Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.391
Revised: August 20, 1999
Accepted: September 20, 1999
Published online: October 15, 1999
AIM: To investigate the pathophysiology of the digestive tract in patients with liver cirrhosis.
METHODS: In 42 cirrhotic patients and 20 control subjects, the following fecal proteins were measured by enzyme-linked immunosorbent assay: albumin (Alb), transferrin (Tf), and α1-antitrypsin (α1-AT) as a marker for intestinal protein loss, hemoglobin (Hb) for bleeding, PMN-elastase for intestinal inflammation, and secretory IgA for intestinal immunity.
RESULTS: The fecal concentrations of Hb, Alb, Tf, α1-AT, an d PMN-elastase were increased in 13 (31%), 8 (19%), 10 (24%), 6 (14%), and 11 (26%) cases among 42 patients, respectively. Fecal concentration of secretory IgA was decreased in 7 (17%) of 42 patients. However, these fecal concentrations were not related to the severity or etiology of liver cirrhosis. The serum Alb level was significantly decreased in patients with intestinal protein loss compared to that in patients without intestinal protein loss.
CONCLUSION: These findings suggest that: ① besides the well-known pathological conditions, such as bleeding and protein loss, intestinal inflammation and decreased intestinal immunity are found in cirrhotic patients; ② intestinal protein loss contributes to hypoalbuminemia in cirrhotic patients, and ③ intestinal inflammation should not be over looked in cirrhotic patients, since it may contribute to or cause intestinal protein loss and other various path ological conditions.
- Citation: Saitoh O, Sugi K, Kojima K, Matsumoto H, Nakagawa K, Kayazawa M, Tanaka S, Teranishi T, Hirata I, Katsu KI. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol 1999; 5(5): 391-396
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/391.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.391
Gastrointestinal symptoms are common in patients with liver cirrhosis and portal hypertension. Their pathophysiology remains, for the most part, obscure. It is well known that esophageal varices and portal hypertensive gastropathy[1,2] are common causes of upper gastrointestinal (GI) bleeding in patients with liver cirrhosis. Recently, portal hypertensive colopathy, such as colonic vascular ectasia and rectal varices, were recognized as causes of lower gastrointestinal bleeding in cirrhotic patients[3,4]. Other than bleeding, various pathological conditions such as malabsorption[5,6] and intestinal protein loss[7,8] have been reported in cirrhotic patients. We developed fecal tests that are useful for evaluating the various pathophysiologies of the digestive tract[9-12]. Fecal hemoglobin (Hb) is a useful marker of bleeding. Fecal albumin, transferrin (Tf), and α1-antitrypsin (α1-AT) are useful markers of intestinal protein loss. Fecal secretory IgA (sIgA) level reflects the condition of local immunity in the intestine and an increase in fecal neutrophil granule-derived proteins, such as PMN-elastase, indicates the presence of intestinal inflammation. In the present study, we examined the fecal protein profile to investigate the pathophysiology of the digestive tract in cirrhotic patients.
Forty-two patients with liver cirrhosis aged 59.0 ± 8.7 years (mean ± SD) were evaluated. The diagnosis of liver cirrhosis in each case was confirmed by a combination of clinical, biochemical, radiological, and pathological methods. The clinical severity of the liver disease in each case was determined using the Pugh modification of Child’s original classification[13]. These cases consisted of thirty-one males and eleven females. The number of patients with Child A, Child B, and Child C were 27, 6, and 9, respectively. The etiology of the liver cirrhosis was as follows: 33 patients with hepatitis C, 3 with hepatitis B, and 6 with alcoholism. All patients had no obvious gastrointestinal bleeding. The control group consisted of 20 subjects aged 41.0 ± 18.1 years with no demonstrated abnormality in the upper or lower digestive tract. Informed consent was obtained from each subject in accordance with the Helsinki Declaration.
Patients were instructed to defecate into a polystyrene container (diameter 15 cm, depth 12 cm). The stool samples, collected at 4 °C over a period of 48 h-72 h, were homogenized with a small amount of water, then stored at -80 °Cuntil the time of measurement. The concentrations of fecal Hb, Alb, Tf, α1-AT, PMN-elastase, and sIgA were measured by enzyme-linked immunosorbent assay (ELISA) as described previously[9-11]. Briefly, anti-human Hb antibody (Institute of Immunology, Japan), anti-human albumin antibody (Dakopatts, Glostrup, Denmark), anti-human Tf antibody (Dakopatts), anti-human α1-AT antibody (Dakopatts), anti-human PMN-elastase antibody (Serotec, Oxford, England), or anti-human secretory component antibody (Dakopatts) was coated in the wells of a 96-well microplate. The diluted fecal samples (100 to 10000 fold) were added to each well. After reaction at 37 °C for 1 h, the wells were washed with water. The samples were then reacted with the respective alkaline phosphatase-labeled antibody except alkaline phosphatase labeled anti-human IgA antibody (Dakopatts) for ELISA of sIgA. The enzyme reaction was then carried out, and color deve lopment was measured with a microplate colorimeter at 510/630 nm.
Values were expressed as medians (25%, 75%). The Mann-Whitney U tests and/or χ2 tests were used to compare groups. All P values were two-tailed; P values less than 0.05 were considered statistically significant.
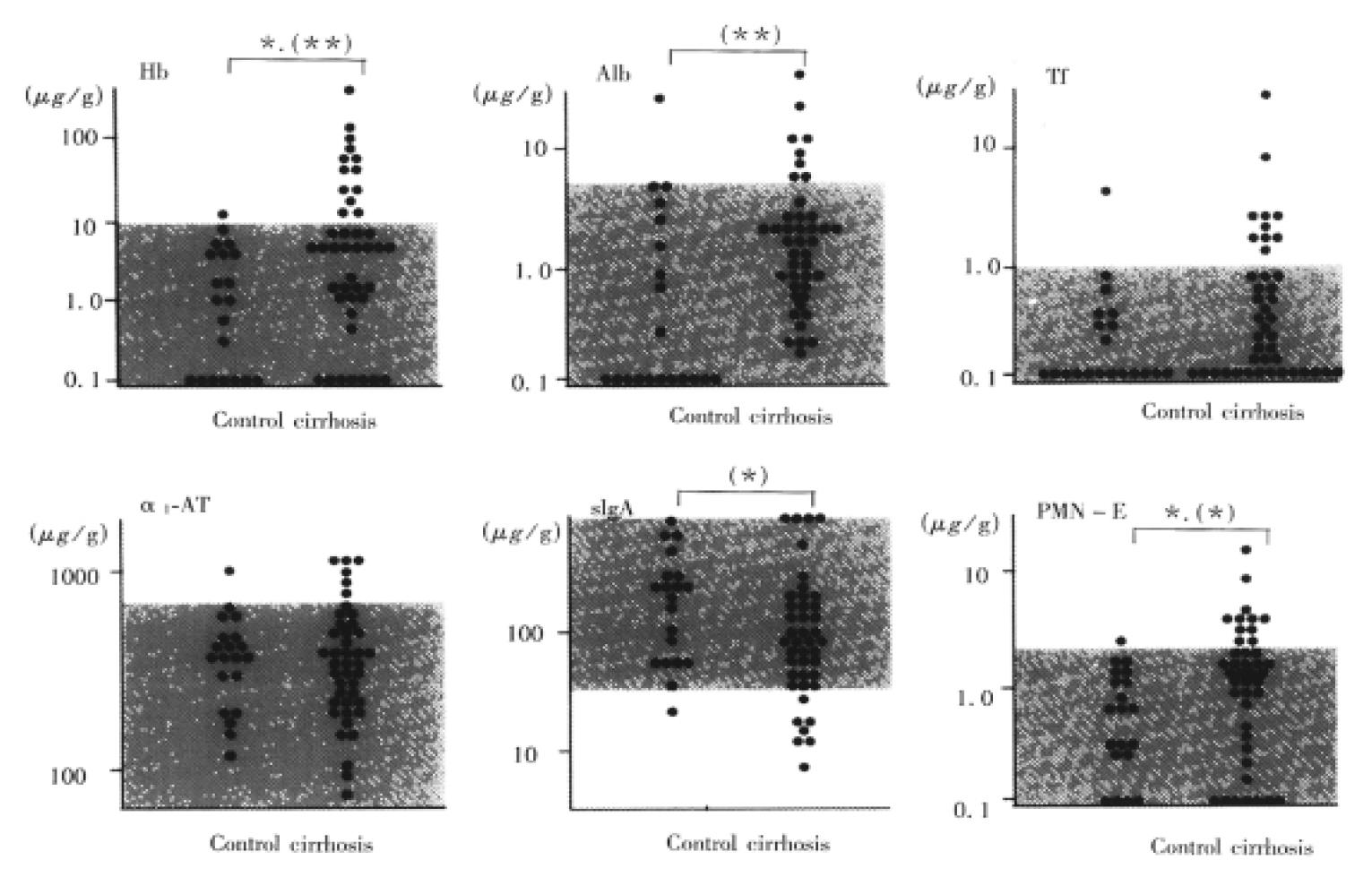
Results are shown in Figure 1. Daily stool weight (g/day) was 140 (100, 230) in control subjects. The concentrations of fecal Hb, Alb, Tf, α1-AT, sIgA, and PMN-elastase were 1.5 (0.1, 4.0), 0.1 (0.1, 1.8), 0.1 (0.1, 0.4), 327.4 (201.1, 421.3), 214.9 (56.6, 244.1), and 0.6 (0.3, 1.2), respectively. The 95th percentile of the control subjects was used as the cut-off value (10.6, 5.3, 1.0, 771, and 2.2 μg/g for Hb, Alb, Tf, α1-AT, and PMN elastase, respectively). sIgA was important when not only increasing, but also decreasing. Therefore, the lower limit of the normal range was defined as the 5th percentile (26.8 μg/g) of the control subjects for sIgA.
Daily stool weight (g/day) was 140 (100, 180). In terms of daily stool weight, there was no significant difference between the cirrhotic patients and control subjects. As shown in Figure 1, the fecal concentrations of Hb, Alb, Tf, α1-AT, and PMN-elastase were increased in 13 (31%), 8 (19%), 10 (24%), 6 (14%), and 11 (26%) among 42 patients, respectively. Conversely, the fecal concentration of sIgA was decreased in 7 (17%) of 42 patients. There were significant differences in fecal Hb, Alb, sIgA, and PMN-elastase concentrations between the cirrhotic patients and the controls.
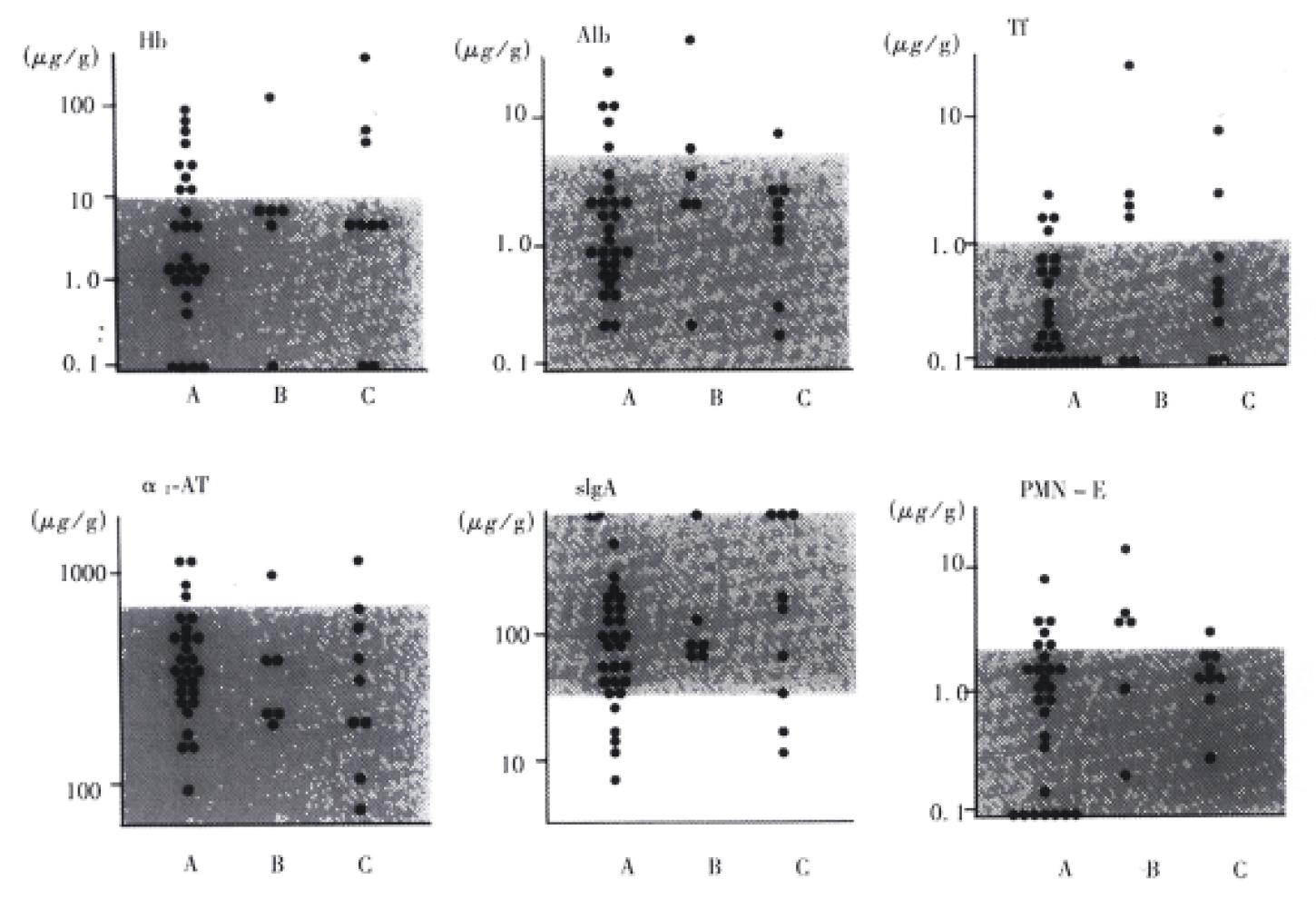
As shown in Figure 2, there was no relationship between the concentrations of these fecal proteins and the severity of the disease.
As shown in Figure 3, fecal Hb concentration demonstrated a significant difference between the cirrhotic patients and the controls.
There was no association between fecal protein concentrations and the etiology of the liver disease (data not shown).
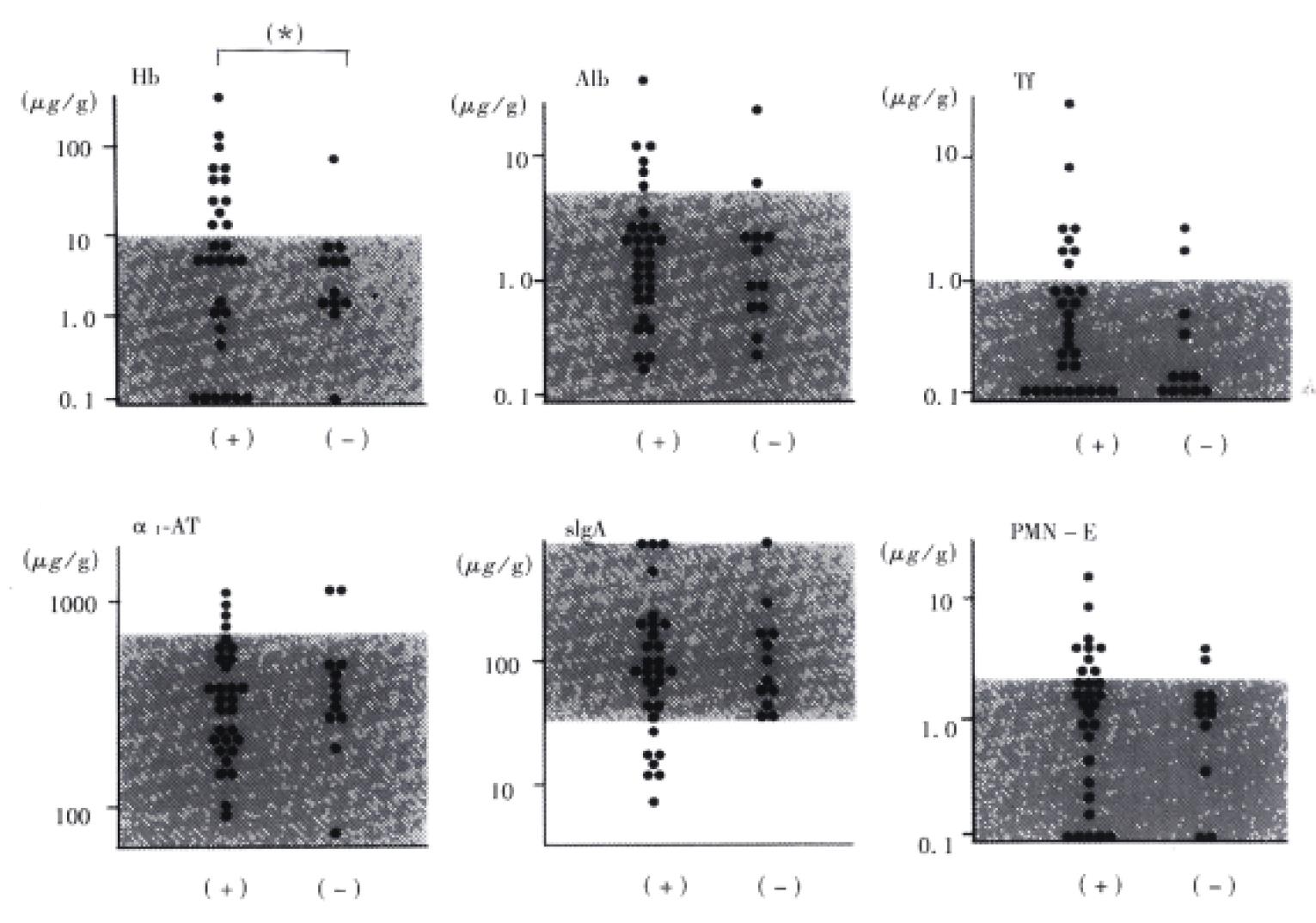
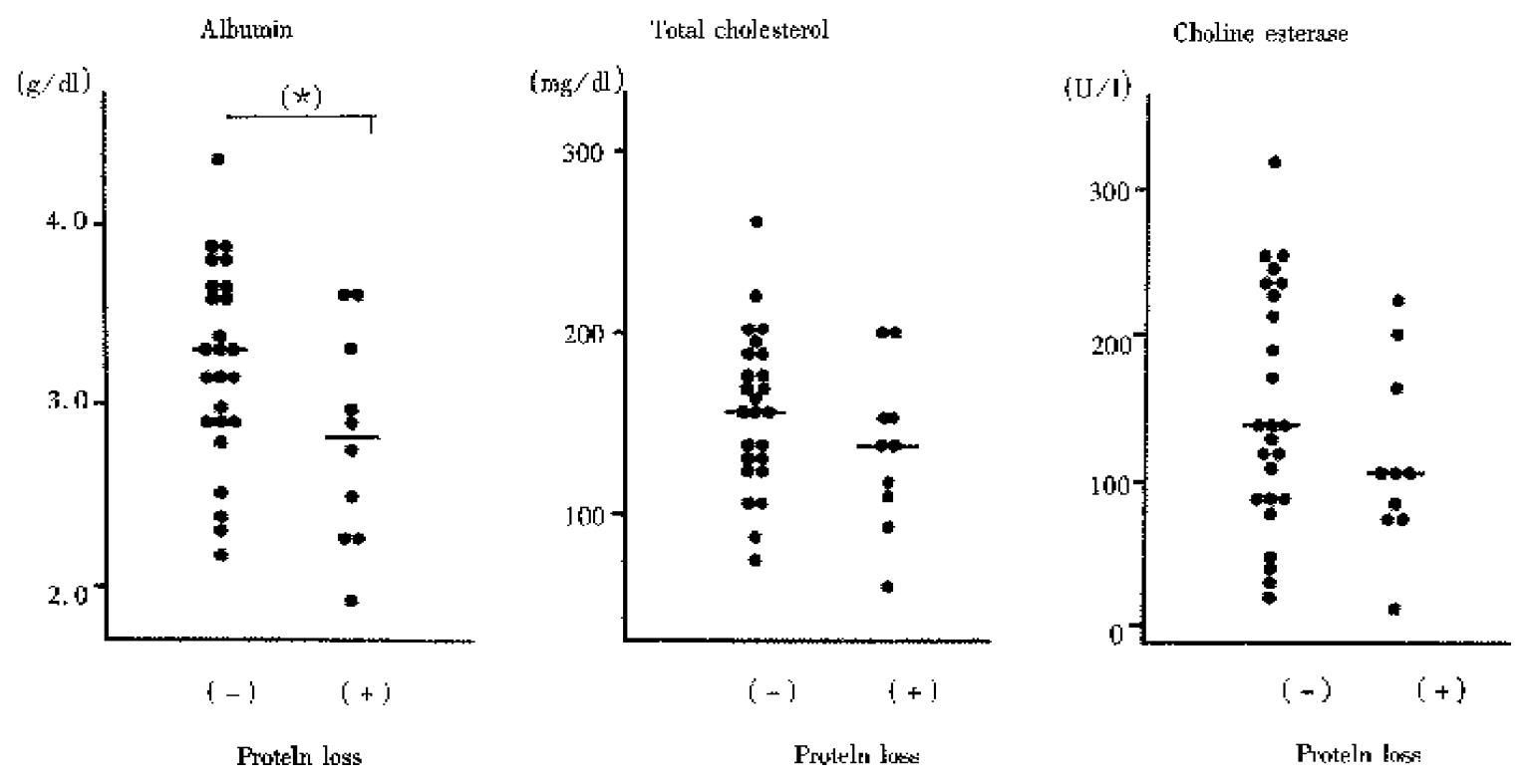
Results are shown in Figure 4. The patient was defined as having intestinal protein loss, when a patient had increased concentrations of at least one of Alb, Tf, and α1-AT. The serum Alb level was significantly decreased in patients with intestinal protein loss compared to that in patients without intestinal protein loss. The findings suggest that intestinal protein loss contribute to the development of hypoalbuminemia in cirrhotic patients.
The present study showed that 26% of the cirrhotic patients had elevated fecal PMN-elastase concentrations and suggested an increased prevalence of intestinal inflammation in cirrhotic patients. Furthermore, occult GI bleeding and intestin alprotein loss were observed commonly even in cirrhotic patients who had no massive GI bleeding. The conditions such as bleeding, intestinal protein loss, impaired intestinal immunity, and intestinal inflammation can be considered to be included in portal hypertensive enteropathy, because these seemed to be related not to the severity of the liver disease, but to portal hypertension. Recently, Stanley et al[14] first demonstrated that many of the manifestations of portal hypertensive enteropathy can be corrected by a trasjugular intrahepatic portosystemic stent-shunt (TIPS) procedure. They described a cirrhotic patient with diarrhea and hypoalbuminemia. Protein losing enteropathy was confirmed by analysis of whole gut lavage fluid. They then performed a TIPS procedure, which eliminated the portal hypertension. Both the patient’s diarrhea and elevated whole gut lavage fluid protein concentrations were reduced.
It is well known that the mechanism of hypoalbuminemia observed in cirrhotic patients is multifactorial. Decreased albumin production in the liver is one of the causes. In the present study, the serum Alb level was decreased significantly in patients with intestinal protein loss compared to that in patients without intestinal protein loss. The findings suggest that intestinal protein loss contributes to the development of hypoalbuminemia in cirrhotic patients.
Little attention has been paid to the relation between liver cirrhosis and intestinal inflammation. The prevalence of colitis diagnosed by colonoscopy in cirrhotic patients is not well established in the literature, and ranges from 10% to 57.9%[3,15-18]. Such a wide distribution is considered to be due to differences in the definition of inflammation and wide variation between observers in describing the mucosal appearance. The highest value, 57.9%, was reported by Scandalis N et al[16]. In their study, inflammatory changes included mucosal edema as well as erythema, granularity, and fragility of the mucosa. In contrast to colonoscopy, stool test is presumably a relatively objective test for assessment of inflammation. The value obtained in the present study (26%) was highest among the reported prevalence except for 57.9% reported by Scandalis N et al. The following possible reasons should be taken into consideration. ① Fecal PMN-elastase, a marker of intestinal inflammation in the present study, reflects mucosal inflammation of the small intestine as well as the colon. The prevalence of inflammation of the small intestine has not been studied so far. ② Difference in sensitivity between the stool test and colonoscopy: the stool test seems to be more sensitive than colonoscopy, since colonoscopy does not always detect inflammation if the inflammation is minimal or localized.
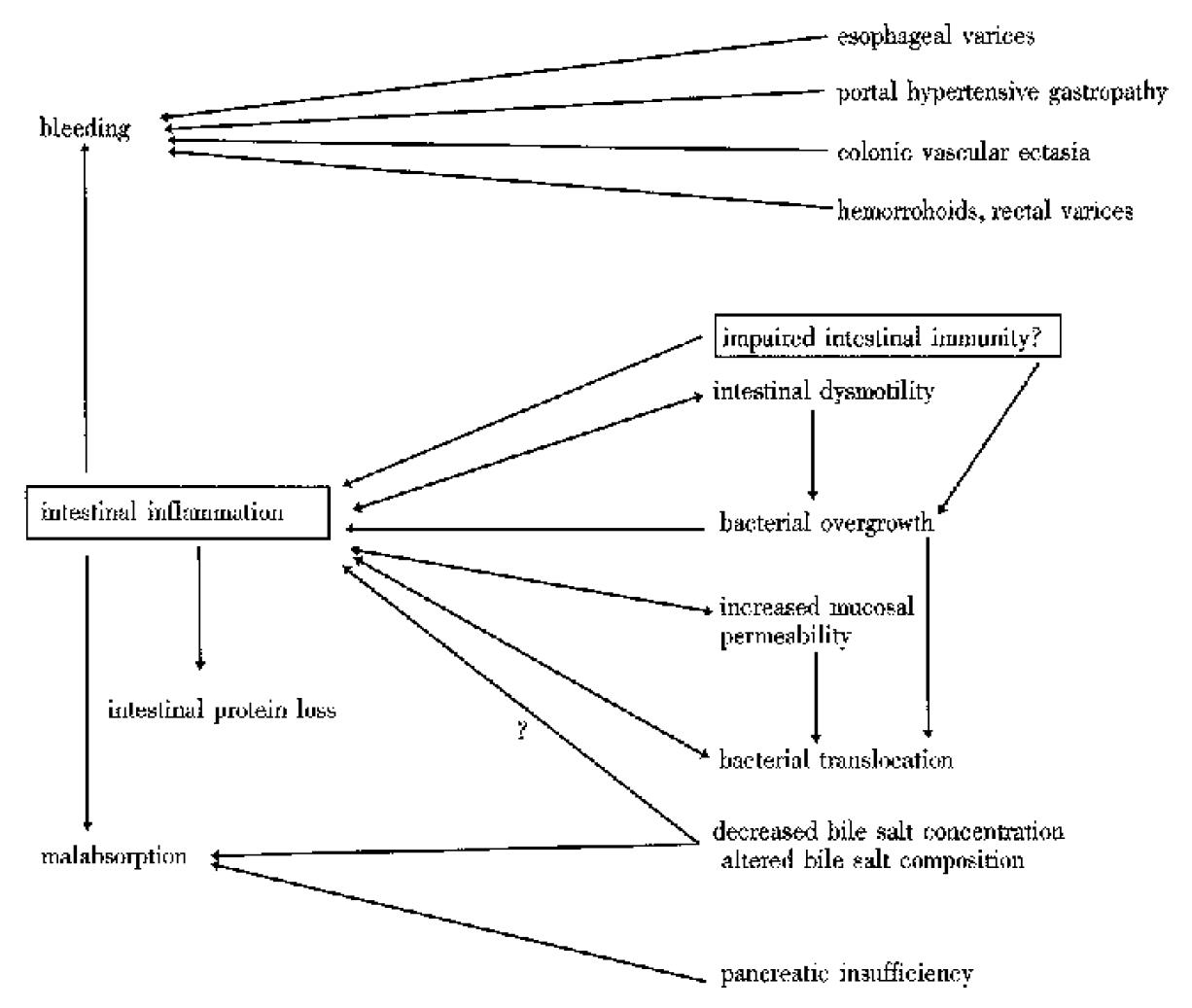
Our proposed pathology of the digestive tract in cirrhotic patients is shown in Figure 5. The present study suggested that impaired intestinal immunity might be one of the predisposing factors to intestinal inflammation. In the present study, 17% of the cirrhotic patients showed decreased fecal sIgA concentrations. This is the first report concerning fecal sIgA in cirrhotic patients, although serum sIgA in cirrhotic patients was shown previously to be elevated[19]. Besides impaired intestinal immunity, predisposing factors for intestinal inflammation include intestinal dysmotility, bacterial overgrowth, bacterial translocation, and increased mucosal permeability[20]. Recently, we reported that bile acids inhibit tumor necrosis factor-α-induced interleukin-8 production in human intestinal cells[21]. A decreased level or an altered composition of the intraluminal bile acids may make cirrhotic patients suscept ible to intestinal inflammation[22,23].
It is well known that GI bleeding, intestinal protein loss, malabsorption, and bacterial translocation develop in patients with inflammatory bowel diseases[10,24,25]. In cirrhotic patients with intestinal inflammation, therefore, such serious pathological conditions may easily occur. Therefore, it is important to evaluate whether a cirrhotic patient has intestinal inflammation or not in order to manage the patient. Measurement of fecal PMN-elastase is preferable to colonoscopy as a screening test for intestinal inflammation in cirrhotic patients, since the former is a sensitive and non-invasive test.
We thank Drs. Ryoichi Matsuse, Kazuo Uchida, and Kazue Tabata of the Kyoto Medical Science Laboratory for their helpful discussion, and are also grateful to other doctors in the Second Department of Internal Medicine, Osaka Medical College, who have contributed to this work.
Supported in part by Grant-in aid Nos.07670630 and 10670518 (to O. Saitoh) for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan
Edited by Ma JY
| 1. | McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226-1232. [PubMed] |
| 2. | Iwao T, Toyonaga A, Sumino M, Takagi K, Oho K, Nishizono M, Ohkubo K, Inoue R, Sasaki E, Tanikawa K. Portal hypertensive gastropathy in patients with cirrhosis. Gastroenterology. 1992;102:2060-2065. [PubMed] |
| 3. | Kozarek RA, Botoman VA, Bredfeldt JE, Roach JM, Patterson DJ, Ball TJ. Portal colopathy: prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192-1197. [PubMed] |
| 4. | Naveau S, Bedossa P, Poynard T, Mory B, Chaput JC. Portal hypertensive colopathy. A new entity. Dig Dis Sci. 1991;36:1774-1781. [PubMed] |
| 5. | Losowsky MS, Walker BE. Liver disease and malabsorption. Gastroenterology. 1969;56:589-600. [PubMed] |
| 6. | Romiti A, Merli M, Martorano M, Parrilli G, Martino F, Riggio O, Truscelli A, Capocaccia L, Budillon G. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Ital J Gastroenterol. 1990;22:118-123. [PubMed] |
| 7. | Iber FL. Protein loss into the gastrointestinal tract in cirrhosis of the liver. Am J Clin Nutr. 1966;19:219-222. [PubMed] |
| 8. | Conn HO. Is protein-losing enteropathy a significant complication of portal hypertension? Am J Gastroenterol. 1998;93:127-128. [PubMed] |
| 9. | Sugi K, Saitoh O, Nakagawa K, Sugimori K, Takada K, Yoshizumi M, Miyoshi H, Hirata I, Ohshiba S, Matsumoto H. Fecal secretory IgA levels in patients with colorectal diseases. Digestion and Absorption. 1993;16:28-33. |
| 10. | Saitoh O, Matsumoto H, Sugimori K, Sugi K, Nakagawa K, Miyoshi H, Hirata I, Matsuse R, Uchida K, Ohshiba S. Intestinal protein loss and bleeding assessed by fecal hemoglobin, transferrin, albumin, and alpha-1-antitrypsin levels in patients with colorectal diseases. Digestion. 1995;56:67-75. |
| 11. | Saitoh O, Sugi K, Matsuse R, Uchida K, Matsumoto H, Nakagawa K, Takada K, Yoshizumi M, Hirata I, Katsu K. The forms and the levels of fecal PMN-elastase in patients with colorectal diseases. Am J Gastroenterol. 1995;90:388-393. [PubMed] |
| 12. | Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927-934. [PubMed] |
| 13. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 14. | Stanley AJ, Gilmour HM, Ghosh S, Ferguson A, McGilchrist AJ. Transjugular intrahepatic portosystemic shunt as a treatment for protein-losing enteropathy caused by portal hypertension. Gastroenterology. 1996;111:1679-1682. [PubMed] |
| 15. | Rabinovitz M, Schade RR, Dindzans VJ, Belle SH, Van Thiel DH, Gavaler JS. Colonic disease in cirrhosis. An endoscopic evaluation in 412 patients. Gastroenterology. 1990;99:195-199. [PubMed] |
| 16. | Scandalis N, Archimandritis A, Kastanas K, Spiliadis C, Delis B, Manika Z. Colonic findings in cirrhotics with portal hypertension. A prospective colonoscopic and histological study. J Clin Gastroenterol. 1994;18:325-328; discussion 329. [PubMed] |
| 17. | Tam TN, NG WW, Lee SD. Colonic mucosal changes in patients with liver cirrhosis. Gastrointest Endosc. 1995;42:408-412. [PubMed] |
| 18. | Bresci G, Gambardella L, Parisi G, Federici G, Bertini M, Rindi G, Metrangolo S, Tumino E, Bertoni M, Cagno MC. Colonic disease in cirrhotic patients with portal hypertension: an endoscopic and clinical evaluation. J Clin Gastroenterol. 1998;26:222-227. [PubMed] |
| 19. | Pelletier G, Briantais MJ, Buffet C, Pillot J, Etienne JP. Serum and intestinal secretory IgA in alcoholic cirrhosis of the liver. Gut. 1982;23:475-480. [PubMed] |
| 20. | Quigley EM. Gastrointestinal dysfunction in liver disease and portal hypertension. Gut-liver interactions revisited. Dig Dis Sci. 1996;41:557-561. [PubMed] |
| 21. | Saitoh O, Nakagawa K, Sugi K, Matsuse R, Uchida K, Kojima K, Tanaka S, Teranishi T, Hirata I, Katsu K. Bile acids inhibit tumour necrosis factor alpha-induced interleukin-8 production in human colon epithelial cells. J Gastroenterol Hepatol. 1998;13:1212-1217. [PubMed] |
| 22. | Vlahcevic ZR, Juttijudata P, Bell CC, Swell L. Bile acid metabolism in patients with cirrhosis. II. Cholic and chenodeoxycholic acid metabolism. Gastroenterology. 1972;62:1174-1181. [PubMed] |
| 23. | Jönsson G, Hedenborg G, Wisén O, Norman A. Presence of bile acid metabolites in serum, urine, and faeces in cirrhosis. Scand J Clin Lab Invest. 1992;52:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Laffineur G, Lescut D, Vincent P, Quandalle P, Wurtz A, Colombel JF. [Bacterial translocation in Crohn disease]. Gastroenterol Clin Biol. 1992;16:777-781. [PubMed] |
| 25. | Saitoh O, Nakagawa K, Sugi K, Kojima K, Teranishi T, Tanaka S, Kayazawa M, Maemura K, Hirata I, Katsu K. Plasma endotoxin level as a marker of disrupted intestinal mucosal barrier in inflam-matory bowel disease. Bull Osaka Med Coll. 1997;43:95-101. |