Published online Feb 15, 2001. doi: 10.3748/wjg.v7.i1.42
Revised: October 12, 2000
Accepted: October 20, 2000
Published online: February 15, 2001
AIM: To evaluate the antifibrotic effect of different doses of recombinant human Gamma-Interferon (IFN-γ) in two rat models of hepatic fibrosis, and to observe its effect on moderate chronic hepatitis B virus fibrosis.
METHODS: Hepatic fibrosis was successfully induced in 150 and 196 rats by subcutaneous injection of carbon tetrachloride (CCla4) and intraperitoneal injection of dimethylnitrosamine (DMN), respectively. Each of the two model groups was divided into: ① fibrotic model group; ② colchicine treatment group (0.1 mg/kg/day, gastrogavage for 8 weeks); ③ high-dose IFN-γ group (15 MU/kg per day, i.m. for 8 weeks); ④ medium-dose IFN-γ group (5 MU/kg daily, i.m. for 8 weeks); and ⑤ low-dose IFN-γ group (1.67 MU/kg daily, i.m. for 8 weeks). Another group of 10 rats without any treatment was used as normal controls. At the end of the experiment, semi-quantitative histopathological scores of inflammation and fibrosis, liver α smooth muscle actin (α-SMA) expression level, liver hydroxyl proline content and serum hyaluronic acid levels were compared. And 47 medium chronic hepatitis B viral fibrosis patients were studied. They were given IFN-γ treatment, 100 MU/day i.m. for the first three months and 100 MU qod i.m. for the next six months. Semi-quantitative pathological scores of inflammation and fibrosis and serum hepatic fibrosis indices were compared within the 9 months.
RESULTS: In animal experiment, the pathological fibrosis scores and liver hydroxyl proline content were found to be significantly lower in rats treated with different doses of IFN-γ as compared with rats in fibrotic model group induced by either CCla4 or DMN, in a dose-dependent manner. For CCla4-induced model, pathological fibrosis scores in high, medium and low doses IFN-γ groups were 5.10 ± 2.88, 7.70 ± 3.53 and 8.00 ± 3.30, respectively, but the score was 14.60 ± 7.82 in fibrotic model group. Hydroxyl proline contents were 2.83 ± 1.18, 3.59 ± 1.22 and 4.80 ± 1.62, in the three IFN-γ groups, and 10.01 ± 3.23 in fibrotic model group. The difference was statistically significant (P < 0.01). Similar results were found in DMN-induced model. Pathological fibrosis scores were 6.30 ± 0.48, 8.10 ± 2.72 and 8.30 ± 2.58, in high, medium and low doses IFN-γ groups, and 12.59 ± 3.57 in fibrotic model group. Hydroxyl proline contents were 2.72 ± 0.58, 3.14 ± 0.71 and 3.62 ± 1.02, in the three IFN-γ groups, and 12.79 ± 1.54 in fibrotic model group. The difference was statistically significant (P < 0.01). Serum hepatic fibrosis indices decreased significantly in the 47 patients after IFN-γ treatment (HA: 433.38 ± 373.00 vs 281.57 ± 220.48; LN: 161.22 ± 41.02 vs 146.35 ± 44.67; PC(r): 192.59 ± 89.95 vs 156.98 ± 49.22; C-I: 156.30 ± 44.01 vs 139.14 ± 34.47) and the differences between the four indices were significant (P < 0.05). Thirty-three patients received two liver biopsies, one before and one after IFN-γ treatment. In thirty of 33 patients IFN-γ had better effects according to semi-quantitative pathological scores (8.40 ± 5.83 vs 5.30 ± 4.05, P < 0.05).
CONCLUSION: All the three doses of IFN-γ are effective in treating rat liver fibrosis induced by either CCla4 or DMN, the higher the dose, the better the effect. And IFN-γ is effective for patients with moderate chronic hepatitis B viral fibrosis.
- Citation: Weng HL, Cai WM, Liu RH. Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol 2001; 7(1): 42-48
- URL: https://www.wjgnet.com/1007-9327/full/v7/i1/42.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i1.42
Hepatic fibrosis can cause liver cirrhosis in chronic liver diseases (viral, schistosomal, alcoholic). Regardless of causes, hepatic fibrosis is characterized by a large deposition of extracellular matrix, collagen being a major component. Studies on interferon-gamma (IFN-γ ) in vitro or in vivo are increasing[1-7]. Although the interferons were initially described for their antiviral properties, IFN-γ has also been found to have significant effects against hepatic fibrosis and to inhibit the synthesis of extracellular matrix[8-14]. However, there have been few studies on the relationship between different doses and effects of IFN-γ for hepatic fibrosis. Our previous study showed that IFN-γ could lower serum hyaluronic acid levels in chronic hepatitis patients with hepatic fibrosis[15]. But no reports concerning histological changes after IFN-γ treatment have ever been found. We were prompted to systematically observe the effect of IFN-γ and investigate its effective doses on rat hepatic fibrosis induced by intraperitoneal injection of two different toxins, carbon tetrachloride (CCl4) and dimethylnitrosamine (DMN). Once hepatic fibrosis emerged in two rat models, different doses of IFN-γ were injected intramuscularly to observe the following changes after IFN-γ treatment: pathological fibrosis scores, liver hydroxylproline content and serum hyaluronic acid levels. In the meantime, we observated 47 chronic hepatitis B patients with hepatic fibrosis who received IFN-γ treatment for 9 months. Liver biopsy and other laboratory tests were performed within the 9 months, the effect of IFN-γ was assessed.
Sprague-Dawley rats (n = 483) weighing 200 gm-250 gm were used. They were fed with Good Laboratory Practice diet in pellets (provided by Zhejiang University Animal Study Center). Rats were maintained under 12 h light/dark cycles and allowed free access to food and water. Experiments were performed in accordance with the institutional ethical guidelines.
Hepatic fibrosis was induced by intraperitoneal injections of CCl4 or DMN. Recombinant human IFN-γ was kindly provided by Shanghai Clonbiotech Co., Ltd (Shanghai, China, Batch Number 970521).
Rats (n = 205) were subcutaneously administered dissolved CCl4 in olive oil (a proportion of 4:6) at 0.3 mL/kg of body weight, i.p., for 2 consecutive days a week for 16 weeks. Five rats were killed at the end of each week to examine their pathological changes.
For DMN-induced fibrosis, 278 rats were injected i.p. repeatedly, for 3 consecutive days at the first week and 2 consecutive days for the next 5 weeks. Five rats were killed at the end of each week to examine their pathological changes.
According to histological sections, hepatic fibrosis appeared at the end of the fourth week from the start of the study. One hundred and fifty rats in CCl4 induced model and 196 rats in DMN-induced model could be used for the next study. Rats in the two models were divided into five groups: ① fibrotic model group; ② colchicine treatment group (0.1mg·kg-1·day-1, gastrogavage); ③ high-dose IFN-γ group (15 MU·kg-1·day-1, i.m.); ④ medium-dose IFN-γ group (5 MU·kg-1·day-1, i.m.); and ⑤ low-dose IFN-γ group (1.67 MU·kg -1·day-1, i.m.). In addition, 10 rats were chosen as the normal control group. Rats in the normal control and fibrotic model groups were given 0.9% sodium chloride by intramuscular injection instead of IFN-γ .
Rats of CCl4 induced model received 8-week treatment. At the end of the eighth week, 10 rats of each group were killed, and histological sections of their livers were evaluated by hematoxylin and eosin and Sirius red stains. α-smooth muscle actin (α-SMA) was examined with immunohistochemical technique. Liver hydroxylproline content and serum hyaluronic acid level were also detected. In the next 4 weeks, all treatments stopped. The rest of the rats were killed at the end of the sixteenth week from the start of the experiment.
Rats of DMN-induced model received 4-week treatment. At the end of the fourth week, 10 rats of each group were killed. All items performed in CCl4-induced model were detected, too. The other rats were killed at the end of the 12th week from the start of the study.
Excised liver tissues from rats were fixed in 10% neutralized formaldehyde, embedded in paraffin, and then stained with hematoxylinandeosin. Alternatively, the Sirius red stain method was used to specifically stain fibrous tissue components. These sections were observed under polarization microscopy (Leica DMLB, Leica Wetalar, Germany.) to distinguish type I and III collagen[16]. Specimens were reviewed under code by a single pathologist who graded and scored the specimens according to the criteria of Scheuer and Chevallier et al[17,18]. In Scheuer criteria, the transformation of grade and stage was as follows: stage 0, = 2°; grade or stage 1, = 21; grade or stage 2, = 22; grade or stage 3, = 23; grade or stage 4, = 24.
α-SMA, a marker of activated hepatic stellate (HSC)[19,20], was detected in formaldehyde-fixed paraffin-embedded section. Briefly, liver tissue was incubated with a monoclonal IgG2a recognizing α-SMA (Dako M851) and reactive sites were detected using the labeled streptavidin-biotin technique (Dako M680), resulting in a brown staining after incubation with Tris-buffered saline containing diammobenzidine and H2O2. In control slides, the primary antibody was substituted by the Tris-buffers.
The liver was homogenated into powder and hydrolyzed with 6 mol/L HCl. The hydroxyl proline content was measured by the means of Kivirikko et al[21]. The routine serum biochemical tests, including serum aspartate transaminase and alanine transaminase, were performed.
Serum hyaluronic acid level was detected by radioimmunoassay technique. The kit was provided by the Shanghai Navy Medical Institute. The operations were performed according to the manufacturer’s instructions.
Fourty-seven hepatic fibrosis patients with chronic hepatitis B, 37 males and 10 females, were chosen for the study. They were confirmed to have moderate hepatic fibrosis pathologically according to Scheuer and Chevallier scoring system[17,18] and had chronic hepatitis B for 6 months to 30 years. Additional criteria included: age between 18 and 65 years; presence of HBsAg in serum for at least 6 months; positive serum tests for anti-HBe documented on four occasions, at least 1 month apart, during the 6 months before entry into the study. Among four serum hepatic fibrosis indices, including hyaluronic acid (HA, upper nomal limit < 110 μg/L), laminin (LN, upper normal limit < 150 μg/L), type III procollagen (PCIII, upper normal limit < 120 μg/L) and type IV collagen (C-IV, upper normal limit < 80 μg/L), two indices were higher than upper normal limit. Patients were excluded when the haemoglobin ≤ 10 g/dL, platelet count ≤ 50000/mL, and white cell count ≤ 4000/mL. Other exclusion criteria were patients with decompensated liver disease (serum albumin < 3.5 g/dL; serum total bilirubin ≥ 85.5 μmol/L; prothrombin time prolongation > 3sec), histories of hepatic encephalopathy or ascites, esophageal or gastric varices at risk of bleeding, hepatitis delta virus, hepatitis C virus infections, causes of liver disease other than HBV, intravenous drug abuse, pregnancy, malignancy, chronic heart and/or renal failure, anti-virus therapy (such as IFN-α, lamivudine, Famciclovir and Penciolovir therapy) and immune modulation therapy (such as Thymosin α1) within a period of 3 months, and anti-hepatic fibrosis drugs (such as colchicines, herbal recipe 861 and Extractum Semen Percise).
In this open label study, all the patients were given routine therapy and received rhIFN-γ (kindly provided by Shanghai Clonbiotech Co., Ltd, Shanghai, China, Batch Number 981210) at a dose of 1 MU intramuscularly daily for the first three months and every other day for the following six months. They were asked to take rhIFN-γ in local hospitals near their homes. They were visited every three months during the treatment and thereafter followed up for 3 months after the treatment stopped. At each visit, clinical and laboratory assessments were made, including serum biochemical tests, such as serum hepatic fibrosis indices, routine serum biochemical tests, such as ALT, AST and γ-glutamyl transpeptidase, alkaline phosphatase, albumin, total bilirubin and alfha fetoprotein (AFP), routine blood tests, and HBV serology. Ethical Committee of the Centers involved approved the study. All the patients were volunteers.
Liver biopsies were performed twice within the 9 months, one before the treatment and one after it. All liver biopsy specimens were stained with hematoxylin-eosin and Sirius red. A single pathologist who was blinded with respect to treatment regimen evaluated specimens according to the criteria of Scheuer and Chevallier et al[9,10].
Results were expressed as mean ± standard deviation and compared when appropriate, by Student’s two-tailed paired and unpaired t test and Kruskall Wallis nonparametric one-way ANOVA.
In normal control group, Sirius red staining showed a normal distribution of collagen in a small amount in the portal tracts and around the terminal hepatic veins.
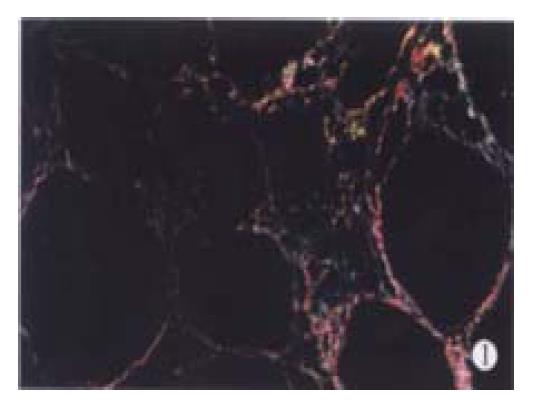
CCl4-induced model showed different pathological changes from DMN-induced model. In the former, besides hepatocyte necrosis, fatty degeneration was the major feature[22] while in the latter, lymphomonocyte infiltration and local hepatocyte necrosis were the main characteristics[23]. However, after 12 weeks of CCl4 and 8 weeks of DMN treatment, all the rats in fibrotic model groups showed complete fibrotic septum, forming a pattern of micronodular cirrhosis to the parenchyma (Figure 1).
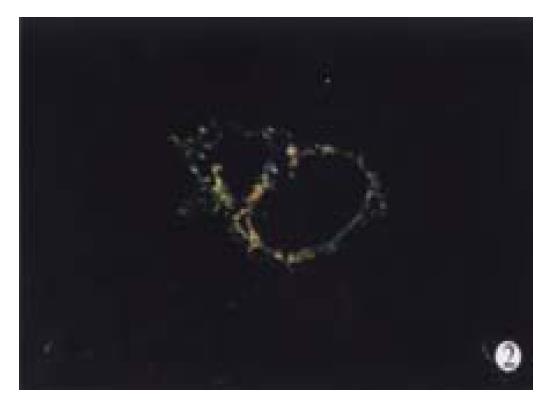
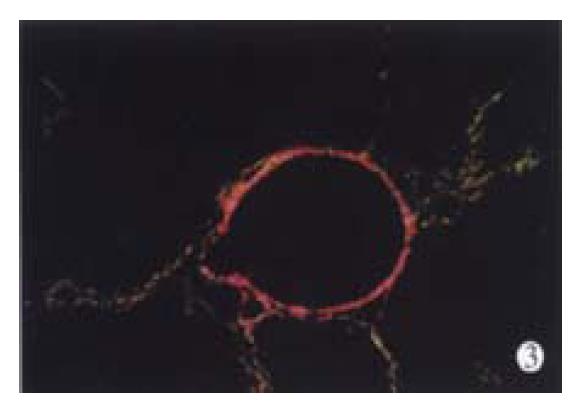
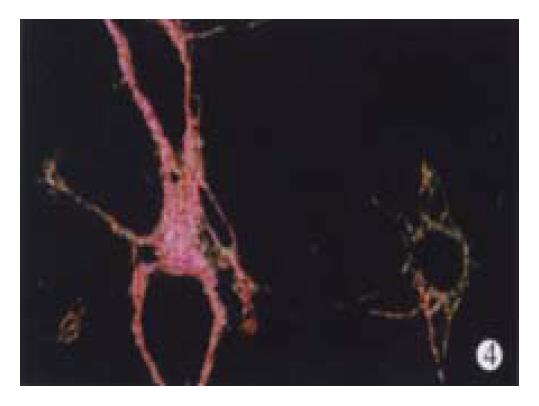
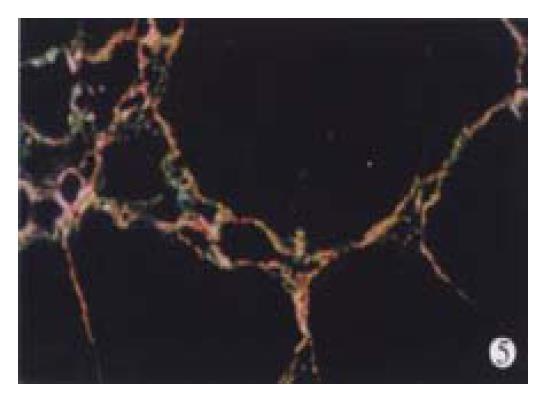
In animal treated with high-dose of IFN-γ, Sirius red staining showed a small collagen deposition in the portal tracts and lobules. Only collagen fibers around the terminal hepatic veins were observed (Figure 2). Collagen depositions in liver sections taken from animals treated with medium or low dose of IFN-γ were particularly evident but only thin bands of collagen which formed short, incomplete septum could be seen (Figure 3, Figure 4). In colchcine treated animals, complete fibrotic septum departing from the central vein were present, and no cirrhosis could be observed (Figure 5). Table 1 and Table 2 show the histological stage and grade of different groups.
| Groups | 2g* | 2s* | SSS△ |
| Normal control group | 1.00 ± 0.00 | 1.00 ± 0.00 | 0 |
| Fibrotic model group | 9.20 ± 5.01 | 10.40 ± 5.06 | 14.60 ± 7.82 |
| Colchicine treatment group | 10.40 ± 5.06 | 7.40 ± 4.99a | 10.60 ± 3.34a |
| High-dose IFN-γ group | 9.60 ± 3.37 | 3.00 ± 1.05bc | 5.10 ± 2.88bc |
| Medium-dose IFN-γ group | 6.00 ± 4.22 | 5.60 ± 4.20bc | 7.70 ± 3.53bc |
| Low-dose IFN-γ group | 7.60 ± 4.79 | 5.40 ± 2.32bc | 8.00 ± 3.30bc |
| Groups | 2g* | 2s* | SSS△ |
| Normal control group | 1.00 ± 0.00 | 1.00 ± 0.00 | 0 |
| Fibrotic model group | 7.00 ± 2.16 | 9.60 ± 4.70 | 12.60 ± 3.57 |
| Colchicine treatment group | 6.80 ± 4.13 | 7.00 ± 3.92a | 11.30 ± 2.37a |
| High-dose IFN-γ group | 6.40 ± 2.07 | 2.60 ± 0.97bc | 6.30 ± 0.48bc |
| Medium-dose IFN-γ group | 4.80 ± 2.35 | 4.00 ± 2.32bc | 8. 10 ± 2.72bc |
| Low-dose IFN-γ group | 5.00 ± 2.71 | 4.40 ± 2.07bc | 8.30 ± 2.58bc |
In the normal control group, α-SMA positive cells were detected mainly in the portal space as elements of vascular walls or as fibroblastlike cells scattering in the connective tissue or near bile ductules. In lobule, α-SMA positive cells were present on the walls of large and medium sized terminal hepatic veins. The pattern of distribution of α-SMA positive cells was modified in CCl4 or DMN treated animals as compared with the normal control rats. In fibrotic model groups, α-SMA positive cells were detected in portal space, sinusoid, lobule and areas where fibrotic septum appeared. Different doses of IFN-γ appeared to strikingly decrease the activation of HSC. After 8 weeks of IFN-γ treatment in CCl4 model or 4 weeks in DMN model, only thin and incomplete parenchymal α-SMA positive septum joining thickened centrilobular veins were observed. α-SMA positive cells were mainly found in portal space and areas around fibrotic septum. Few α-SMA positive cells were present in sinusoid and lobule.
Analysis of total collagen content in liver substantiated the histological impression. Hydroxylproline evaluation in fibrotic model groups showed that the amount of Hydroxyl proline content increased approximately 5-fold (in CCl4 model) or 6-fold (in DMN model) as compared with the normal control group, whereas, figures of different doses of IFN-γ groups were lower (< 2-fold). The results in Table 3 and Table 4 indicate the serum hyaluronic acid level of different groups. Except for low-dose IFN-γ group in CCl4-induced model, other IFN-γ treatment groups had obvious decrease of serum hyaluronic acid level in both models.
| Groups | HA (μg/L) | Hyp (mg/g liver dry weight) |
| Normal control group | 147.64 ± 19.28 | 2.43 ± 0.33 |
| Fibrotic model group | 900.04 ± 508.84 | 10.01 ± 3.23 |
| Colchicine treatment group | 745.57 ± 170.44a | 6.90 ± 1.70 |
| High-dose IFN-γ group | 482.05 ± 210.57 | 2.83 ± 1.18 |
| Medium-dose IFN-γ group | 765.51 ± 586.65a | 3.59 ± 1.22bd |
| Low-dose IFN-γ group | 981.52 ± 509.95 | 4.80 ± 1.62 |
| Groups | HA (μg/L) | Hyp (mg/g liver dry weight) |
| Normal control group | 147.64 ± 19.28 | 2.43 ± 0.33 |
| Fibrotic model group | 969.52 ± 257.80 | 12.79 ± 1.54 |
| Colchicine treatment group | 323.72 ± 388.45a | 6.40 ± 1.84a |
| High-dose IFN-γ group | 144.25 ± 105.66 | 2.72 ± 0.58 |
| Medium-dose IFN-γ group | 334.10 ± 420.06 | 3.14 ± 0.71 |
| Low-dose IFN-γ group | 407.95 ± 386.61a | 3.62 ± 1.02bd |
All the 47 patients completed the 9-month treatment. Table 5 shows the changes of symptoms after the 9-month IFN-γ treatment. Except for these changes, 12 patients (12/47, 25.5%) had a better appetite than before the treatment.
| Fatigue | Abdominal distention | Nausea | Liver area pain | |
| Before treatment | 33 | 31 | 12 | 22 |
| After treatment | 4 | 4 | 0 | 4 |
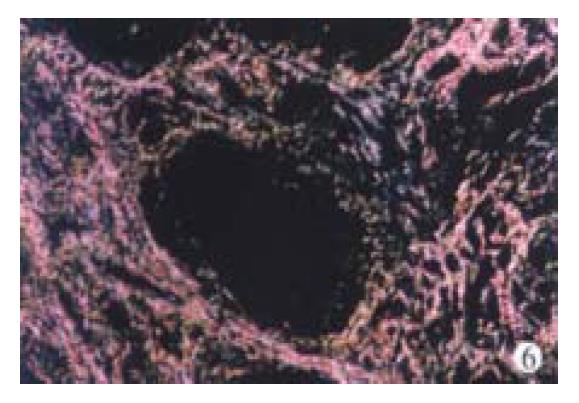
Thirty-three patients were given a second biopsy after the 9-month treatment (33/47, 70.2%). According to Chevallier’s criteria, semi-quantitative system score (SSS) was calculated. After the 9-month IFN-γ treatment, the SSS decreased significantly in 14 patients (14/33, 42.4%) and the SSS dropped over 2 points, increased in 3 patients (3/33, 9.1%), and remained unchanged or dropped 1 point in the remaining 16 patients (16/33, 48.5%). Table 6 shows the mean SSS change during the treatment. A significant decrease of hepatic fibrosis score in accordance with the SSS was seen at the end of the IFN-γ treatment (8.40 ± 5.83 vs 5.30 ± 4. 05, P < 0.05). In the meantime, a decrease of inflammation score in accorda nce with the SSS was also found although the difference was not statistically significant (P > 0.05). Figure 6 display the collagen deposition changes within the IFN-γ treatment.
| Grade* | Score of grade△ | Stage* | Score of stage△ | |
| Before treatment | 2.30 ± 0.98 | 7.30 ± 5.39 | 2.60 ± 0.88 | 8.40 ± 5.83 |
| After 9-month treatment | 1.95 ± 0.69 | 4.90 ± 2.27 | 2.05 ± 0.76 | 5.30 ± 4.05 |
| t | 2.719 | 2.719 | ||
| P | 0.051 | 0.014a |
Table 7 shows the mean serum hepatic fibrosis indices, including HA, LN, PCIII and C-IV, and changes during the entire study. Decreases were present in the four indices (HA: 433.38 ± 373.00 vs 281.57 ± 220.48; LN: 161.22 ± 41.02 vs 146.35 ± 44.67; PCIII: 192.59 ± 89.95 vs. 156.98 ± 49.22; C-IV: 156.30 ± 44.01 vs. 139.14 ± 34.47) and the differences between the four indices were significant (P < 0.05).
As described by Cannon et al[24], the main side-effects in our study during the IFN-γ treatment were: fever (25) , joint pain (13), nausea (1), myagias (18), headache (10), leukopenia (5) and platelet decreases (7). Fever presenting in some patients was temporary and only 1 person had persistent fever (about 38 °C), which disappeared when the treatmentended.
We also performed other routine serum biochemical tests, including ALT, AST and γ-GT, AFP. The results showed that the mean serum ALT, AST, γ-GT levels lowered in those patients as compared with those of IFN-γ treatment. No obvious change in serum AFP level was present within the 9 months (data not shown).
CCl4 is one of the most widely used hepatic toxins for experimental induction of liver fibrosis cirrhosis in laboratory[22,25,26]. The model was used even in 1936. But, disadvantages of the model were apparent, such as higher mort ality rate. For this reason, 205 rats were used to produce CCl4-iduced hepatic fibrosis model in our study. By killing some animals at the end of each week, we successfully got the hepatic fibrosis model at the fourth week. A total of 150 rats were used for the following study. We used DMN-induced hepatic fibrosis model. The characteristics of this model had been described by Madden et al[23]. Low mortality rate, shortproduction period and easy reproduction were the main advantages. We could investigate the effect of IFN-γ on hepatic fibrosis caused by different toxins because the two models had different mechanisms of inducing hepatic fibrosis.
Earlier report on the effect of IFN-γ anti-fibrosis could be found in 1984[8]. Studies in vitro or in vivo later proved that IFN-γ had a better effect on hepatic fibrosis[9-14]. However, IFN-γ and toxins were used at the same time in these studies. In the strict sense, their results only demonstrated that IFN-γ could prevent hepatic fibrosis, but failed to prove the effect of IFN-γ. In this study, IFN-γ was used on the first day of the fifth week when hepatic fibrosis had appeared so as to observe its effect on hepatic fibrosis.
In our study, we substantiated the impression that pathological changes taking place in CCl4-induced model were different from those in DMN-induced model[22,26]. But, all the animals in fibrotic model groups showed complete fibrotic septum, forming a pattern of micronodular cirrhosis. Treatments with different doses of IFN-γ indicated significant decreases in the SSS, liver hydroxylproline content and serum hyarunic acid level. Among the three IFN-γ treatment groups, the best effect was present in the high-dose IFN-γ group.
To our knowledge, this is the first study on the effect of IFN-γ using different doses in animals. Our results showed that the effect of IFN-γ was associated significantly with doses. The higher the dose, the better the effect (Table 1, Table 2, Table 3, Table 4). The dose of IFN-γ used for virus is as high as 600 MU/day. However, in our study, the medium dose of IFN-γ (5 MU/kg/day) used in rats is equal to 100 MU/day used in human. Whether the dose of IFN-γ could be increased clinically needs further studies.
Colchicine was used as the control drug in the experiment. Its effect on hepatic fibrosis in rats was not as good as that of IFN-γ according to the pathological and biochemical examinations (Table 1, Table 2, Table 3, Table 4).
In order to complete the animal experiment, we substantiated the effect of IFN-γ on chronic hepatitis B patients with hepatic fibrosis. Fourty-seven patients conforming to the study criteria were chosen in our study. After a 9-month IFN-γ treatment, 33 patients underwent a second liver biopsy. The results showed that the SSS decreased or unchanged in 30 patients (Table 5). The mean fibrosis score in accordance with SSS decreas ed from 8.40 ± 5.83 to 5.30 ± 4.05 (Table 6). And serum indices (including HA, C-IV, PC III and LN) strengthened the pathological impression. All the four indices decreased significantly after the IFN-γ treatment (Table 7).
It was concerned that IFN-γ would increase inflammation[27]. However, our study showed a decrease of inflammation as compared with the previous IFN-γ treatments although the difference was not statistically significant (Table 6). We chose chronic hepatitis patients for the experiment so as not to cause patients any worries at the stage of acute inflammation. The results showed that IFN-γ treatment was safe for chronic hepatic patients. In the study, we also performed routine serum biochemical tests, including ALT, AST, and γ-GT, AFP. The mean serum ALT, AST, and γ-GT level lowered in those patients as compared with those of IFN-γ treatment. No obvious change in serum AFP level was found within the 9 months.
It is well known that collagen type I is the predominant extracellular matrix protein in fibrosis and cirrhosis but few techniques could discriminate different collagens in the same section[28,29]. We used Sirius red stain to resolve the problem as described by Zhang et al[16]. Under Polarization microscopy, sections stained with Sirius red showed different colors, red and yellow representing collagen type I and green collagen type III.
In our previous report, decrease of serum hepatic fibrosis indices was observed[15] after 3 months of IFN-γ treatment. So, at the earlier stage of the experiment, we adopted a 6-month treatment. There were 2 patients who received a second liver biopsy. No obvious changes were found in liver sections. We considered that a 9-month IFN-γ treatment was appropriate.
In this study, we paid more attention to side-effects. Symptoms including fever, joint pain, myagias, headache, nausea, leukopenia and platelet decrease were found in the experiment. IFN-γ was first used to treatrheumatoid[30,31], for as long as 24-months[24]. No serious side-effects were reported. However, whether IFN-γ may cause any side effects when it is used to treat hepatic fibrosis needs further observations.
We are indebted to Prof. Pei Hui Chen from the Department of Pathology, Second Affiliated Hospital, Medical College, Zhe jiang University for his help with the pathological technique.
Edited by Ma JY
| 1. | Pan X, Ke CW, Pan W, Wu WB, Zhang B, He X, Cao GW, Qi ZT. Construction of eukaryotic expression vector carrying IFN-β gene under control of human HBV promoter. Shijie Huaren Xiaohua Zazhi. 2000;8:520-523. |
| 2. | Chen SB, Miao XH, Du P, Wu QX. Assessment of natural and interleukin-2-induced production of interferon-gamma in pa-tients with liver diseases. China Natl J New Gastroenterol. 1996;2:173-175. |
| 3. | Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol. 1998;4:137-139. [PubMed] |
| 4. | Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38-40. [PubMed] |
| 5. | Liu ZG, Yang JH, An HZ, Wang HY, He FT, Han ZY, Han Y, Wu HP, Xiao B, Fan DM. Cloning and identification of an angiostatic molecule IP10/crg-2. World J Gastroenterol. 1999;5:241-244. [PubMed] |
| 6. | Yin T, Tong SQ, Xie YC, Lu DY. Cyclosporin A protects Balb/c mice from liver damage induced by superan tigen SEB and D-GalN. World J Gastroenterol. 1999;5:209-212. [PubMed] |
| 7. | Zhong QP. Pathogenic effects of Opolysaccharide from Shigella flexneri strain. World J Gastroenterol. 1999;5:245-248. [PubMed] |
| 8. | Granstein RD, Murphy GF, Margolis RJ, Byrne MH, Amento EP. Gamma-interferon inhibits collagen synthesis in vivo in the mouse. J Clin Invest. 1987;79:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Czaja MJ, Weiner FR, Takahashi S, Giambrone MA, van der Meide PH, Schellekens H, Biempica L, Zern MA. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989;10:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Baroni GS, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 204] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776-784. [PubMed] |
| 12. | Weng HL, Cai WM, Wang GY, Chen F, Liu RH. Experiment study of recombinant human gamma-interferon on anti-hepatic fibrosis. Zhonghua Ganzangbing Zazhi. 1999;7:33-35. |
| 13. | Yang Y, Cai W, Jin G. [Dynamic changes in hepatic myofibroblast of rabbits with Schistosoma japonicum]. Zhonghua Yixue Zazhi. 1999;79:870-873. [PubMed] |
| 14. | Li XQ, Zeng MX, Ling QH. Effects of interferon-γ on DNA synthesis and collagen production of cultured rat hepatocytes. Huaren Xiaohua Zazhi. 1998;6:488-490. |
| 15. | Cai WM, Chen Z, Chen F, Weng HL, Liu RH. The primary obser-vation on the effect of γ -interferon for antifibrosis. Linchuang Gandanbing Zazhi. 1998;14:21-23. |
| 16. | Zhang J, He JW, Wang TL, Zhao JB. Discriminate collagen type I and collagen type III by Sirius red stain and polarization microscopy. Zhonghua Binglixue Zazhi. 1996;25:180-181. |
| 17. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1506] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 18. | Chevallier M, Guerret S, Chossegros P, Gerard F, Grlmaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20:349-355. [RCA] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Lortat-Jacob H, Baltzer F, Desmouliere A, Peyrol S, Grimaud JA. Lobular--but not periovular--inhibition of collagen deposition in the liver of S. mansoni infected mice using interferon-gamma. J Hepatol. 1997;26:894-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 202] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Kivirikko KI, Laitinen O, Prockop DJ. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967;19:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 789] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Jiang Z, You DY, Chen XC, Wu J. Monitoring of serum markers for fibrosis during CCl4-induced liver damage. Effects of anti-fibrotic agents. J Hepatol. 1992;16:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Madden JW, Gertman PM, Peacock EE. Dimethylnitrosamine-induced hepatic cirrhosis: a new canine model of an ancient human disease. Surgery. 1970;68:260-267; discussion 260-267. [PubMed] |
| 24. | Cannon GW, Emkey RD, Denes A, Cohen SA, Saway PA, Wolfe F, Jaffer AM, Weaver AL, Cogen L, Gulinello J. Prospective two-year followup of recombinant interferon-gamma in rheumatoid arthritis. J Rheumatol. 1990;17:304-310. [PubMed] |
| 25. | Wang YJ, Wang SS, Bickel M, Guenzler V, Gerl M, Bissell DM. Two novel antifibrotics, HOE 077 and Safironil, modulate stellate cell activation in rat liver injury: differential effects in males and females. Am J Pathol. 1998;152:279-287. [PubMed] |
| 26. | Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Calès P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Alpini G, Elias I, Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Francis H, Lasater J, Richards M, LeSage GD. gamma-Interferon inhibits secretin-induced choleresis and cholangiocyte proliferation in a murine model of cirrhosis. J Hepatol. 1997;27:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Brenner DA, Westwick J, Breindl M. Type I collagen gene regulation and the molecular pathogenesis of cirrhosis. Am J Physiol. 1993;264:G589-G595. [PubMed] |
| 30. | Firestein GS, Weisman MH, Zvaifler NJ. Parenteral IFN-γ in rheu-matoid arthritis: Effect on disease activity and peripheral blood monocytes (abstr). Arthritis Rheum. 1987;30:S115. |
| 31. | Machold KP, Smolen JS. Recombinant human gamma interferon (gifn) in the treatment of rheumatoid arthritis (RA): Double-blind placebo-controlled crossover study (abstr). Arthritis Rheum. 1989;32:S131. |