCT1 (SLC22A1) is expressed specifically in the liver (Figure 1) and mediates the hepatic clearance of small type I cations such as tetraethylammonium, N-methylnicotinamide, dopamine and choline[81,82]. No studies investigating the role of the OAT/OCT/ OCTN transporter family in human liver disease have been performed to date.
Basolateral efflux pumps The basolateral membrane also possesses several members of the multidrug resistance protein family (MRPs) belonging to the superfamily of ATP-binding cassette (ABC) transporters (Figure 1). MRP1 (ABCC1) mediates the ATP-dependent efflux of glutathione S-conjugates[83], leukotriene C4, steroid conjugates such as estradiol-17β-D-glucuronide and glucuronidated or sulfated bile salt conjugates[84]. MRP1 is normally expressed at very low levels in hepatocytes, but expression levels are increased in human hepatoblastoma HepG2 cells and SV40 large T antigen-immortalized human hepatocytes[85]. MRP3 (ABCC3) is expressed at the basolateral hepatocyte membrane[86] and mediates basolateral efflux of the organic anions estradiol-17β-D-glucuronide and S-(2,4-dinitrophenyl) glutathione, the anticancer drugs methotrexate and etoposide [87,88] and even of monovalent bile salts[89]. MRP5 (ABCC5) appears to be an anion transporter, however its expression level in the adult liver is very low[90]. MRP6 (ABCC6) is localized at the lateral membrane of hepatocytes and transports the cyclic pentapeptide and endothelin antagonist BQ-123[91-93]. Interestingly, mutations in the MRP6 gene have been shown to be the cause of pseudoxanthoma elasticum[94].
Bile salt excretion Canalicular excretion represents the rate-limiting step in the overall secretion of bile salts from blood into bile. Whereas bile salt concentrations within the hepatocyte are in the micromolar range, canalicular bile salt concentrations are more than 1000fold higher, necessitating active transport across the canalicular hepatocyte membrane. Characterization of ATP-dependent taurocholate transport in canalicular membrane vesicles indicated the presence of a specific carrier system for monovalent bile salts[95,96], with an apparent Km for ATP-dependent taurocholate transport of-2-20 µM[95-98].
The chief transport system that mediates the canalicular excretion of monovalent bile salts is the so-called “bile salt export pump” or Bsep (ABCB11), first cloned from pig[99] and subsequently from rat[100] and mouse liver[101,102]. Rat Bsep is a 1321 amino acid protein with 12 putative membrane-spanning domains, four potential -N-linked glycosylation sites, a molecular mass of-160 kDa and with the structural features of the ABC-transporter superfamily[100]. The amino acid sequence is more homologous with the MDR family of transporters (-50%) than with MRPs. In membrane vesicles from transfected Sf9 insect cells, rat Bsep transports taurocholate with a Km of-5 µM which is comparable to ATP-dependent transport in canalicular rat liver plasma membrane vesicles[100]. Bsep is expressed on the surface of canalicular microvilli as indicated by electron microscopic studies. In addition to taurocholate, rat Bsep also mediates ATP-dependent transport of glycocholate, taurochenodeoxycholate (Km-2 µM) and tauroursodeoxycholate (Km-4 µM).
The locus of the mouse Bsep gene on chromosome 2, band 2C1.3[101], corresponds to the locus of human BSEP on chromosome 2q24[103]. This region has been linked to the Lith1 gene near D2Mit56 that confers genetic gallstone-susceptibility in the C57L/J mouse strain[104-106]. These mice overexpress Bsep[107] and exhibit relative hypersecretion of cholesterol into bile with subsequent cholesterol supersaturation[108]. The exact significance of overexpression of Bsep in these mice is unclear, since it has been shown that functional ATP-dependent taurocholate transport activity in canalicular membrane vesicles is approximately 3fold lower compared to AKR/J gallstone-resistant mice, despite 3fold higher protein levels[109]. The functional decrease in canalicular bile salt excretion could be the cause of increased gallstone susceptibility in C57L/J mice.
The human BSEP gene locus has been identified as the positional candidate for progressive familial intrahepatic cholestasis type 2 (PFIC2), a progressive liver disease characterized by low biliary bile salt concentrations[103]. In PFIC2, BSEP is absent from the canalicular membrane and biliary bile salt concentrations are less than 1% of normal[110].
Excretion of non-bile salt organic anions The excretion of non-bile salt organic anions into bile is mediated by the canalicular multidrug resistance protein 2, MRP2[111]. MRP2 (ABCC2) has a molecular mass of 190 kD and the human protein exhibits 46% amino acid identity to human MRP1. Both rat and human MRP2 are expressed predominantly in the liver with exclusive localization in the canalicular membrane (Figure 1)[112-115]. The spectrum of organic anions transported by MRP2 is qualitatively similar to that of MRP1[84] and includes glutathione conjugates, glucuronides, leukotriene C4 and divalent bile salts, but not monovalent bile salts[114,116]. A role for MRP2 in the canalicular excretion of reduced glutathione (GSH), a major driving force for the maintenance of bile salt-independent bile flow, has also been demonstrated[117]. Various structurally and functionally unrelated xenobiotics such as probenecid, glibenclamide, rifampicin, vinblastine, indomethacin and cyclosporin A were shown to inhibit excretion of the anionic fluorescent dye carboxy-2’,7’-dichlorofluorescein (CF) by primary human hepatocytes, thus suggesting that organic anion excretion by human liver may be impaired by various drugs[118]. Mutations in the MRP2 gene that lead to the synthesis of a truncated, non-functional protein, have been identified as the pathogenetic basis of hereditary chronic conjugated hyperbilirubinemia, discussed further below.
Phospholipid excretion The major lipid that is cosecreted into bile with cholesterol is phosphatidylcholine(PC). Theconstant replenishment of PC molecules from the inner to the outer hemileaflet of the canalicular membrane is mediated by the concerted action of ATP-dependent[119] and ATP-independent[120-122] PC “flippases”. The ATP-dependent flippase has been identified as a class III multidrug resistance (MDR) P-glycoprotein, Mdr2 in mice and MDR3 in humans (ABCB4) (Figure 1)[123-125], a 170 kD canalicular protein. Mouse Mdr2 and human MDR3 are present in high concentrations in the canalicular membrane of hepatocytes. Mice lacking this protein are unable to secrete phosphatidylcholine (PC) into bile[123]. Conversely, in fibroblasts from transgenic mice expressing the human MDR3 gene under a vimentin promoter, the transfer of radiolabeled PC from the inner to the outer leaflet of the plasma membrane is stimulated [124]. In addition, expression of mouse Mdr2 in secretory vesicles from the yeast mutant sec6-4, results in a time-and temperature-dependent enhancement of PC translocation to the inner leaflet of the membrane[126]. These data indicate that both mouse Mdr2 and human MDR3 function as physiological phospholipid translocators.
Copper excretion The liver is the central organ of copper homeostasis with a great capacity to store and excrete this metal. The degree of biliary copper excretion is directly proportional to the size of the hepatic copper pool, indicating that hepatocytes can sense the copper status in the cytoplasm and regulate copper excretion into bile accordingly[127]. The biliary excretion of heavy metals such as copper is an important detoxifying mechanism of the liver. Copper excretion is mediated by a copper transporting P-type ATPase called ATP7B that is expressed predominantly in the liver[128-130] (Figure 1). This 160 kD protein is localized to the trans-Golgi network[131] where it mediates the incorporation of copper into cuproenzymes such as ceruloplasmin. A truncated 140 kD isoform of ATP7B is localized to mitochondria[132], possibly explaining the abnormalities of mitochondrial morphology in Wilson’s disease. Immunohistochemical studies in human liver indicate additional weak staining of ATP7B at the canalicular membrane[133]. A green fluorescent GFP-ATP7B fusion construct transfected into human hepatoma Huh7 cells localizes neither to the trans-Golgi network nor to the canalicular membrane, but to so-called late endosomes[134]. Copper incorporated into late endosomes is probably transported to lysosomes and subsequently excreted into bile by a process known as biliary lysosomal excretion[134].
Copper is presumably taken up into human hepatocytes via the copper transporters hCTR1 and hCTR2[135]. As the copper concentration of the hepatocyte increases, ATP7B redistributes from the trans-Golgi network to a cytoplasmic vesicular compartment[131] and to pericanalicular vacuoles[136] (Figure 1). After copper depletion, ATP7B returns to the trans-Golgi network. Thus copper can induce trafficking of its own transporter from the trans-Golgi network to the apical membrane, where it may mediate biliary copper excretion. Copper-induced redistribution of ATP7B may provide a mechanism to preserve copper when it is scarce and to prevent copper toxicity when levels become too high.
Progressive familial intrahepatic cholestasis Progressive familial intrahepatic cholestasis (PFIC) describes a group of autosomal-recessive disorders. The onset is usually during the first months of life, with severe and progressive intrahepatic cholestasis, proceeding to cirrhosis by the second decade. Diagnosis is based on the following criteria: ① progressive intrahepatic cholestasis and liver cell failure, after the exclusion of other causes of liver disease; ② lack of bile duct pathology (intrahepatic and extrahepatic); ③ a normal number of interlobular bile ducts[137]. Other signs and symptoms include pruritus, jaundice, hepatomegaly, wheezing and nosebleeds, cough, fat-soluble vitamin deficiency, cholelithiasis, short stature and delayed sexual development[138].
Three types of PFIC have been described[139] (Table 1). PFIC type 1 (PFIC1, Byler’s disease) is caused by a mutation in the coding sequence of the FIC1 gene (ATP8B1, chromosome 18q 21-22)[140], that is expressed predominantly in liver and small intestine. The FIC1 gene product is a P-type ATP-ase putatively involved in the transport of phosphatidylserine and phosphatidylethanolamine from the outer to the inner leaflet of plasma cellular membranes[141]. Patients with a defective FIC1 protein encounter bouts of jaundice that later become permanent, severe pruritus, chronic watery diarrhea, high serum bile salts, but normal γ-GT and cholesterol levels in serum. Histologically, cholestasis is found with progression to cirrhosis, but without ductular proliferation.
| Species | Transport protein | Gene symbol | Physiologic function | Alteration in liver disease |
| Basolateral transport proteins | ||||
| Rat/human | Ntcp/NTCP | SLC10A1 | Na+ dependent bile salt uptake | Decreased Ntcp expression in rat models of cholestasis[25,26,34] |
| Decreased NTCP expression in human cholestatic liver disease[35] | ||||
| Rat | Oatp1 | Slc21a1 | Multispecific uptake of organic anions | Decreased Oatp1 expression in bile duct ligation[32] and in ethinyl |
| and amphipathic compounds | estradiol induced cholestasis[34] | |||
| Oatp2 | Slc21a5 | Multispecific uptake of organic anions | Not yet investigated | |
| and of cardiac glycosides (digoxin) | ||||
| Oatp4 | Slc21a10 | Multispecific uptake of organic anions | Decreased Oatp4 expression in bile duct ligation and sepsis[59] | |
| and amphipathic compounds | ||||
| Human | OATP-A | SLC21A3 | Multispecific uptake of organic anions | Increased mRNA levels in primary sclerosing cholangitis (PSC)[180] |
| and amphipathic compounds | ||||
| OATP-B | SLC21A9 | Multispecific uptake of organic anions | Not yet investigated | |
| and amphipathic compounds | ||||
| OATP-C | SLC21A6 | Multispecific uptake of organic anions | Decreased mRNA levels in primary sclerosing cholangitis[181] | |
| and amphipathic compounds | Not yet investigated | |||
| OATP8 | SLC21A8 | Multispecific uptake of organic anions | ||
| and amphipathic compounds | ||||
| Rat/human | rOCT1/hOCT1 | SLC22A1 | Uptake of small hydrophilic organic | Not yet investigated |
| cations (TEA, MPP, choline, dopamine) | ||||
| Rat | OAT2 | SLC22A7 | Uptake of glutarate, salicylates, | Not yet investigated |
| methotrexate, PGE2 and PAH | ||||
| Rat | OAT3 | SLC22A8 | Uptake of PAH, estrone-3-sulfate, | Not yet investigated |
| ochratoxin A, cimetidine | ||||
| Rat/human | Mrp1/MRP1 | ABCC1 | Efflux of cytotoxic cations and | Increased expression in hepatoma cells[85] and sepsis[182] |
| non-bile salt organic anions | ||||
| Rat/human | Mrp3/MRP3 | ABCC3 | Efflux of organic anions, bile salts | Increased Mrp3 expression in Eisai Hyperbilirubinemic Rats and |
| and anticancer agents | in bile duct ligation[91] | |||
| Increased MRP3 expression in Dubin-Johnson syndrome and | ||||
| primary biliary cirrhosis[86] | ||||
| Rat/human | Mrp6/MRP6 | ABCC6 | Efflux of BQ-123 | Not yet investigated |
| Canalicular Transport Proteins | ||||
| Mouse/rat/mBsep/Bsep/BSEP | ABCB11 | Canalicular efflux of bile salts | Mutations in the BSEP gene and absence of the protein in patients | |
| Human | with PFIC2, characterized by low γ -GT levels and reduced biliary | |||
| bile acid excretion[103,110] | ||||
| Cis-inhibition by cholestatic drugs such as cyclosporine A[172] | ||||
| Trans-inhibition by the cholestatic estrogen metabolite estradiol- | ||||
| 17β− D-glucuronide[172,175] | ||||
| Increased mBsep expression in C57L/J gallstone-susceptible | ||||
| mice, despite reduced bile salt excretory capacity[107,109] | ||||
| Mouse/rat/ | Mdr2/Mdr2/MDR3 | ABCB4 | Biliary excretion of phospholipids | Mdr2-/- knockout mice exhibit an absence of phospholipids in bile |
| and develop progressive liver disease with portal inflammation, | ||||
| bile duct proliferation and fibrosis[123] | ||||
| PFIC3, characterized by high γ -GT levels and absent lipoprotein | ||||
| X in serum, is caused by mutations in the MDR3 gene | ||||
| (chromosome 7q21)[143] | ||||
| MDR3 mutations in PFIC3 are associated with intrahepatic | ||||
| cholestasis of pregnancy[171] | ||||
| Rat/human | Mrp2/MRP2 | ABCC2 | Canalicular excretion of organic anions | Decreased Mrp2 mRNA and protein levels in bile duct ligation and |
| endotoxinemia[154,183] | ||||
| Decreased canalicular density of Mrp2 transporter molecules in | ||||
| endotoxinemia[183], taurolithocholate cholestasis[184] | ||||
| and bile duct ligation[154] | ||||
| Mutations in the rat Mrp2 gene cause hereditary conjugated | ||||
| hyperbilirubinemia[112] | ||||
| Mutations in the human MRP2 gene cause the Dubin-Johnson | ||||
| syndrome with absent protein expression[147,149] | ||||
| MRP2 function is inhibited by anabolic 17á-alkylated | ||||
| steroids[185,186] | ||||
| Decreased MRP2 mRNA but unchanged protein levels in PBC[187] | ||||
| Decreased MRP2 mRNA levels in PSC[181] | ||||
| Human | FIC1 | ATP8B1 | Putative aminophospholipid | P-type ATPase, positional candidate in genetic linkage analysis of |
| translocator | PFIC1 (Byler’s disease) and BRIC[141] | |||
| Human | AE2 | SLC4A2 | Canalicular Cl-/HCO3- exchange | DecreasedAE2 expression on the luminal surface of cholangiocytes in |
| PBC (increased expression secondary to UDCA treatment)[188] | ||||
PFIC type 2 (PFIC2, Byler syndrome) is caused by mutations in the BSEP gene (located on chromosome 2q24)[142] that lead to an absence of the bile salt export pump from the canalicular hepatocyte membrane[110]. Defective canalicular bile salt excretion results in an accumulation of bile salts within the hepatocyte and toxic damage. PFIC2 resembles PFIC1 clinically, biochemically and histologically, although the initial presentation is more severe with permanent jaundice from onset, and liver failure occurs more rapidly.
PFIC type 3 (PFIC3) is caused by homozygous mutations in the MDR3 gene[143], that lead to an absence of the phospholipid export pump MDR3 from the canalicular membrane and to an absence of the major biliary phospholipid, phosphat-idylcholine, from bile. This results in toxic bile salt induced injury of the biliary epithelium. PFIC3 is characterized by elevated serum γ -GT levels, ductular proliferation, and an inflammatory infiltrate in the early stages which progresses to biliary cirrhosis[143,144]. Mice with a homozygous disruption of the Mdr2 gene (which corresponds to MDR3 in man) represent an animal model of PFIC3 [123]. Overall, the analogy between the murine knockout model and human cholestatic liver disease indicates that the nonsuppurative cholangitis observed in Mdr2/MDR3 deficiency is caused by the high luminal concentration of free bile salts that are not sequestered in mixed micelles in the absence of phospholipids.
The appearance of lipoprotein X in the plasma of cholestatic mice has been attributed to the function of Mdr2. Bile duct ligation in control mice induced a dramatic increase in plasma cholesterol and phospholipid concentrations, mainly as lipoprotein X[145]. In bile duct ligated Mdr2-/-mice, cholesterol and phospholipid concentrations were also increased but plasma fractionation revealed a complete absence of lipoprotein X. Plasma levels of cholesterol and phospholipid during cholestasis correlated very closely with the expression level of Mdr2, indicating first that the shift of hepatocellular lipid secretion from bile to plasma during cholestasis depends upon the formation of lipoprotein X, and second that the concentration of lipoprotein X is modulated by the activity of Mdr2. Thus, the elevation of serum cholesterol that is a common feature of cholestasis in man, could also be dependent upon the function of MDR3.
Benign recurrent intrahepatic cholestasis (BRIC) is also caused by a mutation in the FIC1 gene (ATP8B1) (Table 1). It is characterized by recurrent bouts of cholestasis in the adult, with symptom-free intervals lasting from months to several years. Unlike PFIC1, BRIC is not associated with progressive liver damage. Serum bile salt concentrations are elevated as the earliest markers of cholestasis. FIC1 is also expressed in the small intestine, where it appears to play a role in intestinal bile salt absorption. It is of interest that in non-symptomatic BRIC patients, fecal loss of bile salts due to intestinal malabsorption is increased[146].
Dubin-Johnson syndrome The Dubin-Johnson syndrome is an autosomal recessive disorder that is caused by impaired biliary excretion of certain cholephilic organic anions such conjugated bilirubin (Table 1). It is characterized by conjugated hyperbilirubinemia, increased urinary excretion of coproporphyrin I, deposits of a black pigment in centrolobular hepatocytes, and prolonged BSP retention[147]. In contrast to PFIC, hepatic function is preserved. The syndrome is produced by the absence of MRP2 protein from the canalicular hepatocyte membrane[148] due to mutations of the MRP2 gene (ABCC2)[111,147,149]. Recently, the MRP2 Delta(R,M) mutation, which describes the deletion of Arg1392 and Met1393, was shown to cause disturbed maturation and trafficking of the protein from the ER to the Golgi complex and impaired sorting of this glycoprotein to the apical membrane[150]. Absent MRP2 function may be compensated for by increased expression of MRP3 at the basolateral hepatocyte membrane, as suggested by immunofluorescence studies on liver sections from a Dubin-Johnson patient[86].
Wilson’s disease Wilson’s disease is an autosomal recessive disorder characterized by copper accumulation in the liver, brain, kidney and cornea secondary to inadequate biliary copper excretion. Under physiologic circumstances, biliary excretion represents the sole mechanism for copper excretion, and thus affected individuals have progressive copper accumulation in the liver. When the capacity for hepatic storage is exceeded, cell death ensues with copper release into the plasma, hemolysis, and tissue deposition[127]. The age at onset ranges from 3 to 40 years, with highly variable clinical manifestations. Hepatic dysfunction is the most common initial presentation in childhood, progressing from mild elevation of serum transaminases in asymptomatic individuals to chronic active hepatitis and cirrhosis. In some cases, severe chronic liver disease or fulminant hepatic failure may be the initial manifestations. The laboratory diagnosis of Wilson’s disease is confirmed by decreased serum ceruloplasmin, increased urinary copper content, and elevated hepatic copper concentration.
Wilson’s disease results from the absence or dysfunction of the ATP7B gene product, a copper transporting P-type ATPase encoded on chromosome 13. Molecular genetic analysis is complex, as more than 100 unique mutations have been identified and most individuals are compound heterozygotes. A database of Wilson’s disease mutations can be retrieved online at http://www.medgen.med.ualberta.ca. Of these mutations, the H1069Q mutation accounts for more than 40% of the alleles in affected Northern European patients, whereas the A778L mutation is observed in 30% of alleles of Oriental patients[127]. Expression of the H1069Q mutant in a copper transporter-deficient cell line reveals that this mutation causes a defect in protein folding that results in mislocalization to the ER and rapid degradation[151]. The histidine residue at amino acid position 1069 appears to be essential for trafficking from the trans Golgi network in response to copper[151] (Figure 1).
Extrahepatic cholestasis Extrahepatic cholestasis is produced by an obstruction of the hepatic or common bile duct secondary to cholelithiasis, neoplasms, or sclerosing cholangitis. A major risk factor for hepatocellular injury during bile duct obstruction is the increased intracellular concentration of potentially toxic bile salts[152]. This can be partly counteracted by the decrease in Ntcp expression that occurs in bile duct ligated rats[25,32]. The human NTCP mRNA is also downregulated in cholestasis, as evidenced in 23 patients with a diagnosis of extrahepatic biliary atresia[35]. At the canalicular pole, the expression of the bile salt export pump, Bsep, is reduced to 50% of controls on the protein and to 32% on the RNA level[153]. Bsep expression is thus preserved relatively well compared to the marked decrease in expression of the canalicular multispecific organic anion transporter Mrp2[154]. The relative preservation of Bsep expression during bile duct ligation serves to maintain the canalicular efflux of bile salts that has been demonstrated experimentally[155].
The molecular basis of reduced Ntcp expression in cholestasis has not been resolved. The Ntcp gene promoter appears to contain a response element for the farnesoid X receptor (FXR), a nuclear receptor that is responsive to bile salts[156-159]. This is suggested by recent studies in FXR knockout mice, which are unable to decrease Ntcp mRNA levels in response to bile acid feeding[160]. Since intracellular bile salt levels are elevated in bile duct ligation, decreased Ntcp expression is probably caused by suppression of Ntcp transcription via a cascade involving FXR. In the case of cholesterol 7á-hydroxylase (CYP7A1), the rate-limiting enzyme in cholesterol catabolism to bile salts, repression of gene transcription by bile salts has been extensively studied (for review, see reference[161]). Elevated intracellular bile salts activate FXR. This decreases levels of Ntcp and increases those of Bsep[160]. FXR also induces expression of the “short heterodimer partner” SHP, a nuclear receptor that suppresses bile acid synthesis by antagonizing the function of “liver receptor homolog-1” or LRH-1, an orphan receptor required for expression of CYP7A1[161]. Decreased expression of rat Oatp1 in bile duct ligation may also be mediated by FXR, since in FXR knockout mice bile acid feeding induces expression of (mouse) Oatp1[160]. These elaborate autoregulatory cascades ultimately serve to maintain hepatic cholesterol catabolism, and coordinate regulation of bile acid transporters and synthesizing enzymes is likely.
Sepsis-associated cholestasis Septic patients frequently exhibit cholestasis, the primary clinical manifestation of which is hyperbilirubinemia. In animal models of sepsis, reduced hepatic clearance of bile acids and organic anions is found[27,33,162]. The key mediators of sepsis induced cholestasis are inflammatory cytokines such as tumor necrosis factor alpha (TNF-α ) and interleukin-1β . These are liberated in response to endotoxemic stimuli, which can be induced experimentally by application of bacterial lipopolysaccharide (LPS). Both Na+ dependent basolateral and ATP-dependent canalicular bile salt transport is reduced in hepatocyte plasma membrane vesicles isolated from LPS treated rats[27,33]. Direct administration of either LPS, TNF-α or interleukin-1β causes a decrease in Ntcp mRNA levels[26]. The decrease in Ntcp expression can be explained by decreased binding activity of ① the nuclear transcription factor hepatocyte nuclear factor 1 (HNF1) and ② a heterodimeric complex consisting of the retinoic acid receptor (RARα ) and the retinoid x receptor (RXRα), to the Ntcp gene promoter[163,164]. In the case of the human NTCP, dependence of gene transcriptional activity upon the CCAAT/ enhancer binding protein, the α form of which is reduced in sepsis[165], has been shown[166].
The reduction in bile flow that follows LPS administration is caused primarily by an 86% decrease in GSH secretion and a 25% decrease in HCO3- secretion [167], two major driving forces of bile salt independent bile flow. GSH is a substrate of Mrp2[117], the mRNA and protein levels of which are also reduced following treatment of rats with LPS[154]. The mechanism of decreased Mrp2 expression appears to be similar to Ntcp, since reduced binding of the RXRα: RARα complex to the rat Mrp2 promoter secondary to IL-1β has been shown[164].
Cholestasis of pregnancy Intrahepatic cholestasis of pregnancy (ICP) has a high prevalence in Sweden and Chile and is characterized by pruritus and biochemical cholestasis. It is the clinical correlate of estrogen induced cholestasis. The familial clustering, the higher prevalence among relatives of patients with ICP and the susceptibility to oral contraceptive-induced cholestasis in families with a history of ICP implicates genetic factors in the pathogenesis[168-170]. Mutations of the MDR3 gene in women with PFIC type 3 seem to predispose to ICP, although not all women with the mutation develop cholestasis[143,171].
The susceptibility to ethinyl estradiol in patients with a history of ICP suggests a role for estrogen metabolites in the pathogenesis. The cholestatic estrogen metabolite estradiol-17β -D-glucuronide (E-217G) has been shown to inhibit Bsep transport function[172]. E-217G, which is an Mrp2 substrate[173], probably trans-inhibits Bsep function from within the canalicular lumen, since Mrp2-deficient rat strains that are unable to secrete E-217G into the bile canaliculus do not develop cholestasis[174]. A recent study has confirmed that intact Mrp2 function is a prerequisite for the development of Es217G induced cholestasis [175]. The possible role of as yet unidentified genetic polymorphisms of the BSEP gene in the development of estrogen-induced cholestasis is currently under investigation.
Drug-induced cholestasis The liver is the major site of drug metabolism and elimination from the human body. The importance of drugs as hepatotoxins lies not in the overall number of cases, which is relatively small, but in the severity of some reactions and in their potential reversibility provided the drug etiology is promptly recognized. The most common causative agents include NSAIDs, antibiotics, newer antihypertensive agents, H2-receptor blockers and psychotropic drugs. Drug induced hepatotoxicity can be divided into the three categories cholestatic, hepatocellular or mixed type injury, depending upon serum biochemistry. Cholestasis with hepatitis is seen with many drugs, notably chlorpromazine, psychotropic agents, erythromycins, clavulanic acid and NSAIDs. Pure cholestasis without hepatitis is observed most frequently with estrogens, oral contraceptive steroids and 17α -alkylated androgenic steroids and less frequently with cyclosporine A, tamoxifen, griseofulvin, glibenclamide and others. Steroid jaundice caused by methyltestosterone and other C17-alkylated anabolic steroids is dose-related but is also dependent upon the individual susceptibility of the recipient. Whereas hepatic dysfunction is seen in most recipients of steroids, jaundice in seen in only few. A minor degree of hepatic dysfunction in women taking oral contraceptives which contain C-17 ethinyl estrogen and progesterone derivatives is relatively frequent. As mentioned above, women with a personal or family history of cholestatic jaundice of pregnancy are particularly prone to develop jaundice when taking oral contraceptives.
The following alterations of hepatocellular transporter function can be held responsible for the development of drug induced cholestasis. Selective interference of a drug or its metabolite with bile secretory mechanisms has been shown for C17-alkylated ethinylated steroids, the cholestatic bile acid lithocholic acid, and experimentally for icterogenin. Cis-inhibition of Bsep mediated [3H]-taurocholate transport by cyclosporine A, rifamycin SV, rifampicin and glibenclamide is the likely mechanism for itrahepatic cholestasis caused by these agents[172]. Parenteral administration of cyclosporin A in rats inhibits both bile salt excretion and bile salt-independent bile flow, resulting in cholestasis[176]. In addition, bile salt synthesis decreases by about 50% and the total bile acid pool is reduced in rats following orthotopic liver transplantation. Selective interference with the sinusoidal -uptake of substances such as bilirubin and bromosulphophthalein has been shown for the tuberculostatic agents rifamycin SV and rifampicin. Both are mainly eliminated by hepatic uptake, metabolism and excretion into bile. Rifampicin increases serum bile salt concentrations in 72% of patients after the first dose[177], suggesting acute interference with sinusoidal uptake of bile salts. In the Xenopus laevis-oocyte expression system, rifampicin was shown to inhibit Oatp2 but not Oatp1 mediated taurocholate uptake. Both Oatp1 and Oatp2 were inhibited by 10 μmol/L rifamycin SV, whereas significantly higher concentrations of rifamycin SV and rifampicin were required to inhibit Ntcp[178].
The nonsteroidal anti-inflammatory agent sulindac, an established hepatotoxin, may also cause cholestasis by interference with the canalicular excretion of bile salts. Sulindac has been shown to follow the “cholehepatic shunt” pathway and induce choleresis[179]. However, when coinfused with taurocholate in the isolated perfused rat liver, sulindac causes cholestasis by reducing taurocholate secretion. Sulindac appears to be secreted into the bile canaliculus in unconjugated form via a canalicular bile salt export system and is passively absorbed by the bile duct epithelium, thereby inducing a bicarbonate-rich choleresis. Due to continuous cycling within the cholehepatic shunt pathway, high local concentrations of sulindac could be reached within the hepatocyte that cause cholestasis by inhibition of canalicular bile salt efflux[180-188].
This work was supported by grant 32-59155.99 from the Swiss National Science Foundation.
Edited by Ma JY
| 1. | Reichen J, Paumgartner G. Uptake of bile acids by perfused rat liver. Am J Physiol. 1976;231:734-742. [PubMed] |
| 2. | Hofmann AF. Bile acids. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter D, Shafritz DA, eds. The Liver, Biology and Pathobiology. New York: Raven. 1994;677-718. |
| 3. | Petzinger E. Transport of organic anions in the liver. An update on bile acid, fatty acid, monocarboxylate, anionic amino acid, cholephilic organic anion, and anionic drug transport. Rev Physiol Biochem Pharmacol. 1994;123:47-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Suchy FJ. Hepatocellular transport of bile acids. Semin Liver Dis. 1993;13:235-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Müller M, Jansen PL. The secretory function of the liver: new aspects of hepatobiliary transport. J Hepatol. 1998;28:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Anwer MS, Hegner D. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe Seylers Z Physiol Chem. 1978;359:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Scharschmidt BF, Stephens JE. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes. Proc Natl Acad Sci USA. 1981;78:986-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Yamazaki M, Suzuki H, Hanano M, Sugiyama Y. Different relationships between cellular ATP and hepatic uptake among taurocholate, cholate, and organic anions. Am J Physiol. 1993;264:G693-G701. [PubMed] |
| 9. | Boelsterli UA, Zimmerli B, Meier PJ. Identification and characterization of a basolateral dicarboxylate/cholate antiport system in rat hepatocytes. Am J Physiol. 1995;268:G797-G805. [PubMed] |
| 10. | Kouzuki H, Suzuki H, Ito K, Ohashi R, Sugiyama Y. Contribution of sodium taurocholate co-transporting polypeptide to the uptake of its possible substrates into rat hepatocytes. J Pharmacol Exp Ther. 1998;286:1043-1050. [PubMed] |
| 11. | Caflisch C, Zimmerli B, Reichen J, Meier PJ. Cholate uptake in basolateral rat liver plasma membrane vesicles and in liposomes. Biochim Biophys Acta. 1990;1021:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hofmann AF. Bile secretion and the enterohepatic circulation of bile acids. In: Feldman M, Scharschmidt BF, Sleisenger MH, eds. Gastrointestinal and Liver Disease. Philadelphia, London, Toronto, Montreal, Sydney, Tokyo: W.B. Saunders Company. 1998;937-948. |
| 13. | Hagenbuch B, Stieger B, Foguet M, Lübbert H, Meier PJ. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci USA. 1991;88:10629-10633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 326] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 338] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Cattori V, Eckhardt U, Hagenbuch B. Molecular cloning and functional characterization of two alternatively spliced Ntcp isoforms from mouse liver1. Biochim Biophys Acta. 1999;1445:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604-1617. [PubMed] |
| 17. | Stieger B, Hagenbuch B, Landmann L, Höchli M, Schroeder A, Meier PJ. In situ localization of the hepatocytic Na+/Taurocholate cotransporting polypeptide in rat liver. Gastroenterology. 1994;107:1781-1787. [PubMed] |
| 18. | Ananthanarayanan M, Ng OC, Boyer JL, Suchy FJ. Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am J Physiol. 1994;267:G637-G643. [PubMed] |
| 19. | Boyer JL, Ng OC, Ananthanarayanan M, Hofmann AF, Schteingart CD, Hagenbuch B, Stieger B, Meier PJ. Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am J Physiol. 1994;266:G382-G387. [PubMed] |
| 20. | Kullak-Ublick GA, Ismair MG, Kubitz R, Schmitt M, Häussinger D, Stieger B, Hagenbuch B, Meier PJ, Beuers U, Paumgartner G. Stable expression and functional characterization of a Na+-taurocholate cotransporting green fluorescent protein in human hepatoblastoma HepG2 cells. Cytotechnology. 2000;34:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 240] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340-1347. [PubMed] |
| 23. | Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157-G169. [PubMed] |
| 24. | Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, LaRusso NF. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci USA. 2000;97:11092-11097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, Stolz A, Suchy FJ, Boyer JL. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Green RM, Beier D, Gollan JL. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 1996;111:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 206] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Bolder U, Ton-Nu HT, Schteingart CD, Frick E, Hofmann AF. Hepatocyte transport of bile acids and organic anions in endotoxemic rats: impaired uptake and secretion. Gastroenterology. 1997;112:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Green RM, Gollan JL, Hagenbuch B, Meier PJ, Beier DR. Regulation of hepatocyte bile salt transporters during hepatic regeneration. Am J Physiol. 1997;273:G621-G627. [PubMed] |
| 29. | Liang D, Hagenbuch B, Stieger B, Meier PJ. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology. 1993;18:1162-1166. [PubMed] |
| 30. | Boyer JL, Hagenbuch B, Ananthanarayanan M, Suchy F, Stieger B, Meier PJ. Phylogenic and ontogenic expression of hepatocellular bile acid transport. Proc Natl Acad Sci USA. 1993;90:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kullak-Ublick GA, Beuers U, Paumgartner G. Molecular and functional characterization of bile acid transport in human hepatoblastoma HepG2 cells. Hepatology. 1996;23:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Dumont M, Jacquemin E, D'Hont C, Descout C, Cresteil D, Haouzi D, Desrochers M, Stieger B, Hadchouel M, Erlinger S. Expression of the liver Na+-independent organic anion transporting polypeptide (oatp-1) in rats with bile duct ligation. J Hepatol. 1997;27:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Moseley RH, Wang W, Takeda H, Lown K, Shick L, Ananthanarayanan M, Suchy FJ. Effect of endotoxin on bile acid transport in rat liver: a potential model for sepsis-associated cholestasis. Am J Physiol. 1996;271:G137-G146. [PubMed] |
| 34. | Simon FR, Fortune J, Iwahashi M, Gartung C, Wolkoff A, Sutherland E. Ethinyl estradiol cholestasis involves alterations in expression of liver sinusoidal transporters. Am J Physiol. 1996;271:G1043-G1052. [PubMed] |
| 35. | Shneider BL, Fox VL, Schwarz KB, Watson CL, Ananthanarayanan M, Thevananther S, Christie DM, Hardikar W, Setchell KD, Mieli-Vergani G. Hepatic basolateral sodium-dependent-bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology. 1997;25:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci USA. 1994;91:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 384] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 37. | Noé B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci USA. 1997;94:10346-10350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Cattori V, Hagenbuch B, Hagenbuch N, Stieger B, Ha R, Winterhalter KE, Meier PJ. Identification of organic anion transporting polypeptide 4 (Oatp4) as a major full-length isoform of the liver-specific transporter-1 (rlst-1) in rat liver. FEBS Lett. 2000;474:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Bergwerk AJ, Shi X, Ford AC, Kanai N, Jacquemin E, Burk RD, Bai S, Novikoff PM, Stieger B, Meier PJ. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol. 1996;271:G231-G238. [PubMed] |
| 40. | Eckhardt U, Schroeder A, Stieger B, Höchli M, Landmann L, Tynes R, Meier PJ, Hagenbuch B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am J Physiol. 1999;276:G1037-G1042. [PubMed] |
| 41. | Reichel C, Gao B, Van Montfoort J, Cattori V, Rahner C, Hagenbuch B, Stieger B, Kamisako T, Meier PJ. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology. 1999;117:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy JM, Meier PJ, Wolkoff AW. The choroid plexus epithelium is the site of the organic anion transport protein in the brain. Proc Natl Acad Sci USA. 1997;94:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Gao B, Stieger B, Noé B, Fritschy JM, Meier PJ. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem. 1999;47:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Frimmer M, Ziegler K. The transport of bile acids in liver cells. Biochim Biophys Acta. 1988;947:75-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Shi X, Bai S, Ford AC, Burk RD, Jacquemin E, Hagenbuch B, Meier PJ, Wolkoff AW. Stable inducible expression of a functional rat liver organic anion transport protein in HeLa cells. J Biol Chem. 1995;270:25591-25595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. J Biol Chem. 1997;272:26340-26345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273:16184-16191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 183] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395-22401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Dubuisson C, Cresteil D, Desrochers M, Decimo D, Hadchouel M, Jacquemin E. Ontogenic expression of the Na(+)-independent organic anion transporting polypeptide (oatp) in rat liver and kidney. J Hepatol. 1996;25:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Kakyo M, Sakagami H, Nishio T, Nakai D, Nakagomi R, Tokui T, Naitoh T, Matsuno S, Abe T, Yawo H. Immunohistochemical distribution and functional characterization of an organic anion transporting polypeptide 2 (oatp2). FEBS Lett. 1999;445:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Tavoloni N, Jones MJ, Berk PD. Dose-related effects of phenobarbital on hepatic microsomal enzymes. Proc Soc Exp Biol Med. 1983;174:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Klaassen CD. Effects of phenobarbital on the plasma disappearance and biliary excretion of drugs in rats. J Pharmacol Exp Ther. 1970;175:289-300. [PubMed] |
| 54. | Greenberger NJ, Thomas FB. Biliary excretion of 3 H-digitoxin: modification by bile salts and phenobarbital. J Lab Clin Med. 1973;81:241-251. [PubMed] |
| 55. | Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of cardiac glycosides. J Pharmacol Exp Ther. 1974;191:201-211. [PubMed] |
| 56. | Thompson TN, Klaassen CD. The effects of hepatic microsomal enzyme inducers on the pharmacokinetics of ouabain after portal and systemic administration to rats. J Pharm Pharmacol. 1995;47:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | McClain RM, Levin AA, Posch R, Downing JC. The effect of phenobarbital on the metabolism and excretion of thyroxine in rats. Toxicol Appl Pharmacol. 1989;99:216-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Hagenbuch N, Reichel C, Stieger B, Cattori V, Fattinger KE, Landmann L, Meier PJ, Kullak-Ublick GA. Effect of phenobarbital on the expression of bile salt and organic anion transporters of rat liver. J Hepatol. 2001;34:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Kakyo M, Unno M, Tokui T, Nakagomi R, Nishio T, Iwasashi H, Nakai D, Seki M, Suzuki M, Naitoh T. Molecular characterization and functional regulation of a novel rat liver-specific organic anion transporter rlst-1. Gastroenterology. 1999;117:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159-17163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 374] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 61. | Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161-37168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 62. | König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156-G164. [PubMed] |
| 63. | König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000;275:23161-23168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 390] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 64. | Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 523] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 65. | Ismair MG, Stieger B, Cattori V, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Hepatic uptake of cholecystokinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver. Gastroenterology. 2001;121:1185-1190. [PubMed] [DOI] [Full Text] |
| 66. | Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 287] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 67. | Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73-79. [PubMed] |
| 68. | Bossuyt X, Müller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996;25:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Kullak-Ublick GA, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, Paumgartner G. Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett. 1998;424:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Pascolo L, Cupelli F, Anelli PL, Lorusso V, Visigalli M, Uggeri F, Tiribelli C. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun. 1999;257:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866-871. [PubMed] |
| 72. | van Montfoort JE, Hagenbuch B, Fattinger KE, Müller M, Groothuis GM, Meijer DK, Meier PJ. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. J Pharmacol Exp Ther. 1999;291:147-152. [PubMed] |
| 73. | Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pflugers Arch. 2000;440:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Koepsell H. Organic cation transporters in intestine, kidney, liver, and brain. Annu Rev Physiol. 1998;60:243-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 219] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Koepsell H, Gorboulev V, Arndt P. Molecular pharmacology of organic cation transporters in kidney. J Membr Biol. 1999;167:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272:18526-18529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 448] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 77. | Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, Endou H. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol. 1999;10:464-471. [PubMed] |
| 78. | Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 234] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 79. | Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem. 1999;274:13675-13680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 80. | Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 479] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 81. | Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 482] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 82. | Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol. 1997;51:913-921. [PubMed] |
| 83. | Müller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci USA. 1994;91:13033-13037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 419] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 84. | Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988-994. [PubMed] |
| 85. | Roelofsen H, Vos TA, Schippers IJ, Kuipers F, Koning H, Moshage H, Jansen PL, Müller M. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology. 1997;112:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | König J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 322] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 87. | Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem. 1999;274:15181-15185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 88. | Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA. 1999;96:6914-6919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 450] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 89. | Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem. 2000;275:2905-2910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 90. | McAleer MA, Breen MA, White NL, Matthews N. pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem. 1999;274:23541-23548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 91. | Hirohashi T, Suzuki H, Ito K, Ogawa K, Kume K, Shimizu T, Sugiyama Y. Hepatic expression of multidrug resistance-associated protein-like proteins maintained in eisai hyperbilirubinemic rats. Mol Pharmacol. 1998;53:1068-1075. [PubMed] |
| 92. | Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175-182. [PubMed] |
| 93. | Madon J, Hagenbuch B, Landmann L, Meier PJ, Stieger B. Transport function and hepatocellular localization of mrp6 in rat liver. Mol Pharmacol. 2000;57:634-641. [PubMed] |
| 94. | Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 425] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 95. | Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci USA. 1991;88:6590-6594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Stieger B, O'Neill B, Meier PJ. ATP-dependent bile-salt transport in canalicular rat liver plasma-membrane vesicles. Biochem J. 1992;284:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Müller M, Ishikawa T, Berger U, Klünemann C, Lucka L, Schreyer A, Kannicht C, Reutter W, Kurz G, Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991;266:18920-18926. [PubMed] |
| 98. | Suchy FJ, Sippel CJ, Ananthanarayanan M. Bile acid transport across the hepatocyte canalicular membrane. FASEB J. 1997;11:199-205. [PubMed] |
| 99. | Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer Res. 1995;55:2029-2034. [PubMed] |
| 100. | Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046-10050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 673] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 101. | Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol. 2000;57:24-35. [PubMed] |
| 102. | Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 104. | Khanuja B, Cheah YC, Hunt M, Nishina PM, Wang DQ, Chen HW, Billheimer JT, Carey MC, Paigen B. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci USA. 1995;92:7729-7733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | Bouchard G, Nelson HM, Lammert F, Rowe LB, Carey MC, Paigen B. High-resolution maps of the murine Chromosome 2 region containing the cholesterol gallstone locus, Lith1. Mamm Genome. 1999;10:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Paigen B, Schork NJ, Svenson KL, Cheah YC, Mu JL, Lammert F, Wang DQ, Bouchard G, Carey MC. Quantitative trait loci mapping for cholesterol gallstones in AKR/J and C57L/J strains of mice. Physiol Genomics. 2000;4:59-65. [PubMed] |
| 107. | Lammert F, Beier DR, Wang DQ-H, Carey MC, Paigen B, Cohen DE. Genetic mapping of hepatocanalicular transporters establishes sister Pglycoprotein (spgp) as a candidate for the major gallstone gene (Lith1) [abstract]. Hepatology. 1997;26:358A. |
| 108. | Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395-1411. [PubMed] |
| 109. | Green RM, Ward K. Phenotypic characterization of ATP dependent canalicular bile salt transport in C57L/J and AKR/J mice [abstract]. Hepatology. 2000;32:390A. |
| 110. | Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 274] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 111. | Paulusma CC, Oude Elferink RP. The canalicular multispecific organic anion transporter and conjugated hyperbilirubinemia in rat and man. J Mol Med (Berl). 1997;75:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 537] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 113. | Büchler M, König J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271:15091-15098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 427] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 114. | Madon J, Eckhardt U, Gerloff T, Stieger B, Meier PJ. Functional expression of the rat liver canalicular isoform of the multidrug resistance-associated protein. FEBS Lett. 1997;406:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996;56:4124-4129. [PubMed] |
| 116. | Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. Functional analysis of a canalicular multispecific organic anion transporter cloned from rat liver. J Biol Chem. 1998;273:1684-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 118. | Payen L, Courtois A, Campion JP, Guillouzo A, Fardel O. Characterization and inhibition by a wide range of xenobiotics of organic anion excretion by primary human hepatocytes. Biochem Pharmacol. 2000;60:1967-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Elferink RP, Tytgat GN, Groen AK. Hepatic canalicular membrane 1: The role of mdr2 P-glycoprotein in hepatobiliary lipid transport. FASEB J. 1997;11:19-28. [PubMed] |
| 120. | Berr F, Meier PJ, Stieger B. Evidence for the presence of a phosphatidylcholine translocator in isolated rat liver canalicular plasma membrane vesicles. J Biol Chem. 1993;268:3976-3979. [PubMed] |
| 121. | Kullak-Ublick GA, Gerloff T, Hagenbuch B, Berr F, Meier PJ, Stieger B. Expression of a rat liver phosphatidylcholine translocator in Xenopus laevis oocytes. Hepatology. 1996;23:1254-1259. [PubMed] [DOI] [Full Text] |
| 122. | Fuchs M, Carey MC, Cohen DE. Evidence for an ATP-independent long-chain phosphatidylcholine translocator in hepatocyte membranes. Am J Physiol. 1997;273:G1312-G1319. [PubMed] |
| 123. | Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1045] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 124. | Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, Schinkel AH, Borst P. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994;354:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 125. | van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 626] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 126. | Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 127. | Loudianos G, Gitlin JD. Wilson's disease. Semin Liver Dis. 2000;20:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 128. | Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1311] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 129. | Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 910] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 130. | Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993;197:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 344] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 131. | Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD. Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J Biol Chem. 1997;272:21461-21466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 231] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 132. | Lutsenko S, Cooper MJ. Localization of the Wilson's disease protein product to mitochondria. Proc Natl Acad Sci U S A. 1998;95:6004-6009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Schaefer M, Roelofsen H, Wolters H, Hofmann WJ, Müller M, Kuipers F, Stremmel W, Vonk RJ. Localization of the Wilson's disease protein in human liver. Gastroenterology. 1999;117:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 134. | Harada M, Sakisaka S, Terada K, Kimura R, Kawaguchi T, Koga H, Taniguchi E, Sasatomi K, Miura N, Suganuma T. Role of ATP7B in biliary copper excretion in a human hepatoma cell line and normal rat hepatocytes. Gastroenterology. 2000;118:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 135. | Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481-7486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 413] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 136. | Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 137. | Soubrane O, Gauthier F, DeVictor D, Bernard O, Valayer J, Houssin D, Chapuis Y. Orthotopic liver transplantation for Byler disease. Transplantation. 1990;50:804-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 138. | Whitington PF, Freese DK, Alonso EM, Schwarzenberg SJ, Sharp HL. Clinical and biochemical findings in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 149] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Jacquemin E, Hadchouel M. Genetic basis of progressive familial intrahepatic cholestasis. J Hepatol. 1999;31:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 140. | Carlton VE, Knisely AS, Freimer NB. Mapping of a locus for progressive familial intrahepatic cholestasis (Byler disease) to 18q21-q22, the benign recurrent intrahepatic cholestasis region. Hum Mol Genet. 1995;4:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 505] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 142. | Strautnieks SS, Kagalwalla AF, Tanner MS, Knisely AS, Bull L, Freimer N, Kocoshis SA, Gardiner RM, Thompson RJ. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997;61:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 121] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 143. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 456] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 144. | Deleuze JF, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 1996;23:904-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 171] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 145. | Elferink RP, Ottenhoff R, van Marle J, Frijters CM, Smith AJ, Groen AK. Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse. J Clin Invest. 1998;102:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 146. | Bijleveld CM, Vonk RJ, Kuipers F, Havinga R, Boverhof R, Koopman BJ, Wolthers BG, Fernandes J. Benign recurrent intrahepatic cholestasis: altered bile acid metabolism. Gastroenterology. 1989;97:427-432. [PubMed] |
| 147. | Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, Yoshida I, Kimura A, Sakisaka S, Adachi Y. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet. 1998;7:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 177] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 148. | Kartenbeck J, Leuschner U, Mayer R, Keppler D. Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology. 1996;23:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Paulusma CC, Kool M, Bosma PJ, Scheffer GL, ter Borg F, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25:1539-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 340] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 150. | Keitel V, Kartenbeck J, Nies AT, Spring H, Brom M, Keppler D. Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology. 2000;32:1317-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 151. | Payne AS, Kelly EJ, Gitlin JD. Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci USA. 1998;95:10854-10859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 152. | Hofmann AF. Bile Acids: The Good, the Bad, and the Ugly. News Physiol Sci. 1999;14:24-29. [PubMed] |
| 153. | Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 154. | Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, Suchy FJ, Keppler D, Boyer JL. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 347] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 155. | Buscher HP, Miltenberger C, MacNelly S, Gerok W. The histoautoradiographic localization of taurocholate in rat liver after bile duct ligation. Evidence for ongoing secretion and reabsorption processes. J Hepatol. 1989;8:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 156. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2012] [Cited by in RCA: 2093] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 157. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1754] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 158. | Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1138] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 159. | Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918-10924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 160. | Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1400] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 161. | Chawla A, Saez E, Evans RM. "Don't know much bile-ology". Cell. 2000;103:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 162. | Roelofsen H, van der Veere CN, Ottenhoff R, Schoemaker B, Jansen PL, Oude Elferink RP. Decreased bilirubin transport in the perfused liver of endotoxemic rats. Gastroenterology. 1994;107:1075-1084. [PubMed] |
| 163. | Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest. 1998;101:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 164. | Denson LA, Auld KL, Schiek DS, McClure MH, Mangelsdorf DJ, Karpen SJ. Interleukin-1beta suppresses retinoid transactivation of two hepatic transporter genes involved in bile formation. J Biol Chem. 2000;275:8835-8843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 165. | Yin M, Yang SQ, Lin HZ, Lane MD, Chatterjee S, Diehl AM. Tumor necrosis factor alpha promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J Biol Chem. 1996;271:17974-17978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 166. | McClure MH, Denson LA, Ananthanarayanan M, Suchy FJ, Hardikar W, Karpen SJ. CCAAT/Enhancer Binding Protein α (C/EBPα) and Hepatocyte Nuclear Factor 3β (HNF3β) transactivate the human hepatic Na /taurocholate cotransporter (NTCP) [abstract]. Hepatology. 1998;28:428A. |
| 167. | Trauner M, Nathanson MH, Rydberg SA, Koeppel TA, Gartung C, Sessa WC, Boyer JL. Endotoxin impairs biliary glutathione and HCO3- excretion and blocks the choleretic effect of nitric oxide in rat liver. Hepatology. 1997;25:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 168. | Leevy CB, Koneru B, Klein KM. Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease. Gastroenterology. 1997;113:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 169. | Mella JG, Roschmann E, Glasinovic JC, Alvarado A, Scrivanti M, Volk BA. Exploring the genetic role of the HLA-DPB1 locus in Chileans with intrahepatic cholestasis of pregnancy. J Hepatol. 1996;24:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 170. | Meng LJ, Reyes H, Axelson M, Palma J, Hernandez I, Ribalta J, Sjövall J. Progesterone metabolites and bile acids in serum of patients with intrahepatic cholestasis of pregnancy: effect of ursodeoxycholic acid therapy. Hepatology. 1997;26:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 171. | Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353:210-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 172. | Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 173. | Takikawa H, Yamazaki R, Sano N, Yamanaka M. Biliary excretion of estradiol-17 beta-glucuronide in the rat. Hepatology. 1996;23:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 174. | Sano N, Takikawa H, Yamanaka M. Estradiol-17 beta-glucuronide-induced cholestasis. Effects of ursodeoxycholate-3-O-glucuronide and 3,7-disulfate. J Hepatol. 1993;17:241-246. [PubMed] |
| 175. | Huang L, Smit JW, Meijer DK, Vore M. Mrp2 is essential for estradiol-17beta(beta-D-glucuronide)-induced cholestasis in rats. Hepatology. 2000;32:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 176. | Chan FK, Zhang Y, Lee SS, Shaffer EA. The effects of liver transplantation and cyclosporine on bile formation and lipid composition: an experimental study in the rat. J Hepatol. 1998;28:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 177. | Galeazzi R, Lorenzini I, Orlandi F. Rifampicin-induced elevation of serum bile acids in man. Dig Dis Sci. 1980;25:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 178. | Fattinger K, Cattori V, Hagenbuch B, Meier PJ, Stieger B. Rifamycin SV and rifampicin exhibit differential inhibition of the hepatic rat organic anion transporting polypeptides, Oatp1 and Oatp2. Hepatology. 2000;32:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 179. | Bolder U, Trang NV, Hagey LR, Schteingart CD, Ton-Nu HT, Cerrè C, Elferink RP, Hofmann AF. Sulindac is excreted into bile by a canalicular bile salt pump and undergoes a cholehepatic circulation in rats. Gastroenterology. 1999;117:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 180. | Kullak-Ublick GA, Beuers U, Fahney C, Hagenbuch B, Meier PJ, Paumgartner G. Identification and functional characterization of the promoter region of the human organic anion transporting polypeptide gene. Hepatology. 1997;26:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 181. | Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Downregulation of the hepatocellular organic anion transporters OATP-C and MRP2 in patients with primary scle-rosing cholangitis [abstract]. J Hepatol. 2000;32:118. [DOI] [Full Text] |
| 182. | Vos TA, Hooiveld GJ, Koning H, Childs S, Meijer DK, Moshage H, Jansen PL, Müller M. Up-regulation of the multidrug resistance genes, Mrp1 and Mdr1b, and down-regulation of the organic anion transporter, Mrp2, and the bile salt transporter, Spgp, in endotoxemic rat liver. Hepatology. 1998;28:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 183. | Kubitz R, Wettstein M, Warskulat U, Häussinger D. Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide and dexamethasone. Gastroenterology. 1999;116:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 184. | Beuers U, Bilzer M, Chittattu A, Kullak Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Inhibition of bile flow, hepatobiliary exocytosis, and mrp2 insertion into the canalicular membrane by taurolithocholic acid is reversed by tauroursodeoxycholic acid in the perfused rat liver [abstract]. Hepatology. 1999;30:463A. |
| 185. | Descotes G, Osman H, Gallas JF, Penacchio E, Moreau C. Functional, biochemical, and morphological hepatobiliary effects in rats chronically exposed to a steroidal antiandrogen. Toxicol Appl Pharmacol. 1996;137:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 186. | Silva MO, Reddy KR, McDonald T, Jeffers LJ, Schiff ER. Danazol-induced cholestasis. Am J Gastroenterol. 1989;84:426-428. [PubMed] |
| 187. | Oswald M, Kullak Ublick GA, Beuers U, Paumgartner G. Expres-sion of the hepatocyte canalicular multidrug resistance associated protein 2 (MRP2) in primary biliary cirrhosis [abstract]. Hepatology. 1998;28:544A. |
| 188. | Medina JF, Martínez-Ansó JJ, Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |