TEXT
H. pylori infects half of the world population and the prevalence varies widely in different parts of the world with average rates of 40%-50% in western countries, rising to more than 90% in the developing world[1,2]. Compelling evidence from epidemiological and histopathological studies has linked H. pylori infection to the subsequent development of gastric carcinogenesis[3]. Furthermore, Watanabe and colleagues recently induced gastric adenocarcinoma in 37% of orally infected Mongolian gerbils, which were preceded with a series of premalignant changes in gastric mucosa of these gerbils[4]. However, in spite of the established causal relationship between H. pylori infection and gastric carcinogenesis, the underlying mechanisms remain unknown.
Disturbances in cell turnover in the gastrointestinal tract are believed to predispose to cancer development, and until recently, these changes were considered to be a marker of increased cancer risk[5]. It is clear that this organism is the main cause of chronic gastritis and capable of modifying epithelial cell turnover within gastric glands and in culture gastric epithelial cells, by influencing the balance between cell proliferation and apoptosis[6-9]. We and others have studied the effect of H. pylori infection on gastric epithelial cell turnover and found that patients infected with H. pylori had significantly higher proliferation rates as compared with uninfected controls[6,8-12]. The density of H. pylori may be one of the important determinants as we found that H. pylori at low inocula, stimulates cell proliferation, but at higher inocula (bacterium to cell ratio > 100), it causes a time- and concentration-dependent reduction of cell number and a marked increase in apoptosis and cell cycle arrest at G1 phase[8].
Antioxidant vitamins C, E and β-carotene affect cell growth in various human cells directly or through their a ntioxidant properties[13,14]. Our in vitro studies showed that the above vitamins can also significantly inhibit gastric cancer cell proliferation and induce apoptosis[15]. Investigations on gastric vitamins in patients with and without H. pylori infection suggest that H. pylori infection affects the concentrations of vitamin C, E and β-carotene in the stomach and the CagA+ strains have an even greater ability to reduce gastric juice vitamin C levels[16,17].
Although many factors may be related to H. pylori associated gastric carcinogenesis, the underlying molecular mechanisms are still unknown. However, many mediators and signal transduction pathways are involved in the regulation of gastric epithelial cell homeostasis, some of which may determine the final outcome of H. pylori infection. Understanding the molecular basis of H. pylori associated gastric carcinogenesis is important for determining prognosis, prevention and treatment of H. pylori infection. This review examines the possible molecular mechanisms responsible for H. pylori associated gastric carcinogenes is.
BACTERIAL VIRULENCE FACTORS
Although many factors contribute to H. pylori virulence, few have been directly related to gastric carcinogenesis[18]. Most strains of H. pylori from patients with intestinal-type gastric cancer or with atrophic gastritis are type I and secrete vacuolating cytotoxin (VacA) and carry CagA, a gene that encodes an immunodominant protein of unknown function, whereas many of the strains from asymptomatically infected persons lack this gene[19-21]. It has been shown that patients harbouring CagA+ strains have significantly higher gastric epithelial proliferation rates than patients infected with CagA- strains, but the apoptotic index in patients infected with CagA+ strains are lower than in patients infected with CagA- strains. Increased cell proliferation in the absence of a corresponding increase in apoptosis may explain the increased gastric cancer risk associated with infection by CagA+ strains[10]. Although there is no direct evidence suggesting that the CagA or VacA protein is carcinogenic, the enhanced inflammation and marked reduction of gastric juice vitamin C levels in CagA+ strain infected patients may ply a role in H. pylori associated carcinogenesis[16].
H. pylori expresses a powerful urease enzyme, which catalyses the conversion of urea to ammonia. Individuals with H. pylori infection have higher ammonia concentrations in gastric juice than uninfected controls[22]. A series of studies have demonstrated that concentrations of ammonia comparable to those found in gastric juice of infected individuals can cause gastric atrophy in rats, increase epithelial cell proliferation, and act as a promoter in the methyl-N’nitro-N-nitrosoguanidine (MNNG) rat model of gastric cancer[23-28].
Furthermore, H. pylori phospholipases, proteases and oxidases have been shown to cause degradation of many molecules, such as phospholipids in bio-membranes, transforming growth factor-β (TGF-β) and vitamin C, which are important in preventing carcinogenesis[16,29-31].
OXIDATIVE DNA DAMAGE AND p53
Mutation of the p53 tumor suppressor gene is the most common alteration found in a variety of human tumor cells and is considered to be one of the steps leading to the neoplastic state. The importance of p53 protein may be due to its effect on cell cycle progression, including cell proliferation and apoptosis, in response to cell DNA damage[32]. The activated p53 protein can affect the expression of a number of genes, including the cyclin-dependent kinase inhibitor (CKI) p21, which regulates G1 cell cycle check point and bax, a gene involved in apoptosis. Therefore, the protein can either prevent the cell from entering the S phase until the DNA damage is repaired and/or can turn on the apoptotic pathways to destroy an abnormal cell[33].
The p53 gene abnormalities in gastric cancer are usually point mutations or allelic deletions leading to over-expression of the protein, loss of p53 function and with resulting defects in the protective pathways of cell cycle arrest and apoptosis. Furthermore, the increased p53 expression and gene mutations have also been reported in gastric premalignant mucosa, such as dysplasia, atrophy or even the mucosa without obvious abnormality, suggesting that p53 function is affected from the early stage of gastric carcinogenesis[34].
The role of p53 in H. pylori associated carcinogenesis is still unclear, but some evidence suggests that it may be protective in this process. In p53 knockout mice, atrophic gastritis developed in 2 of 4 animals infected with H. felis within 3 months including one which developed moderate dysplasia. In contrast, these changes were not seen in any of the control animals, suggesting that lack of functional p53 accelerated carcinogenesis in experimental Helicobacter infection[35]. Data from Fox and colleagues further support the protective role of p53. They examined the effect of infection with H. felis in heterozygous mice deficient in one p53 allele[36]. One year after infection, the wild-type and p53 heterozygous mice both showed severe adenomatous and cystic hyperplasia of the surface foveolar epithelium. However, infected p53 heterozygous mice had a higher proliferative index than the infected wild-type mice.
Whether H. pylori and its associated inflammation induces p53 mutations or affects the activity of the protein is not clear, but p53 function may be defective at an early stage in H. pylori associated gastric carcinogenesis. Some studies have reported increased expression of p53 protein in gastric mucosa infected with H. pylori. However, the enhanced p53 expression failed to have any effect on gastric epithelial cell proliferation or apoptosis, and there appeared to be a positive relationship between the accelerated cell turnover and p53 over-expression. The accumulation of p53 was also not associated with expression of the CDI p21, a down-stream effector of p53[37,38]. We have recently initiated a study investigating the role of p53 in H. pylori infected gastric cell lines and found that H. pylori associated apoptosis is independent to p53 status of gastric cells[39]. These findings suggest that p53 function is defective in H. pylori infected mucosa. There are several mechanisms which may lead to the loss of p53 function. Firstly, recent studies suggest that p53 mediated apoptosis is suppressed by signals from growth factors, such as inte rleukin-6 (IL-6), interferon-γ (IFN-γ) and protein kinase C, which are shown to be up-regulated by H. pylori infection[40-42]. Secondly, it is likely that H. pylori and its associated inflammatory responses cause p53 gene mutation. H. pylori infection induces increased production of reactive oxygen metabolites (ROMs) by persistent inflammatory cell infiltration in gastric mucosa[43-45]. Many studies have indicated that ROMs can directly interact with genomic DNA and cause damage in specific genes that control cell growth and differentiation[46-48]. Furthermore, it has been reported that intact H. pylori, as well as isolated cellular components, stimulate nitric oxide (NO) synthesis[49-51]. High concentrations of NO induce wild-type p53 protein accumulation[52,53], and the NO-related deamination of DNA has been reported to cause GC-AT transitions, which are frequently found in p53 mutations in gastric cancer[23,54]. In addition, H. pylori possesses several proteases which may directly affect p53 activity though there is no direct experimental evidence of a relationship between these proteases and p53 protein[55].
BCL-2 AND OTHER APOPTOSIS RELATED MOLECULES
Bcl-2 protein is a part of a large group of proteins encoded by specific genes belonging to the bcl-2 family, which are known to play an important role in regulation of apoptosis. Some of these proteins (bcl-2 and bcl-xL) support survival, whereas others (bax, bad, bcl-x5) are apoptosis inducers[56]. Over-expression of bax and bak proteins encoded by the two pro-apoptotic members of the bcl-2 gene family has been associated with H. pylori infection and to induce apoptosis in the AGS gastric epithelial cell line and in gastric mucosal biopsies from patients colonized by H. pylori[57]. In contrast, the expression of bcl-2 protein was not affected or even suppressed by this organism[37]. However, over-expression of bcl-2 with abnormal distribution of apoptotic cells along the glands, which are usually found at the extremities of normal gastric glands, has been described in both intestinal metaplasia and gastric dysplasia, which occur as a result of long-term H. pylori infection[58,59].
Several other molecules may also play a role in H. pylori associated apoptosis. Treatment of gastric cells with TNF-α and IFN-γ markedly potentiate H. pylori induced apoptosis[60]. Rudi et al[61] have recently reported that H. pylori up-regulates the expression of the CD95 (APO-1/FAS) and CD95 ligand (CD95L), which are involved in initiating apoptosis. In fact, the enhanced CD95 expression observed in this study was associated with increased rates of apoptosis in gastric epithelial cell lines and in gastric mucosa. The importance of this study is not only the demonstration of the involvement of CD95 mediated apoptotic pathway in H. pylori associated apoptos is, but also the expression of CD95L mRNA on surface epithelium and pyloric gland cells of H. pylori infected mucosa at levels comparable to those found on lamina propria lymphocytes. CD95L is normally expressed by activated T cells and recently it has been found that many tumors, including gastric adenocarcinomas express CD95L[62]. CD95L can induce apoptosis of activated immunocytes and is thought to contribute to tumor immune escape[62]. The enhanced expression of CD95L mRNA in H. pylori infected gastric mucosa may suppress normal immune responses by inducing immunocyte apoptosis, which may further potentia te genetic instability due to the defect in the DNA damage-p53 mediated protective pathway.
TELOMERASE ACTIVITY
Activity of telomerase, which synthesizes the telomeric DNA to replaces the loss that occurs at each cell division, is suppressed in most normal human somatic cells but induced in most human cancers. Normal human cells progressively lose telomere sequences due to the lack of telomerase activity. In contrast, most immortalized cell lines and malignant human tumors appear to maintain constant telomere length via the activation of telomerase[63,64]. Reactivation of telomerase is thought to be an important step in carcinogenesis.
Expression of human telomerase RNA (hTR) and telomerase activity have been studied in gastric cancer and corresponding non-cancerous mucosa[65]. Telomerase activity was detected in 23 of 26 carcinoma tissues. Although all tumor specimens and non-cancerous mucosa expressed various levels of hTR, 21 cases expressed hTR at a higher level in the tumor than that in the adjacent normal mucosa. Nine of 26 non-cancerous mucosa showed telomerase activity and all of them contained intestinal metaplasia. The incidence of telomerase-positive mucosa in grade II intestinal metaplasia was significantly higher than that in mucosa with grade I intestinal metaplasia or without intestinal metaplasia, whereas hTR over-expression was found both in mucosa with and without intestinal metaplasia regardless to their grades. The level of hTR expression and telomerase positivity was shown to increase in parallel with the degree of H. pylori infection. These results suggest that H. pylori infection may be a strong trigger for hTR over-expression in intestinal metaplasia, and this may lead to telomerase reactivation[65,66].
Recently, Chin and colleagues examined the interaction between telomere dysfunction and p53 in cells and organs of telomerase-deficient mice. Coincident with severe telomere shortening and associated genomic instability, p53 is activated, leading to growth arrest and/or apoptosis. Deletion of p53 significantly attenuated the adverse effects of telomere dysfunction, but only during the earliest stages of genetic crisis. Correspondingly, the loss of telomere function and p53 deficiency cooperated to initiate the transformation process[67]. These findings suggest that p53 deficiency and telomerase reactivation in H. pylori infected mucosa may play an important role in H. pylori associated gastric carcinogenesis.
HOST GENETIC FACTORS
Beales et al[68] reported that the frequency of DQ 5, one of the D-related human leukocyte antigen (HLA) molecules, was significantly higher in individuals with gastric atrophy or metaplasia than in those without gastric atrophy or intestinal metaplasia and in uninfected individuals. Azuma and colleagues have studied the relationship between H. pylori infection and the genotyping of another D-type HLA molecule, DQA1 in 82 gastric adenocarcinoma patients and 167 unrelated controls. They found that the allele frequency of DQA1 was significantly lower in H. pylori positive atrophic gastritis group than in H. pylori positive superficial gastritis and normal control groups. In addition, the allele frequency of DQA1 also was significantly lower in H. pylori positive intestinal type gastric adenocarcinoma group than in normal control, H. pylori positive superficial gastritis, and diffuse type gastric adenocarcinoma groups[69].
The importance of host genetic factors in determining the outcome of H. pylori infection was further demonstrated by Sakagami and colleagues. They assessed H. pylori infection in different strains of mice and found that the level of bacterial colonisation, the severity of gastritis and the development of gastric atrophy varied within these mice. For example, in infected SJL, C3H/He, DBA/2, and C57BL/6 mice, moderate to severe chronic active gastritis was observed only in the body of the stomach, which increased in severity over time with specialised cells in the body glands being replaced. As the severity of this damage in the body increased and atrophic changes were seen, the level of bacterial colonisation of the antrum decreased. In contrast, in BALB/c and CBA mice, there was only mild gastritis in the antrum, no remarkable changes were detected in the gastric body mucosa, and no atrophy was seen over time. In both these strains of mice, heavy bacterial colonisation was seen, which tended to increase over the period of the experiment[70]. These findings suggest that the host genetic factors are important in H. pylori associated gastric carcinogenesis.
SUMMARY
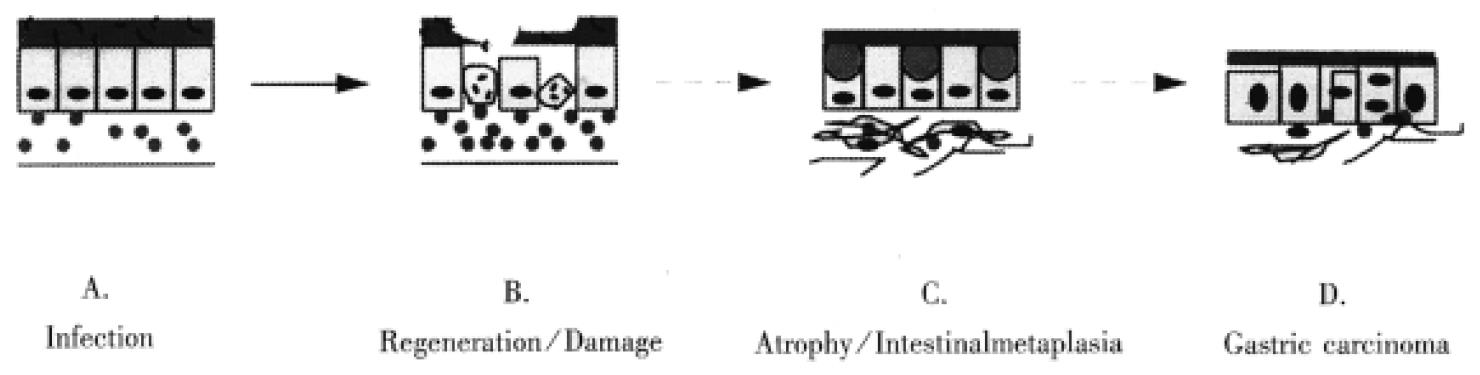
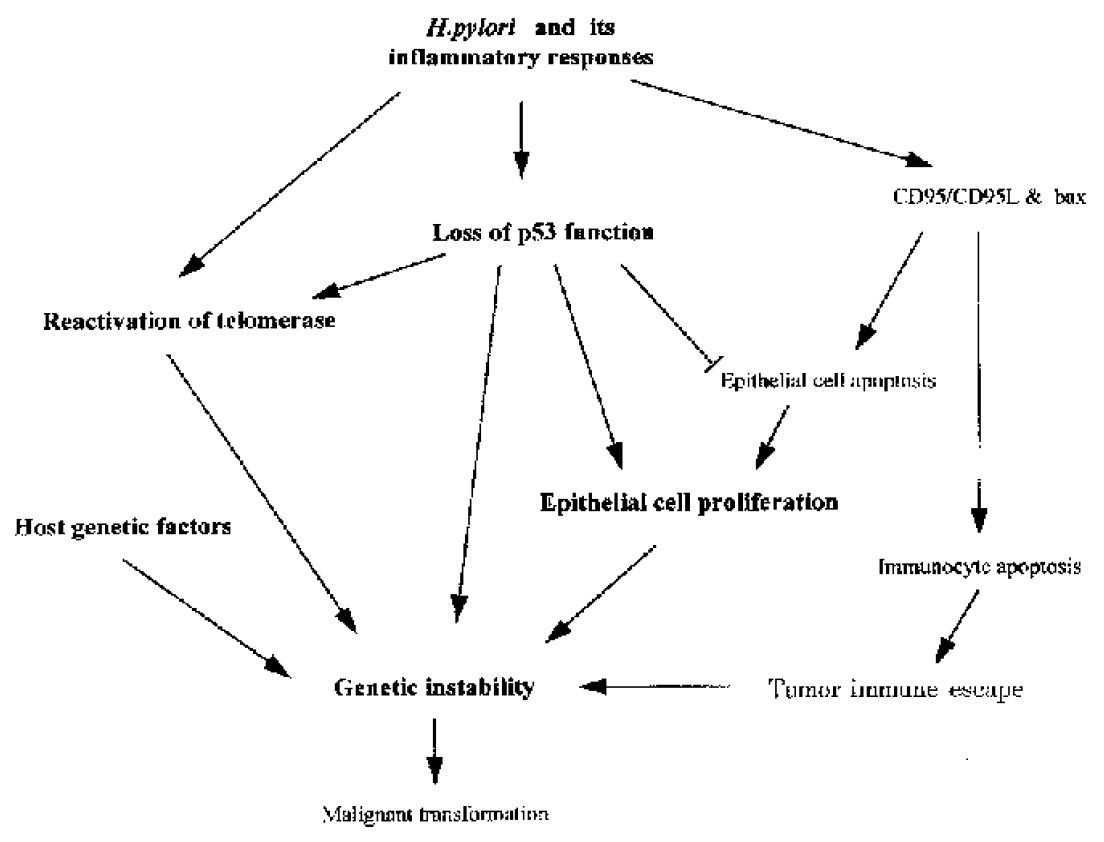
Many molecules are involved in H. pylori associated gastric carcinogenesis. However, how the organism and its associated inflammation, interact with these molecules in gastric mucosa to induce carcinogenesis is still unknown. Over many years, H. pylori infected mucosa may experience sequential exposure to “damage-regeneration” (Figure 1). Following the long-standing repeated damage-regenerate cycle, gastric atrophy and intestinal metaplasia gradually develop, which finally results in adenocarcinoma. During this process, the loss of p53 function may play an important role. As mentioned above, H. pylori infection may cause deficiency of p53 function and subsequently, this may not only lead to defects in the DNA damage-p53 mediated protective pathway, but the mutated p53 protein may also provide a possible selective advantage for tumor cell proliferation by attenuating apoptosis[71]. Furthermore, H. pylori infection has been shown to induce the reactivation of telomerase and to cause telomere dysfunction, which may cooperate with p53 deficiency to accelerate carcinogenesis[67]. In early stages of H. pylori infection, CD95 and bax mediated apoptosis may play an important role in eliminating damaged DNA or gene mutated cells, thereby maintaining genetic stability. However, H. pylori infection induces CD95L expression, which may suppress host immune responses by causing immunocyte apoptosis. Therefore, as shown in Figure 2, the loss of p53 function, reactivation of telomerase activity, inhibition of host immune responses, together with host genetic factors, may play important roles in the development of H. pylori associated carcinogenesis.
Figure 1 Long-term H.
pylori infection may induce a repeated “damage-regeneration” process which gradually leads to gastric atrophy, intestinal metaplasia and finally carcinoma. A. H. pylori infected gastric mucosa with inflammatory cell infiltration. B. Gastric epithelial cells are damaged, become apoptotic and then are regenerated, with enhanced inflammatory cell infiltration and disturbance of mucus layer. C. Atrophy and intestinal metaplasia develop; fibrosis and thinning of the lamina propria; finally H. pylori is lost due to inhospitable mucosa. D. Gastric carcinoma is induced.
Figure 2 Possible molecular mechanisms of H.
pylori associated carcinogenesis. H. pylori and its associated inflammatory responses cause reactivation of telomerase, the loss of p53 function and also induce CD95 and bax mediated apoptosis. CD95L produced by gastric epithelial cells m ay also cause intra-mucosal immunocyte apoptosis, which could facilitate tumor immune escape. However, mutated p53 may attenuate epithelial cell apoptosis, providing a possible selective advantage for tumor cell proliferation. Furthermore , p53 deficiency may cooperate with telomere dysfunction to accelerate carcinogenesis. All of these changes, together with host genetic factors may play important roles in the development of H. pylori associated carcinogenesis.