Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.440
Revised: June 16, 1999
Accepted: August 21, 1999
Published online: October 15, 1999
- Citation: Xu W, Wu SG. The possible relationship between hepatomegaly and release of HGF into plasma induced by clofibrate in rats. World J Gastroenterol 1999; 5(5): 440-442
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.440
Hepatocyte growth factor (HGF) is a newly discovered multifunctional growth factor, and the most potent mitogen known for hepatocyte[1]. It has been shown that recombinant human HGF can protect animal liver against the damaging action of carbon tetrachloride[2]. However, the generation of HGF is also associated with certain liver diseases and several extra-hepatic diseases[3-5]. For example, after partial hepatectomy or carbon tetrachloride poisoning, HGF level in rat plasma increases markedly[3,4]. Therefore, it is believed that HGF plays an important role in the restoration and regeneration of liver after injury.
As hypolipidemic drugs, such as clofibrate, may cause experimental hepatomegaly, proliferation of hepatic peroxisomes and hepatic carcinoma, they are taken as a unique class of carcinogens[6-8]. How the HGF level changes after liver damage due to clofibrate or the like has not been reported. This study was designed for examination of the relation between hepatomegaly and the changes of plasma HGF levels in rats caused by clofibrate.
Male Wistar and Sprague-Dawley rats were supplied by the Laboratory Animal Center of the First Military Medical University.
Clofibrate ( bought from Shanghai 19th Pharmaceutical Factory) was given at doses of 500, 400, 300, 200 or 100 mg·kg-1·d-1 by adding it into food.
At different time intervals after clofibrate administration, the rats were killed with their livers weighed, and the weight was expressed as the percentage of body weight.
At different time intervals after clofibrate administration, the tail of the rat was severed with a knife and the whole blood was collected. After centrifuge, the serum was stored at -20 °C in a freezer.
Isolation of rat hepatocytes Male Sprague-Dawley rats were supplied by the Laboratory Animal Center of the First Military Medical University. The rats were 6-7 weeks old, weighing about 200 g. They were fasted for 8 h-10 h before operation, then were anesthesized with ether. Ane sthesia was discontinued when the respiration turned from rapid and shallow to slow and deep. The rats were fixed on a wooden plate, and the whole abdomen was sterilized with 750 mL/L (V/V) alcohol. The peritoneal cavity was cut open along the midline, and the portal vein and the inferior vena cava were exposed accordingly. The distal part of the portal vein was ligated, and a cannula was inserted into the proximal portal vein. D-Hank’s solution (preheated to 37 °C) was used for the infusion of liver, and at the same time, the inferior vena cava was cut open for blood-letting. The rate of infusion was about 30 mL·min-1-40 mL·min-1 from slow to rapid to wash out any stagnant blood from the liver. After infusion, the surface of liver became smooth and wet, and light-yellow in color. The infusion fluid was then changed to 0.3 g·L-1 collagenase solution. Small vacuoles formed slowly on the connective tissue membrane of the liver surface with gaps in the liver tissue under the vacuoles. Eye-forceps were used to press lightly on the surface, and when the pressure was relieved, the infusion was stopped. The capsule of the liver was then torn open very carefully, and the liver tissue was put into Ham-F12 hepatocyte culture medium (preheated to 37 °C), and was blown mildly with a pipette into dispersed free liver cells, which were then filtered through nylon nets of 200 and 400 mesh size, and were centrifuged with 50 × g three times at 4 °C. Thus, the hepatocytes with more than 90% viability could be obtained. The cell density was adjusted to 1.5 × 105/mL, and the cells were plated in a 96-well culture plate at 200 μL per well. These liver cells were incubated in 50 mL/L CO2 for 24 h. After the cells adhe red to the vessel wall, the culture media were replaced by serum-free ones and incubated for another 24 h. Afterwards, 3H-TdR (185 MBq·L-1) and the animal serum to be tested (which was diluted to different concentrations) were added and incubation continued for 24 h. Standard recombinant human HGF (to replace the animal serum) was used as the positive control. After incubation, the cells were harvested with a multi-channel cell collector and cpm values were measured with a liquid scintillation counter (Beckman Co., USA, Model LS9800).
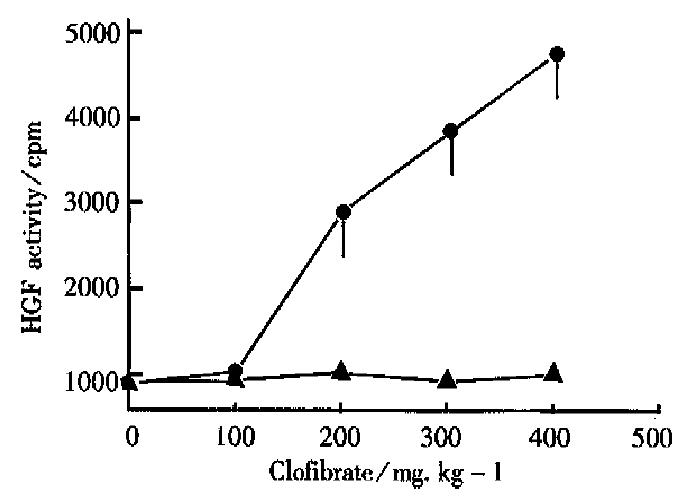
As shown in Figure 1, in the normal control group, the cpm value caused by the incorporation of 3H-TdR in the primary liver cells was 800 ± 150, as it was promoted by the serum of the normal rats. However, in the clofibrate (100 mg·kg-1·d-1) group, the undiluted rat serum caused significant increase of the cpm value to 1010 ± 180. Moreover, in pace with the increasing dosage of clofibrate, the cpm value increased also. When the dose of clofibrate increased to 500 mg·kg-1·d-1, the cpm value reached as much as 4800 ± 810, so the dose-effect relationship was eminent.
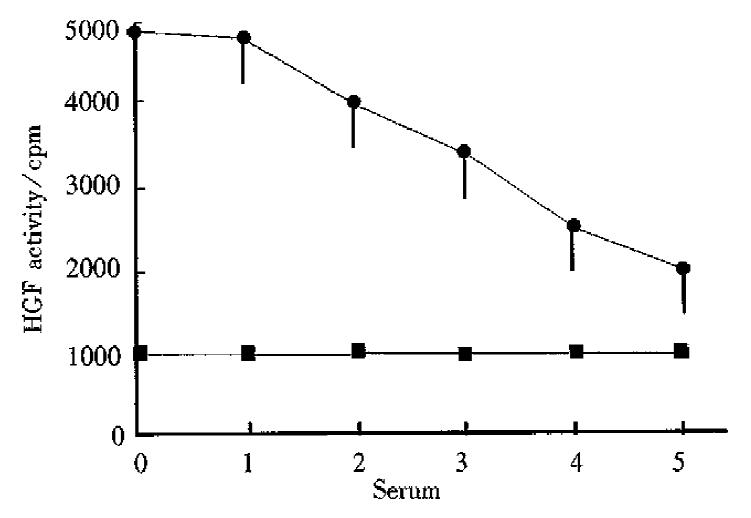
Rats were given 500 mg·kg-1·d-1 of clofibrate with their serum collected 4 d after. We could see that when the serum was diluted sequentially, the increased cpm value would fall down gradually, showing good dose-effect relationship. After the serum was diluted five-fold, the cpm value was still higher than that of the normal control group (Figure 2).
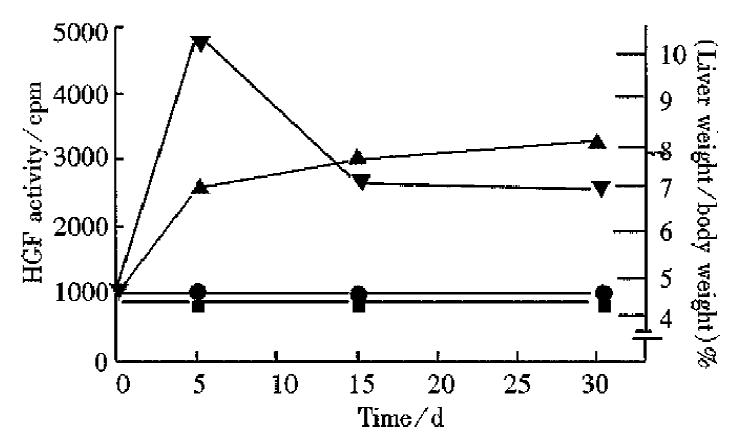
As shown in Figure 3, 4 d after clofibrate administration, HGF level in serum reached its peak, and then fell gradually. Two weeks after taking clofibrate, HGF level still remained high, about 2-fold of the normal level while hepatomegaly also reached its highest level 2 weeks after intake of drug, and remained at this high level (1.8-fold above the normal level).
It was discovered in our study that after administration of clofibrate which can induce hepatomegaly, the HGF activity in rat serum increased significantly.
Previous studies by other investigators indicated that 24 h after carbon tetrachloride (a hepatotoxin) administration, the HGF level in rat plasma increase d by over 20-fold of the normal level[3]. In patients with partial hepatectomy or with severe fulminating hepatitis, the releasing of HGF into plasma was closely related to liver injury[4]. Many experiments indicate that clofibrate-related compounds can induce hepatomegaly, proliferation of hepatic peroxisomes, and hepatic carcinoma[6]. It is shown in our experiments that the release of HGF induced by clofibrate reached a high level 4 d after the drug administration, and could maintain for a long time, while hepatomegaly reached its peak 2 weeks after drug administration and remained at a relatively steady level. So the HGF level is somewhat related to hepatomegaly, suggesting that hepatomegaly induced by clofibrate may depend partly upon the promoting action of HGF on liver regeneration. Although the mechanism of liver injury by clofibrate and carbon tetrachloride is different, both of them can induce liver injury, which is a signal leading to the generation of HGF. Via the action of HGF. DNA synthesis in liver is promoted, which ultimately results in repair and regeneration of the injured liver. Therefore, HGF plays an import ant role in the repair and regeneration of liver. However, the mechanism through which the release of HGF is promoted by these two compounds (clofibrate and carbon tetrachloride) is still unclear and needs further studies.
Edited by Wang XL
| 1. | Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, McMaster P. Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest. 1991;87:1853-1857. [PubMed] |
| 2. | Takehara T, Nakamura T. Protective effect of hepatocyte growth factor on in vitro hepatitis in primary cultured hepatocytes. Biomed Res. 1991;12:354-358. |
| 3. | Asami O, Ihara I, Shimidzu N, Shimizu S, Tomita Y, Ichihara A, Nakamura T. Purification and characterization of hepatocyte growth factor from injured liver of carbon tetrachloride-treated rats. J Biochem. 1991;109:8-13. [PubMed] |
| 4. | Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (Hepatopoietin A) rapidly increases in plasma before DNA syn-thesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743-749. |
| 5. | Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, Shin S. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630-1633. [PubMed] |
| 6. | Reddy JK. Hepatic peroxisome proliferative and carcinogenic ef-fects of hypolipidemic drugs. In: Remo Fumagalli, eds. Drugs af-fecting lipid metabolism, Paolett. New York: Remo Fumagall, David Kritchevsky and Rodolfo. 1980;301-309. |
| 7. | Wu SG, Shen YA, Zheng Z, Long K. [Effects of clofibrate, silybin, safflower oil and lamindran on liver peroxisome proliferation and enzyme activities in rats]. Zhongguo Yaoli Xuebao. 1986;7:283-284. [PubMed] |
| 8. | Wu SG, Long K. Hypolidemic drug and oncogenesis. Guowai Yixue Zhongliuxue Fence. 1985;12:76-78. |
| 9. | McGowan JA, Strain AJ, Bucher NL. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981;108:353-363. [PubMed] |