Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.458
Revised: July 25, 1999
Accepted: September 18, 1999
Published online: October 15, 1999
- Citation: Yuan Z, Wu GY, He YS, Shao CM, Zhan Y. Islet separation and islet cell culture in vitro from human embryo pancreas. World J Gastroenterol 1999; 5(5): 458-460
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.458
Diabetes mellitus is the most common disease and its death rate ranks the 8th in the world. Up to now, its incidence has a tendency to increase[1]. Since disorder of sugar metabolism might result in microvascular degeneration and injury of important human organs, it will endanger human health seriously. In 1969, Younszai first reported the method that could decrease diabetes symptoms by islet tissue transplantation. In 1981, our country began to treat diabetes type I by transplanting cultured islet tissues. In our study, we digested the pieces of pancreas with collagenase and made morphological observation on islet cells cultured for 3 d, 5 d, 7 d, 9 d and 13 d respectively, and the contents of insulin and C-peptide in the supernatant were detected by radioimmunoassay. The experimental model of rabbit diabetes was established, and certain curative effect was achieved in the treatment of experimental diabetes in rats.
Four cases of 18 wk-28 wk human embryo induced by hydrostatic bag were sterilized with 75% alcohol, and their pancreas were removed with the surrounding connective tissues eliminated. After the pancreas were washed with cold Hank’s balanced salt solution, they were cut into 1 mm fragments digested three times with 0.5 g/L collagenase (Sigma Type V 663 U/mg) and shaken thoroughly. Then the islets were carefully isolated under stereoscope (islets are white with different dimensions), and washed two times with Hank’s balanced salt solution and put into glass bottles to be cultured.
Islet cells were cultured with RPMI-1640 medium containg 20% bovine serum, 10 mmol/L glutamine, 80 U penicillin and 0.5 g streptomycin. Approximately 30 islets were inoculated in the 5 mL bottles, and were cultured in CO2 incubator (95% atmosphere, 5% CO2, 37 °C). The cells were digested by 0.25% sodium citrate and their fibroblast was cleaned[2]. The medium solution was replaced every 2 d after the 3 d. One mL-2 mL culture solution was put into clean bottles which were placed into refrigerater (4 °C) to detect the contents of insulin and C-peptide.
In the process of culture, growth of islet cells was observed under invert microscope, and the islet cells cultured for 3 d, 5 d, 7 d, 9 d and 13 d respectively were observed under transmission electron microscope. The samples were fixed with 1.25% glutaraldehyde, and embedded with epoxy 618, sectioned with LKB-V ultramicrotome, and observed under JEM-100CX electron microscope.
FT-630G computer with multiprobe γ counter and kit of De Pu Company were used to collect the supernatant fluid of islet cells cultured for 3 d, 5 d, 7 d, 9 d and 13 d respectively by strict standard operation, and contents of insulin and C-peptide in the culture suspension were measured by radioimmunoassay (RIA) with antigen labeled by radionuclide 125I.
Fasting blood sugar, insulin and C peptide were detected in 18 adult rabbits (9 male and 9 female weighing 1.5 kg-2.0 kg). All rabbits were given injection of alloxan of 150 mg/kg into the posterior auricular vein to establish the model of rabbit experimental diabetes. Their biological behavior was observed after 48 h, and the contents of blood sugar, insulin and C-peptide were measured[3]. The cultured rabbit embryo islet cells were injected (1.1 × 107 cells/each) into their pancreatic artery after 72 h and the experimental treatment was evaluated.
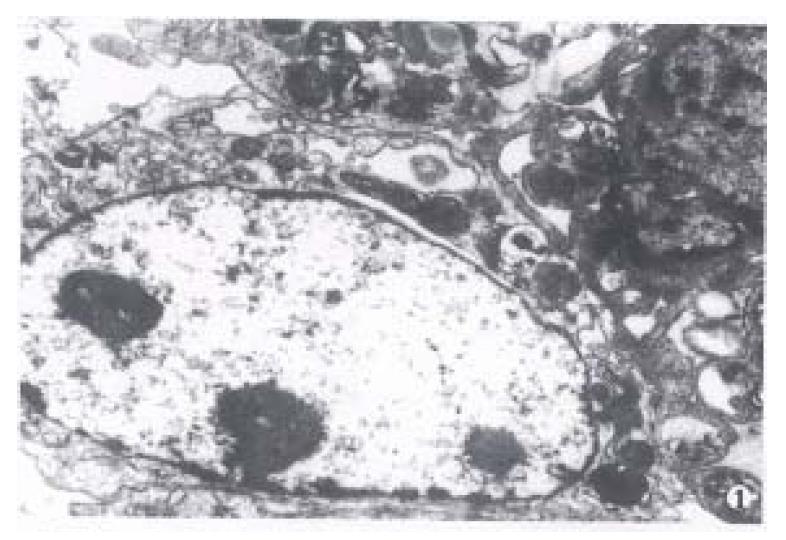
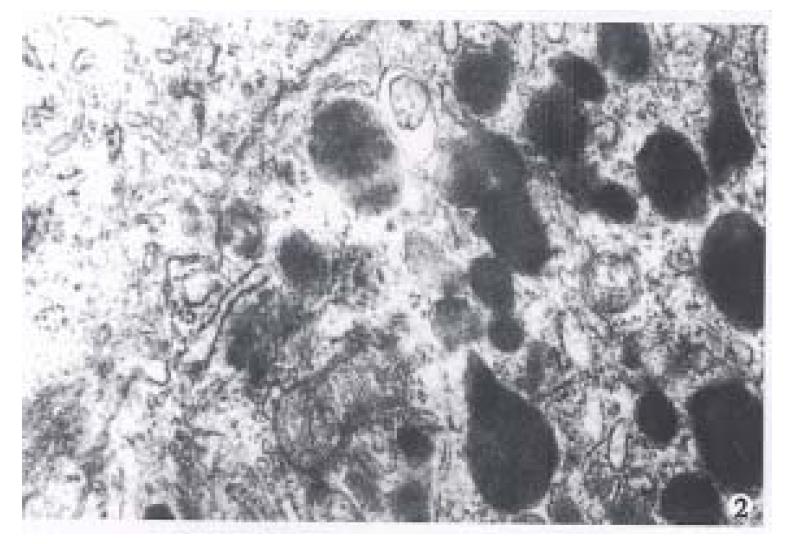
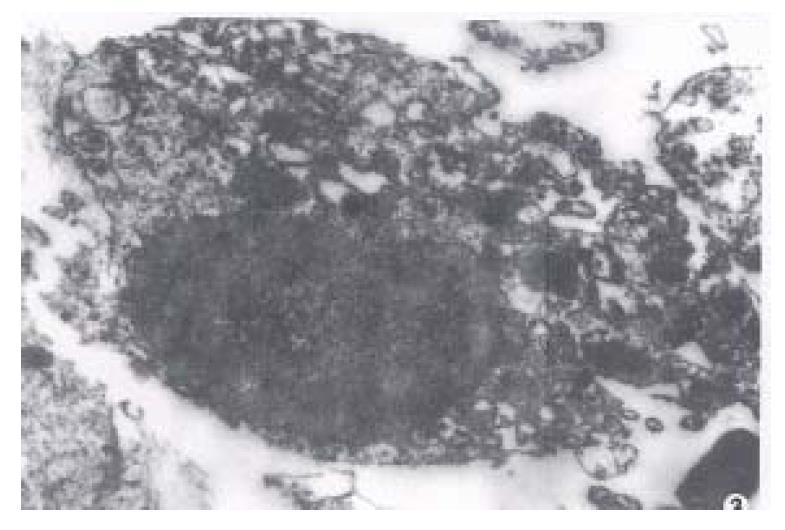
After the islets were cultured for 24 h, fibroblast growth was found on the wall of glass bottle, but no islet cell mass was found. After the fibroblasts were eliminated with citrate sodium, islet cells began to grow and form a single layer of cells with typical morphology. Under invert microscope, the cells were found growing quite well, most of them were of epithelioid type with plenty of cytoplasm. The cell number was counted directly after stained with trypan blue and their survival rate reached up to 90%. Many cells were observed under transmission electron microscope and most of them were found to be beta cells with m any β-granules, alpha cells, and a few extracrinous cells and macroph0ages. These beta cells after cultured for 5 d-9 d with a high density cytoplasm and cytoplasmic β-granule developed well. But eleven days later, the number of cytoplasmic granules decreased with karyopyknosis and degeneration in them(Figure 1, Figure 2, Figure 3).
| Islet cells (culture days) | Contents of insulin (IU/L) | Contents of C-peptide (mg/L) |
| 3 | 47.30 | 3.05 |
| 5 | 64.75 | 6.05 |
| 7 | 72.30 | 6.20 |
| 9 | 72.70 | > 6.00 |
| 11 | 68.75 | 5.15 |
| 13 | 72.20 | > 6.00 |
The quality and quantity of islet cells play a crucial role in the effectiveness of transplantation. An ideal preparative method can provide sufficient pure, viable and functional islet cells. We have achieved good digestive effect by digesting pancreas with collagenase at optimum pH and temperature[4]. In order to obtain good islets, it is necessary to control digestive time and mix the pieces of pancreas with collagenase thoroughly. The islet cells obtained were growing and their structure was not mature before they were cultured for 5 d. But after 5 d-9 d, they grew well with their structure fully matured. The survival rate was 90% according to the trypan blue stainning. However, eleven days later, most cells became ageing with karyopyknosis and cytoplasmiclysis.
Activity and function are the important indexes in assessing the effect of islet cells. The contents of insulin and C-peptide in the culture suspension measured by radioimmunoassay were two times higher than those in normal control serum, indicating that our method is rather good. The highest amount of contents of insulin and C-peptide was found after they were cultured for 13 d, but was not in accordance with the morphological data. The reason why their contents increased in insulin culture solution was aging and released the remaining insulin that made insulin contents of the degeneration of most cells and release of the remaining insulin from cells from the 11th day.
We have used the same method to culture rabbit islet cells and made experimental treatment of rabbit diabetes model after rabbit islet cells were cultured for 7 d. Blood sugar was 1.052 g/L ± 0.5012 g/L and 4.7979 g/L ± 0.9233 g/L in normal and experimental rabbits. Three days after the rabbits were given an injection via pancreatic artery at a density of 1.1× 107 cells/each islet cell, their blood sugar decreased to 3.3193 g/L ± 0.4110 g/L. Compared with diabetes rabbit (P < 0.01), the difference was significant. Serum insulin contents increased from 2.65 IU/L ± 1.4 IU/L to 13.88 IU/L ± 1.5 IU/L (P < 0.05), the difference was also significant. All these indicated that experimental treatment was effective.
In the past ten years, most tissue transplantation focused on subcutaneous and intramuscular transplantation of cultured islets. Present method is to treat diabetes type I with cultured islet cells which were planted by portal vein[5].
Therefore, we believe that the culture of the islet cells by this method can be used to treat diabetes type I, and the optimum time of transplantation is 5-9 d after the islet cells were cultured.
Edited by Wang XL
| 1. | Fu YF, Gu BF, Zhang HD, Ye RS. The present clinical situation of islet transplantation in our country-analysis of clinical material in 416 cases diabetes patients. Zhonghua Qiguanyizhi Zazhi. 1988;9:102-104 (in Chinese with English abstract). |
| 2. | Burghen GA, Murrell LR. Factors influencing isolation of islets of Langerhans. Diabetes. 1989;38 Suppl 1:129-132. [PubMed] |
| 3. | Li SG. Animal models of diabstes. In: Xi SY, Bian RL, Chen X, eds. The experimental methodology of pharmacology. Beijing, People's Health Publishing House. 1982;987-989 (in Chinese). |
| 4. | Maitland JE, Parry DG, Turtle JR. Perifusion and culture of human fetal pancreas. Diabetes. 1980;29 Suppl 1:57-63. [PubMed] |
| 5. | Lacy PE. Treating diabetes with transplanted cells. Sci Am. 1995;273:50-51, 54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |