INTRODUCTION
Liver is one of the important metabolizing organs, and is closely related to many kinds of diseases, such as hereditary disease, infectious disease, metabolic disease, tumors and so on. Since the development of chemical drugs for the trea tment of these diseases is comparatively slow, gene therapy has opened up a prospective way for them[1-5].
The efficient delivery and the high expression of exogenous genes in specific cells or tissues are critical steps for gene therapy both in vitro and in vivo[6-15]. As for the gene therapy of hepatic diseases, efficient delivery of the exogenous genes to the liver and its high expression could increase its local accumulation while minimize the side-effects on other tissues and organs as well.
Both liposome and glyco-poly-L-lysine (G-PLL) are often used as carriers to deliver exogenous genes to the liver in gene therapy experiment[16-22]. Most of the liposomes are cationic when used as the carriers of gene transfe rence. Both in vitro and in vivo studies have showed that liposomes could be applied to transfer exogenous genes to hepatocytes[23,24], but its specificity varies in different studies[24-26]. Poly-L-lysine is a kind of polycation which can be bound to DNA by interacting with the oppositeele ctric charge on DNA. After binding with other specific ligands through covalent linkage, the resulting ligand-poly-L-lysine-DNA complex can be formed[27] and can thus be used to deliver foreign DNA to specific cells or tissues. Similarly, after being saccharified by galactose, glyco-poly-L-lysine (G-PLL) formed is then capable of delivering exogenous genes to the liver specifically [28-30].
Using rats as the experimental animal, we compared the in vivo potency of l iposomes and glyco-poly-L-lysine on delivering the plasmid, which could be expressed in eucaryotic cells.
MATERIALS AND METHODS
Preparation of the carriers
The original plasmid of rat interstitial collagenase was kindly provided by Prof. John J Jeffrey[31], and we reconstructed it with the plasmid of pTa rgetT (TM) (Promega Co., Madison, MI, USA), which could be expressed in eucaryotic cells. We also inserted a segment of nucleotides (GACTACAAGGACGACGATGATAAG) before the terminator codon (TAA) of the rat interstitial collagenase. The ‘Flag Domain’ peptide (DYKDDDDK) encoded by the segment of nucleotide above, which was usually called ‘Tag’, could be fused in the rat interstitial collag enase[32-33] and could be specifically recognized by a M2 monoclonal an tibody (Kodak, New Haven, CT, USA). This recombinant plasmid was named pTM/MMP- 1. The plasmid of pTM/MMP-1 was extracted and purified using QIAGEN-Tip 500 kit (QIAGEN lnc., Valencia, CA, USA) according to manufacturers of the instructions. The plasmid was mixed with different amounts of lipofectamine (GIBCO, Grand I sland, NK, USA) or galactose-terminal glyco-poly-L-lysine (kindly provided by Dr. Shou-Ming Wen of Air-force General Hospital of PLA, China. The mean mol ecu lar weight of this kind of G-PLL was 8500 and the ratio of glactose to poly-L-lysine was 15: 28). The optimal proportion of the plasmid pTM/MMP-1 to lipo fec tamine or galactose-terminal glyco-poly-L-lysine was determined by elect rophoresis in 1% agarose gel.
Animal experiments
Eighteen male Wistar rats, with body weight 130-150 g, were randomly divided into three groups. Lipofectamine intravenious (LI) group: 50 μg of plasmid pTM/ MMP-1 encapsulated by lipofectamine was given through cauda vein; Poly-L-lysi ne intravenous (PI) group: 50 μ plasmid pTM/MMP-1 binding to gala ctose-termi nal glyco-poly-L-lysine was administred through cauda vein; Normal group: control animal. Twenty-four hours, 48 h, 72 h, 1 wk, 2 wk, 3 wk af ter the administration of the plasmid, one rat from each group was randomly selected and anaesthetized with 2% pentobarbital sodium intraperitoneally. Then 1 mL blood from each rat was obtained by cardiac puncture for the assay of alanine transaminase (ALT), aspartic transaminase (AST), and creatinine (Cr) to follow the functions of important organs. After perfusion of the whole body with 20 mL phosphate-buffered saline and 40 mL precold 4% paraformaldehyde through ventricula rinjection, the tissues of liver, spleen, lungs and kidneys were collected and fixed in 4% paraformaldehyde, encapsulated in paraffin and cut into sections of 4 μm thick. The animal experiments above were approved by the Laboratory Animal Committee of Shanghai Medical University.
Immunohistochemistry
Immunohistochemistry was performed according to the literature[34,35]. The first antibody used was M2 monoclonal antibody which was specific for flag-domain tag (Kodak, New Haven, CT, USA) and the second antibody used was Horse anti-mouse IgG, labeled with biotin(Vecter, Burlingame, CA, USA). After the treatment with avidin and biotin (ABC kit, Vecter, Burlingame, CA, USA), color development was followed using dimethylaminoazobenzene (DAB) and counterstained with hematoxylin. Five fields were observed under high power from every immunostained section and the positive signals were counted.
In situ hybridization
The procedure of in situ hybridization was also described previously[34,35]. To state briefly, the oligonucleotide probe (5’-TGGTGTGACTACAAGGACGACGATGATAAG-3’) was synthesized in Cell Biology Institute of Chinese Academy of Sciences (Shanghai), which could hybridize with the (mRNA) of the flag-domain tag in the plasmid pTM/MMP-1, and the 5’ of the probe was labeled with biotin. After the hybridization of the target mRNA with the probe, the rest of the procedure was similar to that followed in immonohistochemistry excluding the step of hematoxylin counterstaining.
Other biochemical assays
ALT, AST, and Cr were assayed using the 7170A Automatic Analyzer (HITACHI, Jap an) to observe the changes in the important organs’ function.
Data analysis
The data was analysed using the software SPSS 7.0 for windows (One-way ANOVA).
RESULTS
Ratio of liposomes and G-PLL to plasmid
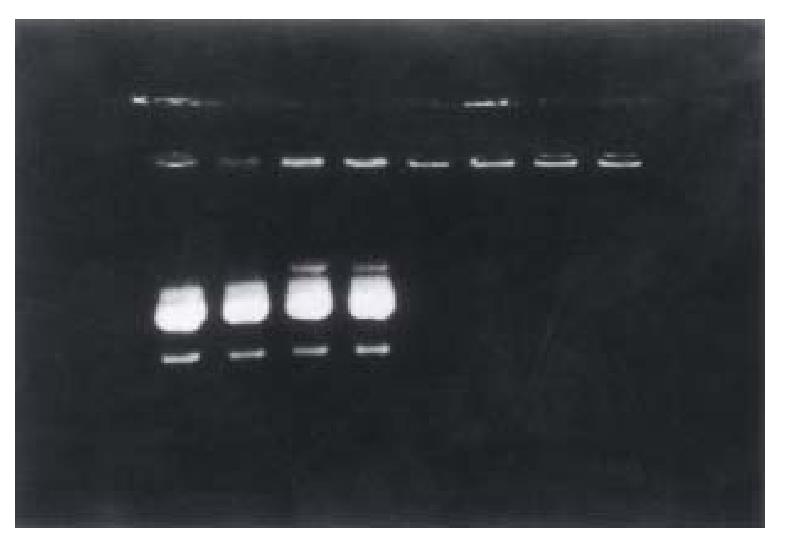
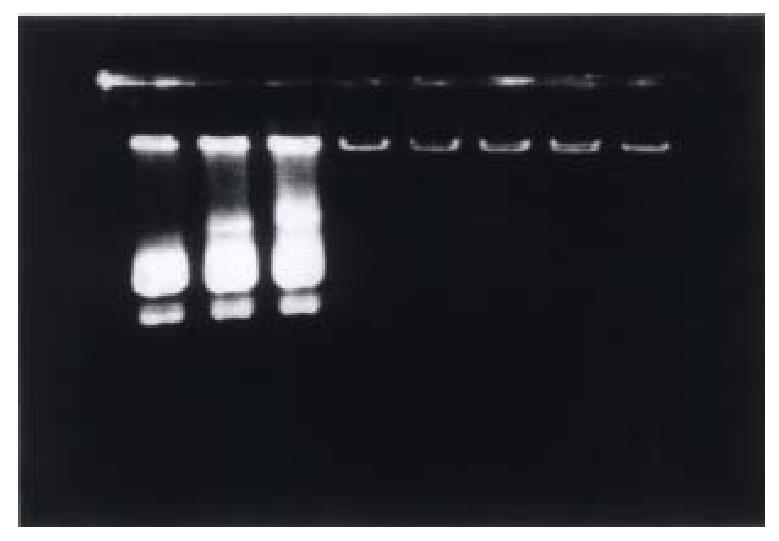
According to the electrophoresis results, we found that 5 μL of li pofectamine could encapsulate 1 μg of plasmid pTM/MMP-1 completely; While 0. 3 μg of galactose-terminal glyco-poly-L-lysine throughly could bind to 1 μg of the pl asmid, which meant that about 72 molecules of G-PLL could carry one molecule of the plasmid pTM/MMP-1 (Figures 1 and 2).
Figure 1 Determination of the optimal proportion of liposome bound to plasmid by 1% agarose electrophoresis.
Lane 1-8 are respectively 1-8 μL lipofectamine mixed with 1 μg pTM/MMP-1 plasmid. Plasmid 1 μg could only be encapsulated complet ely by more than 5 μL lipofectamine.
Figure 2 Determination of the optimal proportion of G-PLL bound to plasmid by 1% agarose electrophoresis.
Lane 1-8 are respectively 0.05 μg, 0.1 μg, 0.2 μg, 0.3 μg , 0.4 μg, 0.5 μg, 1.0 μg, and 1.5 μg G-PLL mixed with 1 μg pTM/ MMP-1 plasmid. Plasmid 1 μg could only been bound completely by more than 0.4 μg G-PLL.
The changes in the functioning of important organs
Compared with the normal group, there was no obvious elevation of the ALT, AS T, and Cr levels in the LI, PI groups.
The distribution and expression of the plasmid in liver, spleen, lung and kidney
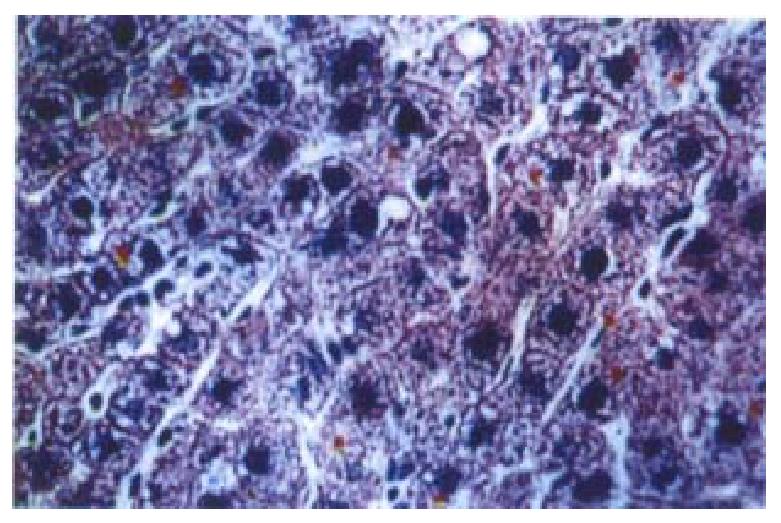
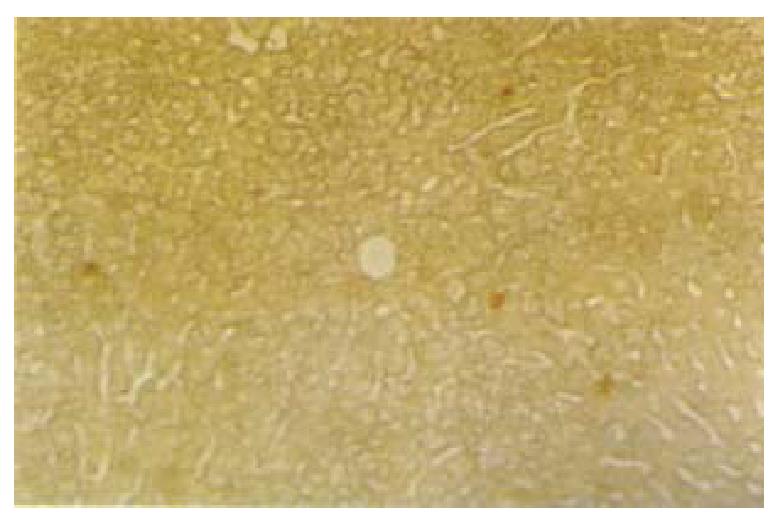
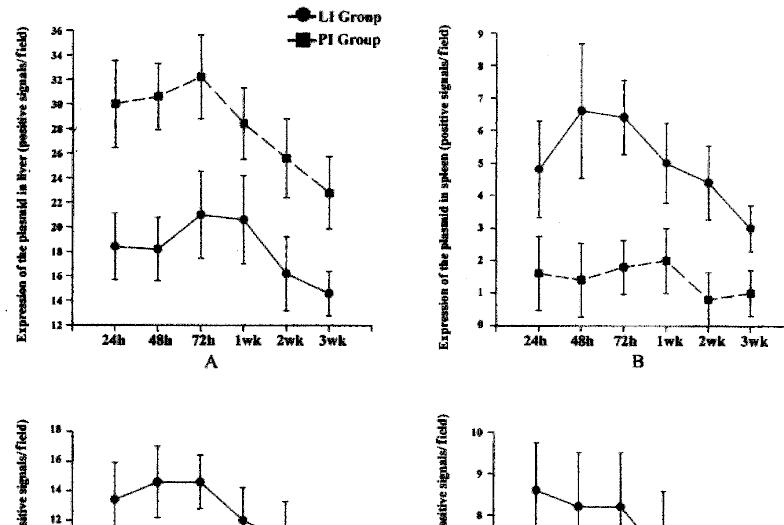
The results of the immunohistochemistry and in situ hybridization showed that the plasmidbinding to liposomes or G-PLL could be expressed in vivo, and the results of immunohistochemistry were more sensitive and stable. In addition, the protein product of the plasmid could be secreted extracellularly (Figures 3 and 4), similar to the expression of interstitial collagenase in the phy siological state[36]. We found that both liposomes and G-PLL could deli ver the plasmid to the liver very efficiently, making liver as its major distribution organ. The obvious expression of the plasmid could be observed 24 h after the administration and began to decrease one week later, although it could still be observed weakly even two or three weeks later (Figure 5). Among the three groups, we also observed that the expression and distribution of the plasmid in the liver was most in the PI group, followed by the LI group. Besides the liver , the exogenous gene could also be expressed highly in lungs, and expressed in ki dney in a relatively lower level in the LI group; while for the PI group, a relatively lower level of the expression could be seen in the kidney, the spleen, and the lung.
Figure 3 Immunostaining of flag-domain tag in the liver of rat 24 h after the admini stration of the plasmid bound to G-PLL (galactose-terminal glyco-poly-L-lysine) via cauda vein.
× 200
Figure 4 In situ hybridization with biotin labeled oligonucleotide probe in the liver 3 wk after the administration of the plasmid encapsulated by liposome (lipofectamine) via cauda vein.
× 100
Figure 5 The distribution and expression of the plasmid bound to liposomes or G-PLL (gal actose-terminal glyco-poly-L-lysine) in different tissues and at differe nt time points.
A: liver, B: spleen, C: lung, D: kidney. LI: plasmid encapsulate d by liposomes given through cauda vein, PI: plasmid bound to G-PLL introduced intravenously.
DISCUSSION
Both liposomes and G-PLL can be used as the targeted carriers to deliver drugs or nucleotides to liver[17-21,24-29], but the comparison of their effects on the liver targeted uptake have not been reported extensively.
Gene transfer mediated by receptors is carried out by high affinity linkage between the ligands (binding to the foreign gene) and specific receptors on the surface of different kinds of cells, and then the foreign gene can be delivered into the cells by phagocytosis[37-40]. There also exist some specific receptors on the surface of hepatocytes such as asialoglycoprotein receptor (ASG P-R)[41,42], which facilitate the delivery of exogenous genes into hepa tocytes specifically using the ligand-receptor interaction. Galactose-terminal glyco-poly-L-lysine contains the saccharide group of galactosan that can be specifically ligated to the ASGP-R on the surface of hepatocytes. At the same time, the cationic poly-L-lysine can bind to nucleotides with high affinity , thus forming a good carrier to deliver exogenous DNA to liver specifically and ste adily[28-30]. It has been reported in the literature, that the ratio of the liver targeted delivery could reach up to 70%-90% in vivo[27,43]. In our experiments we found that in addition to its main locati on in the liver, the plasmid binding to G-PLL could be expressed in kidney, spleen, and lung also. It may be due to the existence of ASGP-R in other extrahepa tic tissues[44,45].
Liposomes are a kind of annular closed vesicles made from double layers of lipid molecules[46]. Having no toxicity and no immunogenicity[47]. Gene transfer mediated by liposomes is achieved by the fusion between liposomes and the membrane of the cells. Liposomes encapsulating the foreign DNA can be integrated with the membrane of the cells, and thus the foreign gene can be deli vered inside the cells by phagocytosis[24,48-50]. Because it contains lecithin and lactosylceramide that can bind to ASGP-R on the surface of hepato cytes specifically, liposome could also be used as targeted delivery carrier to the liver[51,52]. Our experiment al results demonstrated that the plasmid encapsulated by liposomes could also be expressed in lung and spleen to a certain extent despite its major expression in the liver, maybe due to the macrophage system which existed in the lung, spleen, and other tissues. This phenomenon demonstrates further that the efficacy of liver targeted delivery by liposomes is inferior to that of G-PLL.
In this study, we also observed whether liposomes and G-PLL, both binding to the plasmid, would induce some toxicity to the body or not. We did not find any detrimental effects on the functioning of important organs like liver, heart, and kidney and this indicated further that liposomes and G-PLL could be used in vivo safely as delivery carriers for drug or nucleotides.
In conclusion, we found that the plasmid binding to liposomes or G-PLL could be delivered to the liver efficiently and G-PLL was better than liposomes regar ding the distribution and expression of the plasmid in the liver. Both liposomes and G-PLL can be used as carriers to deliver drugs or nucleotides to rat liver, but whether they can be used in human beings in the future deserves further in vestigation.