Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.455
Revised: June 20, 1999
Accepted: August 20, 1999
Published online: October 15, 1999
- Citation: Ji F, Wang WL, Yang ZL, Li YM, Huang HD, Chen WD. Study on the expression of matrix metalloproteinase-2 mRNA in human gastric cancer. World J Gastroenterol 1999; 5(5): 455-457
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.455
During tumor invasion and metastasis, malignant cells in primary site acquire the ability to degrade extracellular matrix (ECM) and penetrate tissue barriers. Among the proteolytic enzymes which degrade ECM, matrix metalloprotenase (MMP) is one of the important ones. MMP2 (72 kDa type IV collagenase) is a member of the MMPs gene family which degrades the macromolecules of connective tissue and ECM, such as collagen, proteoglycans, laminin and fibronectin. Thus MMP2 is believed to play an important role in tumor invasion and metastasis. Several immunohistochemical studies have shown that MMP2 mRNA is overexpressed in gastric cancer and related to the clinical stage of cancer[1,2]. However, samples were not enough and lack completeness in these studies. Reverse trans criptase-polymerase chain reaction (PT-PCR) was used in our study to examine the expression of MMP2 mRNA in tumor and normal tissues adjacent to human gastric cancer.
The samples, including tumour tissue and tumour-adjacent normal tissue (2 cm and 5 cm or over 5 cm distance from the tumour) came from twenty gastric cancer patients at the surgery department of our hospital and People’s Hospital of Yun-He County, Zhejiang. Gastric tissues from five benign ulcer patients after partial gastrectomy were used as controls. The histological diagnosis was made by the pathologists of these two hospitals. Patients’ clinical features are shown in Table 1. Among these cancer patients, five and fifteen were cases of early and advanced cancer respectively. All samples were quenched in liquid nitrogen immediately after operation and were then stored at -70 °C until used for the study.
| No. | Age/sex | Location | Histology | Clinical stageb | MMP2 mRNA expression | ||
| T | 2 cm | ≥ 5 cm | |||||
| 1a | 64/M | Pylorus | Poor | T1N0M0 (I) | - | - | - |
| 2 | 48/M | Body | Poor | T4N0M0 (IV) | + | + | - |
| 3 | 72/F | Lesser curve | Poor | T4N2M1 (IV) | + | + | - |
| 4 | 75/F | Lesser curve | Poor | T3N0M0 (II) | + | + | - |
| 5 | 71/M | Body | Poor | T2N1M0 (IVA) | + | + | - |
| 6 | 63/M | Lesser curve | Well | T4N2M0 (IV) | - | - | + |
| 7a | 73/F | Lesser curve | Poor | T1N1M0 (IIIA) | + | - | - |
| 8a | 46/M | Lesser curve | Poor | T1N0M0 (I) | - | - | - |
| 9 | 60/F | Body | Poor | T3N0M0 (II) | + | - | - |
| 10 | 57/M | Pylorus | Poor | T3N1M1 (IV) | + | + | + |
| 11 | 53/M | Body | Poor | T4N1M0 (IV) | + | + | - |
| 12a | 72/F | Antrum | Poor | T1N0M0 (I) | - | - | - |
| 13 | 59/M | Body | Poor | T3N2M0 (IIIB) | + | - | - |
| 14a | 38/M | Antrum | Well | T1N0M0 (I) | - | - | - |
| 15 | 66/F | Cardia | Poor | T3N1M0 (IIIA) | + | + | + |
| 16 | 55/M | Antrun | Well | T2N0M0 (IB) | - | - | - |
| 17 | 74/M | Body | Poor | T4N2M1 (IV) | + | + | + |
| 18 | 67/M | Cardia | Well | T3N2M0 (IVB) | - | - | - |
| 19 | 67/F | Body | Poor | T4N2M1 (IV) | + | + | + |
| 20 | 56/M | Body | Poor | T4N2M1 (IV) | + | + | + |
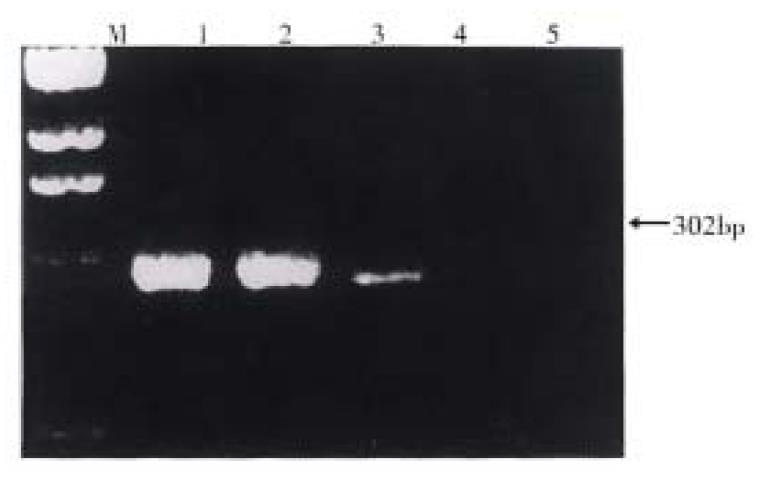
The dNTP, RNAsin and MMLV reverse transcriptase and Taq DNA were provided by Str atagene, La Jolla, CA, U.S.A. MMP2 primer pair was synthesized by Shanghai Cell Research Institute, Chinese Academy of Sciences[2] and its sense and anti-sense were 5’-ACAAAGAGTGGCAGTGCAA-3’ and 5’-CACGAGCAAAGGCATCATCC-3’ respectively. The expected size of MMP-2 product was 302 bp.
Total RNA was extracted from frozen tissues by cesium chloride purifing method. A total amount of 20 µL reaction solution contained 5 µg RNA sample tissue, 1 mmol/L dNTP, 10 U RNAasin, 100 mmol/L Tris-HCl pH8.4, 50 mmol/L KCl, 2.5 mmol/L MgCl2, 100 mg/mL BSA, 100 pmol-random six-polyoligo-nucleotide and 100 U MMLV reverse transcriptase. The reverse transcriptional condition was 37 °C for 1 h, and 95 °C for 5 min. Twenty µL cDNA reverse transcriptase product was put in PCR reaction solution containing 100 mmol/L Tris-HCl, pH8.4, 50 mmol/L KCl, 2.5 mmol/L MgCl2, 100 mg/mL BSA, 30 pmol sense and anti-sense primers, and then 2 U Taq DNA polymerse was added in the solution. The PCR amplification condition was: denatured at 95 °C for 1 min, annealed at 65 °C for 1 min and extended at 72 °C for 1min. The number of cycles was 35.10 µL DNA, amplificating product was subjected to electrophresis in 4% agarose gel, stained with ethidium bromide and observed under ultroviolet light. The photographs of PCR results were used to measure the level of optical density (OD) of MMP-2 cDNA bands with densitometry (Backman CD 2000).
The significance of differences in expression rates and OD levels among groups was determined by χ2 test and Student’s t test respectively.
In 20 cases of gastric cancer, MMP2 mRNA was expressed in 13 tumor tissues, 11 in 2 cm and 6 in ≥ 5 cm adjacent tissues respectively (Table 1). The positive rate of MMP2 mRNA expression in tumor tissues was significantly higher than that in ≥ 5 cm adjacent tissues (P < 0.05). There was no positive expression of MMP2 mRNA in the 5 samples of the control group.
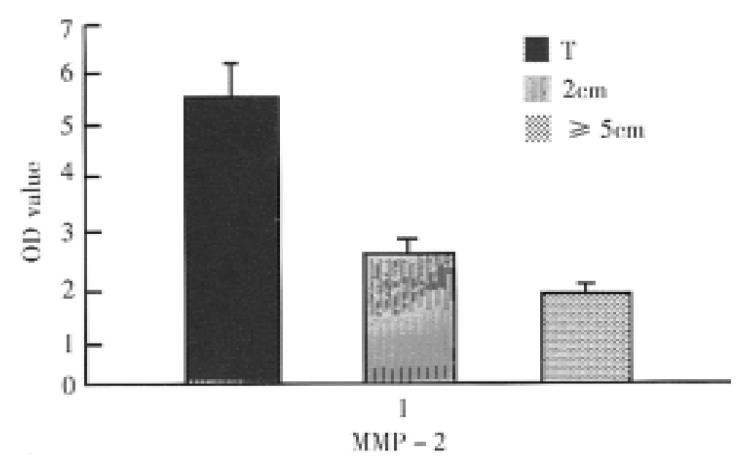
The OD of MMP-2 detected cDNA signals ranged from 1.10 to 19.23 (mean 5.38 ± 0.98) in tumor tissues, 0.86 to 4.17 (mean 2.41 ± 0.30) in 2 cm and 0.78 to 3.80 (mean 1.88 ± 0.22) in ≥ 5 cm adjacent tissues respectively. There was significant difference in OD levels between tumor tissues group and 2 cm or ≥ 5 cm tumoradjacent tissues one (P < 0.01), and no significant difference in OD levels between the two adjacent tissues groups.
The MMP-2 cDNA signals in tumour and 2 cm adjacent tissues of 15 advanced cancer were significantly higher than those in the two corresponding tissues of 5 early cancer respectively (P < 0.05). In the ≥ 5 cm adjacent tissues, there was no-significant difference in the signals between the advanced and early cancer.
In 20 gastric cancer cases, 13, 11 and 6 cases positively expressed MMP-2mRNA in tumor, 2 cm adjacent and ≥ 5 cm adjacent tissues respectively. The cases with MMP-2 mRNA expression in tumor tissues and their corresponding tumor adjacent normal tissues (2 cm and/or ≥ 5 cm) were almost poorly differentiated adenocarcinoma and in higher clinical stage. MMP-2 mRNA was seldom expressed in tumor and tumor adjacent tissues of well differentiated or early carcinoma. These results showed that proliferative and invasive gastric cancer cells had higher MMP-2 secretion. In ultrastructural study, MMP-2 mRNA was expressed markedly in cancer cells with rich false feet and rapid movement in culture, but insignificantly or with few false feet in cancer cells from unmetastatic and uninvasive gastric cancerous tissues, indicating that MMP-2 secretion was correlated with the invasion and metastasis of gastric cancer[3]. In addition, some immunohistochemistry studies have shown that the positive rate of staining cells for MMP-2 protein was consistently higher in poorly differentiated and diffuse gastric carcinoma than that in well differentiated and early gastric carcinoma[1,4]. The results of these studies were very similar to those of MMP-2 mRNA expression in our study, indicating that the increased MMP-2 positive staining was related to the overexpression of MMP-2 mRNA. This is probably due to increase in MMP-2 transcriptional activity and MMP-2 protein products in the cell proliferative process of gastric cancer which is often accompanied with the acceleration of cancer invasion and metastasis.
In the current study, although the levels of MMP-2 cDNA signals in tumor-adjacent tissues (2 cm and/or ≥ 5 cm) were lower than those in tumor tissues, MMP-2 mRNA was overexpressed in the tumor adjacent tissues in certain extent, suggesting that both gastric cancer cells and adjacent mesenchymal cells, including fibrocyte, endothelium cell, macrophage and lymphocyte have the ability to secrete MMP-2. There may be information exchange between the cancer cells and these mesenchymal cells through the dissolvable intercellular substance and membrane cement factor, and such information exchange may regulate the production of MMP-2. This may be very important in elucidating the mechanism of invasion and metastasis of cancer cells[5,6]. In our case No.6, MMP-2mRNA was detected only in tumor-adjacent tissues, but not in tumor tissue. The reason for this is unclear. The discrepancy may be due to the necrotic tumor tissue. Overexpression of MMP-2 mRNA only in tumour adjacent tissues also indicates the malignant degree of cancer is rather high.
The prognosis of early gastric cancer is better than that of advanced cancer. Our study showed that the levels of MMP-2 cDNA signal in advanced cancer tissues ( tumor and 2 cm tumor-adjacent tissues) were significantly higher. This suggests MMP-2 may play a role in gastric cancer invasion and metastatic progression, and the overexpression may be associated with poor prognosis. Further study on relationship between the expression of MMP-2 mRNA and the survival rate of gastric cancer is being carried out.
Edited by Ma JY
| 1. | Grigioni WF, D'Errico A, Fortunato C, Fiorentino M, Mancini AM, Stetler-Stevenson WG, Sobel ME, Liotta LA, Onisto M, Garbisa S. Prognosis of gastric carcinoma revealed by interactions between tumor cells and basement membrane. Mod Pathol. 1994;7:220-225. [PubMed] |
| 2. | Huang Y, Ti TK, Moochhala SM. Expression of matrix metallprotinases (MMP-2 and MMP-7) mRNA in human gastric cancer. Med Sci Res. 1997;25:405-407. |
| 3. | Schwartz GK, Wang H, Lampen N, Altorki N, Kelsen D, Albino AP. Defining the invasive phenotype of proximal gastric cancer cells. Cancer. 1994;73:22-27. [PubMed] |
| 4. | D'Errico A, Garbisa S, Liotta LA, Castronovo V, Stetler-Stevenson WG, Grigioni WF. Augmentation of type IV collagenase, laminin receptor, and Ki67 proliferation antigen associated with human colon, gastric, and breast carcinoma progression. Mod Pathol. 1991;4:239-246. [PubMed] |
| 5. | Poulsom R, Pignatelli M, Stetler-Stevenson WG, Liotta LA, Wright PA, Jeffery RE, Longcroft JM, Rogers L, Stamp GW. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol. 1992;141:389-396. [PubMed] |
| 6. | Grigioni WF, D'Errico A, Fiorentino M, Baccarini P, Onisto M, Caenazzo C, Stetler-Stevenson WG, Garbisa S, Mancini AM. Gelatinase A (MMP-2) and its mRNA detected in both neoplastic and stromal cells of tumors with different invasive and metastatic properties. Diagn Mol Pathol. 1994;3:163-169. [PubMed] |