Published online Aug 15, 1999. doi: 10.3748/wjg.v5.i4.365
Revised: July 3, 1999
Accepted: July 19, 1999
Published online: August 15, 1999
- Citation: Li WJ, Gao QX, Zhou GM, Wei ZQ. Micronuclei and cell survival in human liver cancer cells irradiated by 25 MeV/u40 Ar14+. World J Gastroenterol 1999; 5(4): 365-368
- URL: https://www.wjgnet.com/1007-9327/full/v5/i4/365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i4.365
Heavy ions with high linear energy transfer (LET) have the following advantages as compared with the conventional X-rays and γ-rays therapy: a better physical selectivity, a higher relative biological effectiveness (RBE), a greater therapeutic gain factor (TGF), a smaller oxygen enhancement ratio (OER) for LET > 200 keV/μm, positrons emitted from nuclear reaction products offering the condition of monitoring heavy ion position in tissue, and so on.
During the mid 1970s, 450 patients were treated with heavy ions, mostly neon, at the Bevalac of Lawrence Berkeley Laboratory (LBL), United States[1]. Promising results with neon ions were reported when compared with the conventional radiotherapy for soft tissue sarcoma, bone sarcoma and prostate cancer[2,3]. In 1994, the Heavy Ion Medical Accelerator in Chiba (HIMAC) of Japan was designed to deliver beams of ions, helium to argon, to energies in the range of 100 MeV/u-800 MeV/u. Carbon ions were used for clinical treatment. By August 1997, a total of 301 patients had been treated with carbon ions[4]. The treatment was started at GSI, Darmstadt, Germany in December 1997. Two patients suffering from tumors at the base of skull were treated with five and four fractions of carbon ions respectively[5]. In 1995, HIRFL (Heavy Ion Research Facility in Lanzhou, China) began the basic researches of cancer therapy with heavy ions such as carbon, oxygen and argon. In order to collect basic data for clinical therapy, we studied and discussed the dynamic changes of micronuclei and cell survival in human liver cancer cells SMMC-7721 irradiated by 25 MeV/u40-Ar 14+.
Human liver cancer cells SMMC-7721, purchased from Second Military Medical University in Shanghai, were cultivated in RMPI 1640 medium (Gibco product) supplemented with 10% calf serum in standard incubator at 37 °C in air with 5% CO2. On e passage (subculture) of cells every 6-7 d was performed. The cells were shifted to Φ35 mm petri-dishes 2 d before irradiation, each petri-dish had 2 mL cell suspension, and density of the cells was 5 × 104 cells/mL. Each dose had 6 petri-dishes. Before irradiation, cells in each petri-dish were examined under reverse light microscope so as to select materials good in growth and even in density.
Irradiation was performed using 40Ar14+ ion beam with energy of 25 MeV/u and intensity of 0.005 nA (2.1 × 106 p/s). The doses of cells w ere measured by air ionization chamber. Single (0.68, 6.8 and 68 Gy) and fractionated (twice and thrice, at an interval of 2 h, total dose of 68 Gy) irradiations were done.
Four petri-dishes were taken arbitrarily before irradiation, culture suspension, in which adhesive cells were not included, was harvested, and adhesive cells were fixed with Carnoy′s fluid. At the same time, two other petri-dishes were taken and cells were harvested by trypsinzation. Cells were stained with typan blue, and number of dead and living cells were counted, respectively. Covers of petri-dishes were removed under condition of asepsis, and irradiation of sample s was started after culture medium in petri-dishes was drawn out and the mouths of perti-dishes were sealed using 4 μm sterilized mylar films.
As soon as irradiation ended, sealed films were taken out from petri-dishes, 2 mL fresh 1640 culture medium was then added into each petri-dish, petri -dishes with cells were then placed in CO2 incubator and kept on cultivating. Twenty-four, 48, 72, 96 and 120 h after cell culture, cells in 2 or 3 pet ri-dishes from each time point were treated by trypsinzation and stained with typan blue to identify the number of dead and living cells. Medium 1640 in other 2 or 3 petri-dishes from each time point was removed, cells were fixed for 4 h with Carnoy′s fluid, and stained for 10 min with acridine orange (0.01%, pH6.8), rinsed three times with PBS buffer at pH6.8, for 30 min each time. Micronuclei were observed under fluorescence microscope. Materials in which micronuclei had been observed were rinsed with PBS and stained for 8 min with Giemsa (1:20, pH6.8). Each petri-dish was observed under light microscope. Cells in 9 mesh eye piece micro-ruler were counted, and converted into cell numbers per area.
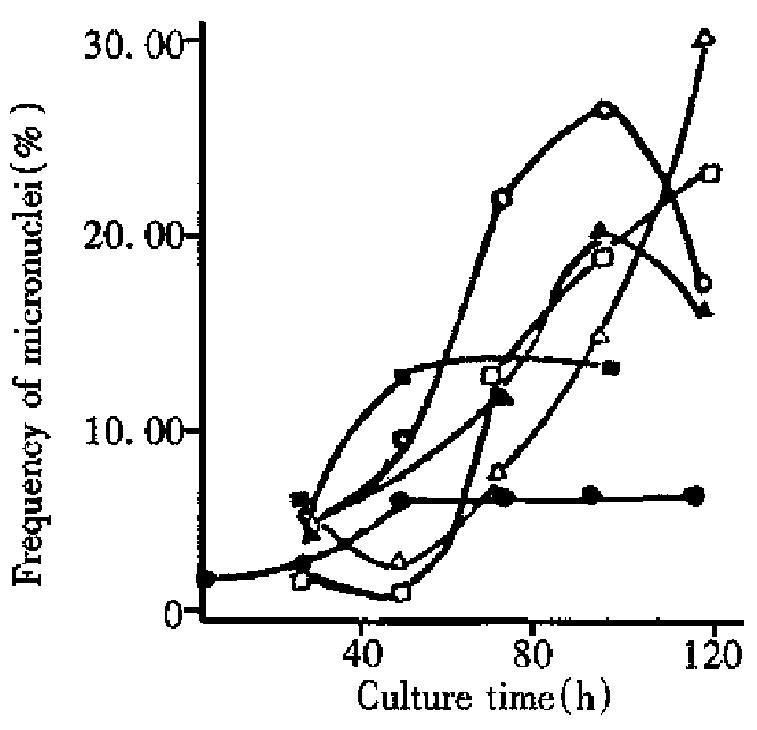
From Figure 1, it can be seen that micronucleus frequencies induced by s ingle radiation of 0.68 Gy and 6.8 Gy and thrice radiation of 68 Gy showed a tendency to rise between 24 h and 96 h culture after radiation. The reasons for this phenomenon might be that: ① micronuclei are delayed to appear following mitoses, as proposed by Mitchell and Norman[5]; ② mitoses take place later in cells with severe chromosomal damage expressed as micronuclei [6]. As compared with single radiation of 6.8 Gy and thrice radiation o f 68 Gy, the micronucleus frequency of single radiation of 0.68 Gy increased over 48 h culture after slight irradiation (the micronucleus frequencies at 48 h and 96 h were 12.86% and 13.45%, respectively). This is because that c ells irradiated with single dose of 0.68 Gy are damaged to a less degree and easy to recover to normal through 48 h culture, thereby the damaged cells inducing micronuclei became so low in number even reaching the number of normal cell s. At about 96 h, the micronucleus frequencies of cells irradiated with single of 6.8 Gy and thrice of 68 Gy tended to fall (Figure 1). The reasons might be that: in these cells with less damage, DNA is repaired fast and easily recovered to normal; after culture for a certain duration, normal cells without micronuclei reproduce faster than the damaged cells with micronuclei, ratio (micronucleus frequencies) between the two was lowered.
Micronucleus frequencies are lowest at 48 h for single and twice radiation of 68 Gy, because the severely damaged cells have not recovered mitoses and only the less-damaged or non-damaged cells have had mitoses. After 48 h culture, the severely damaged cells started mitoses, and the micronucleus frequencies rose significantly (Figure 1).
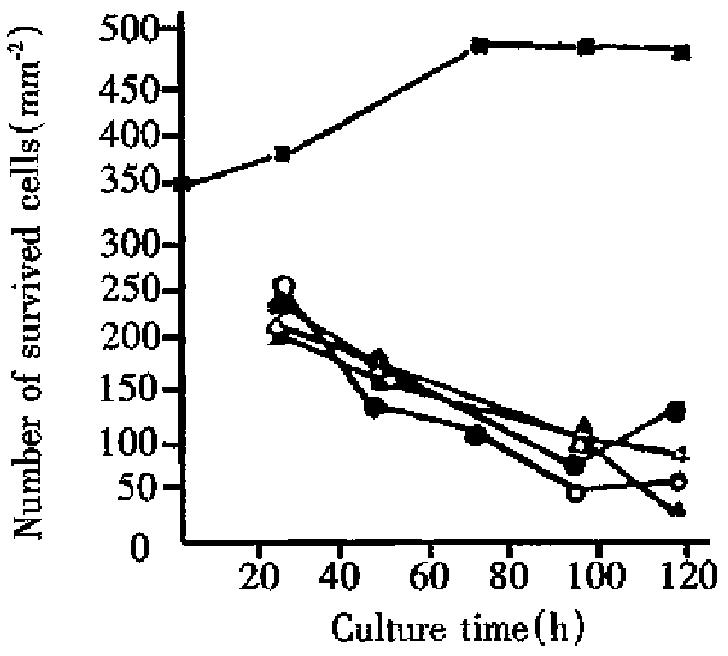
Figure 2 shows the relationship between the number of live cells and culture time for liver cancer cells. It can be seen that irradiated (single or fractionated) liver cancer cells cultured for 24, 96 and 120 h grew much slower than control. This showed that growth of liver cancer cells irradiated with heavy ions was obviously inhibited and some of liver cancer cells with different length of culture either exfoliated or died. As culture time was prolonged, survived control cells showed a rising tendency, whereas those of cells irradiated with heavy ions showed attenuating tendency. The latter resulted from radiation with high LET heavy ions which made the cell potentially lethal damage (PLD) enhance obviously. This appearance was also found in typan blue staining. In harvested cell suspension, some of the suspension cells were not deeply stained but stayed in anabiotic state which was a large proportion in irradiated materials. Comparison of single radiation of 6.8 Gy, single, twice, and thrice of 68 Gy found that the number of survived cells for single and twice radiation of 68 Gy lowered gradually during the whole culture period. The difference is that after 96 h culture, the number of survived cells for single radiation of 68 Gy decayed faster than those for twice radiation, as there was no interval in the single radiation of 68 Gy, i.e., without chance of cell repair, and the number of induced reproductive dead cells increased more obviously than those for twice radiation. The number of survived cells with single radiation of 6.8 Gy and thrice radiation of 68 Gy increased after 96 h culture, which indicates that cell damage by these two kinds of irradiation is slight, with lots of sublethal damage (SLD) cells. These SLD cells reactivated through certain time of culture, therefore, the survived cells increased after 96 h culture.
The results in Table 1 and Table 2 illustrate that micronucleus frequencies and survived cells increase or decrease with culture time with single and twice radiation of 68 Gy (relative coefficient r = -0.97 and r = -0.99, respectively). Micronucleus frequencies were negatively related to the number of survived cells. For single dose of 6.8 Gy and 3 fraction doses of 68 Gy, the micronucleus frequencies both reduced while the number of survived cells increased 96 h after culture. It is indicated again that there was a negative relationship between micronucleus frequencies and the number of survived cells. Therefore micronucleus frequency is an important parameter describing the degree of the damaged cells.
| Cultural time (h) | Dose (Gy) | NO. of scored cells | Frequency of micronuclei (%) | NO. of Survived cells (mm-2) |
| 24 | 6.8 | 2008 | 4.83 | 235 |
| 48 | 6.8 | 2047 | 9.19 | 175 |
| 72 | 6.8 | 2150 | 21.72 | |
| 96 | 6.8 | 1924 | 26.55 | 79 |
| 120 | 6.8 | 2096 | 17.45 | 129 |
| 24 | 68 | 1960 | 5.62 | 207 |
| 48 | 68 | 2150 | 3.00 | 158 |
| 72 | 68 | 2070 | 7.93 | |
| 96 | 68 | 1975 | 14.69 | 107 |
| 120 | 68 | 2040 | 30.39 | 36 |
| Cultural time (h) | Dose (Gy) | Times of irradiation | NO. of scored cells | Frequency of micronuclei (%) | NO. of Survived cells (mm-2) |
| 24 | 68 | 2 | 971 | 5.13 | 213 |
| 48 | 68 | 2 | 1024 | 1056 | |
| 72 | 68 | 2 | 964 | 12.79 | |
| 96 | 68 | 2 | 1152 | 19.01 | 101 |
| 120 | 68 | 2 | 1060 | 23.02 | 90 |
| 24 | 68 | 3 | 974 | 2.47 | 250 |
| 48 | 68 | 3 | 1050 | 136 | |
| 72 | 68 | 3 | 1000 | 12.00 | 109 |
| 96 | 68 | 3 | 1000 | 20.00 | 50 |
| 120 | 68 | 3 | 1116 | 16.13 | 59 |
Micronucleus frequencies of cancer cells after 96 h culture were significantly higher than those of 24 h culture with single radiation of 6.8 Gy, sing le, twice and thrice radiation of 68 Gy. Some researchers obtained a similar rule when studying irradiated lymphocytes[5]. The reasons are that induced micronucleus cells depend on mitosis while mitotic delay of the damaged cells (chromosome lesion) results in delayed production of large amounts of micronuclei. The changing rule of 24 h culture was in agreement with the results by Shibamoto in his studies on lymphocytes[6]. After 96 h culture, micronucleus frequencies of cells irradiated at a dose of 68 Gy fell. This may be because that this kind of cancer cells were damaged more severely and inhibited cell mitotic procedure (mitosis is a prerequisite for micronuclei), therefore damaged cancer cells died before micronuclei were expressed. As compared with cells irradiated with 6.8 Gy following 96 h culture the micronucleus frequencies of cells irradiated with 68 Gy decreased significantly.
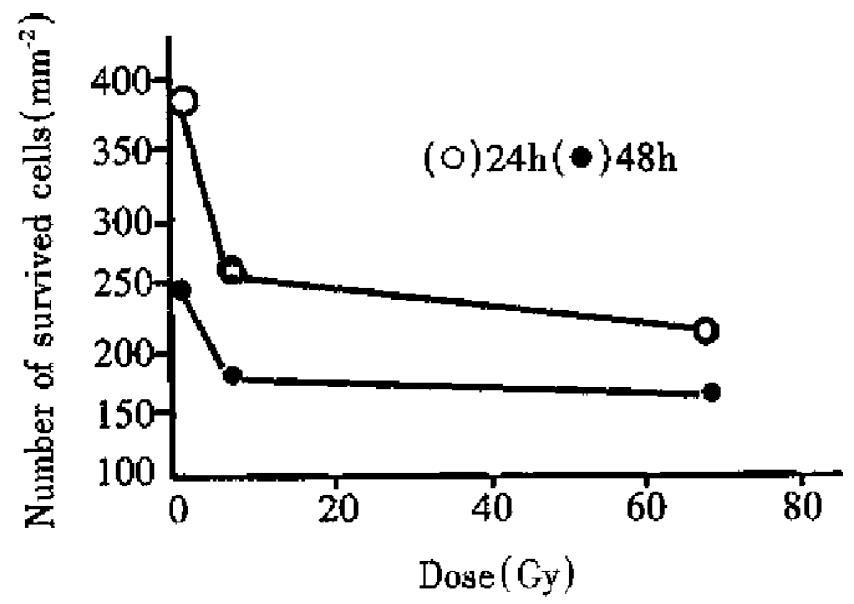
Figure 3 shows the dose response to cell survival in human liver cancer cells. It can be seen that survival of liver cancer cells following 24 h and 96 h culture decreased with increase of radiation dose, i.e. cell survival was negatively related to doses (r24h = -0.68, r48h = -0.70). When radiation dose was the same, the number of survived cells in human liver cancer cells for 48 h culture was lower than that for 24 h culture. The results showed that the number of survived cells of the irradiated liver cancer cells decreased gradually with culture time (survived number of control cells in creased with culture time), which results from reproductive death of lots of potentially lethal cells in irradiated cancer cells through several division cycles.
Wen-Jian Li, male, born on 1959-02-22 in Hebei Province, Associate Professor and Deputy Director of the Second Division of Heavy Ion Application. Graduated and obtained a bachelor degree from Hebei University of Technology in 1982, and a master degree from Institute of Modern Physics, Chinese Academy of Sciences in 1992, specialized in the basic study of radiation biological effects and cancer therapy with heavy ions; having 33 papers published.
Edited by MA Jing-Yun
| 1. | Kraft G. Heavy ion therapy at GSI. GSI Nachrichten. 1993;11:3-5. |
| 2. | Castro JR. Future research strategy for heavy ion radiotherapy. Progress Radiooncol. 1995;5:643-648. |
| 3. | Linstadt DE, Castro JR, Phillips TL. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys. 1991;20:761-769. [PubMed] |
| 4. | Sisterson J. Particles. News Letter. 1998;21:1-11. |
| 5. | Mitchell JC, Norman A. The induction of micronuclei in human lymphocytes by low doses of radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:527-535. [PubMed] |
| 6. | Shibamoto Y, Streffer C, Fuhrmann C, Budach V. Tumor radiosensitivity prediction by the cytokinesis-block micronucleus assay. Radiat Res. 1991;128:293-300. [PubMed] |