Published online Aug 15, 1999. doi: 10.3748/wjg.v5.i4.312
Revised: July 3, 1999
Accepted: July 19, 1999
Published online: August 15, 1999
AIM: To investigate the effects of carnitine on ameliorating hepatic steatosis induced by total parenteral nutrition (TPN) in animal model.
METHODS: Eighteen normal Wistar rats and 19 cirrhotic Wistar rats induced by carbon tetrachloride were randomly divided into three groups, i.e., free access to food and drink (group A), TPN (group B) and TPN + carnitine (group C) for one week, respectively. Hepatic function, histology and its fat content were determined on the 7th day.
RESULTS: Hepatic triglyceride (TG) and cholesterol (CHO) contents were significantly higher in groups B and C than in group A, and significantly lower in group C than in group B in both normal and cirrhotic rats (all P < 0.05). Histopathological examinations revealed that hepatic steatosis was more severe in group B than in group C in both normal and cirrhotic rats.
CONCLUSION: Carnitine can ameliorate hepatic steatosis associated with TPN in both non-cirrhotic and cirrhotic rats.
- Citation: Liang LJ, Yin XY, Luo SM, Zheng JF, Lu MD, Huang JF. A study of the ameliorating effects of carnitine on hepatic steatosis induced by total parenteral nutrition in rats. World J Gastroenterol 1999; 5(4): 312-315
- URL: https://www.wjgnet.com/1007-9327/full/v5/i4/312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i4.312
Long-term total parenteral nutrition (TPN) can frequently lead to the development of hepatic steatosis. Although its incidence has decreased with modifications of the TPN regimen, such as increasing content of lipid and decreasing content of glucose, hepatic steatosis still remains the most common complication of TPN. The exact pathogenesis of TPN-associated hepatic steatosis is still unclear. A number of factors may contribute to the development of hepatic steatosis following TPN. Of them, the absolute or relative deficiency of carnitine is commonly postulated to be an important mechanism. Previous studies showed that patients with long-term TPN had carnitine deficiency[1-4], however, the prophylactic effects of carnitine supplementation on hepatic steatosis have not been well do cumented. In this study, we investigated the effects of carnitine on minimizing TPN-associated hepatic steatosis in both normal and cirrhotic rats.
Normal groups Eighteen male Wistar rats weighing 250-300 g, obtained from the Laboratory Animal Center of our university, served as normal groups. They were randomly divided into three groups: A1, B1 and C1, wit h six in each group.
Cirrhotic groups Twenty-five male Wistar rats, weighing 218-245 g, were used for inducing liver cirrhosis. They were treated with subcutaneous injection of 60% carbon tetrachloride (CCl4) at a dose of 0.3 mL/100 g body weight twice each week for 10 weeks, and 5% ethanol water solution was ingested as drink water through the period of time[5]. A total of six rats died during CCl4 treatment. The remaining 19 rats were taken as cirrhotic groups, and randomly divided into three groups: A2, B2 and C2, with six, seven and six rats, respectively.
Groups A (A1 and A2), B (B1 and B2) and C (C1 and C2) had free access to food and water, carnitine-free TPN, and TPN with carnitine supplementation, respectively, for one week. Both group B and C were fasted when TPN was applied.
TPN solution was prepared according to physiological needs of growing rats[6]. It offered a total non-protein energy of 166 kcal/kg body weight daily, with 40% provided by MCT/LCT lipid emulsion, and a nitrogen quantity of 1140 mg/kg body weight daily. In normal rats, 10% compound amino acids solution was used as source of nitrogen, while 8% essential amino acids and branch-chained amino acids solution was used in cirrhotic rats. In group C, carnitine ("Hepadif", HAN SEO Pharmaceutical Company, Seoul, Korea) was added to TPN solution at a dose of 100 mg/kg body weight per day.
A 0.9 mm silicon cannula was placed into the internal jugular vein of rat under ether anesthesia. The cannula was coursed subcutaneously, and was brought outside and fixed at the back neck. Rats were kept in cages without limiting activity after cannulation, and TPN solution was infused continuously via the cannula over 24 h a day.
By the end of one week feeding, rats were weighed and then sacrificed under ether anesthesia. Blood sample was collected for determining total bilirubin (TBIL), aspartate transaminase (AST), alanine transaminase (ALT) and albumin (ALB). The liver was excised. One part of the liver was taken for quantitative assessment of triglyceride (TG) and cholesterol (CHO) content by using solvent extraction method. The remaining liver was fixed in 10% formalin and embedded in paraffin, and the sections were stained with hematoxylin and eosin (HE) and Sudan IV for histopathological examination. The severity of hepatic steatosis was evaluated semi-quantitatively according to the following grading system suggested by Ruwart et al[7]:
-: absence of steatosis.
+: mild steatosis with presence of lipid, mainly macrovesicular, in no more than 1/3 hepatocytes.
++: Moderate steatosis with presence of both micro and macro-vesic ular lipid in 1/3 to 2/3 of hepatocytes.
+++: severe steatosis with mixed micro and macro-vesicular lipid in more than 2/3 of hepatocytes.
++++: severe steatosis with presence of macrovesicular lipid in all or almost all hepatocytes.
Results were expressed as mean ± SD (mean ± SD). Statistical analysis was performed using commercially available SPSS package, and one-way ANOVA and Student′s t test were employed to compare the means between groups. Statistical significance was considered at P < 0.05.
One rat in group B2 died of pulmonary edema during TPN due to an overly rapid infusion rate, and was excluded from the study. All other rats remained alive up to the end of study. There was no statistically significant difference in the initial body weight among normal groups (A1, B1 and C1) and cirrhotic groups (A2, B2 and C2) (Table 1).
| Normal group | Cirrhotic group | |||||
| A1 | B1 | C1 | A2 | B2 | C2 | |
| n | 6 | 6 | 6 | 6 | 6 | 6 |
| BW (g) | 263.8 ± 13.5 | 268.1 ± 11.8 | 270.8 ± 10.1 | 318.2 ± 7.1 | 318.7 ± 5.3 | 321.8 ± 6.9 |
With respect to liver function, Table 2 shows no significant difference in TBIL, AST, ALT and ALB among normal groups, and no significant difference in TBIL, AST and ALT among cirrhotic groups. ALB was significantly lower in group B2 and C2 than in group A2 (both P < 0.05). Group C2 tended to have a lower ALT and higher ALB than group B2 (Table 2), but the difference was not statistically significant.
| Normal group | Cirrhotic group | |||||
| A1 | B1 | C1 | A2 | B2 | C2 | |
| TBIL (μmol/L) | 12.4 ± 3.2 | 13.8 ± 4.8 | 2.0 ± 5.1 | 13.0 ± 2.7 | 13.2 ± 2.1 | 12.0 ± 2.5 |
| AST (I U/L) | 156.9 ± 28.6 | 187.2 ± 29.6 | 179.7 ± 22.3 | 264.0 ± 23.6 | 269.5 ± 26.7 | 269.5 ± 102.0 |
| ALT (I U/L) | 54.0 ± 11.0 | 43.8 ± 11.7 | 44.8 ± 15.1 | 106.0 ± 24.0 | 117.7 ± 25.0 | 89.2 ± 22.7 |
| ALB (g/L) | 38.8 ± 1.7 | 37.8 ± 1.0 | 39.5 ± 3.1 | 32.2 ± 2.8 | 5.8 ± 1.9 a | 27.2 ± 2.4 a |
In normal groups, hepatic TG and CHO content was remarkably elevated in groups B1 and C1 in comparison with A1 (all P < 0.05), but were significantly lower in C1 when compared with B1 (both P < 0.05, Table 3). Similarly, in cirrhotic groups, B2 and C2 had a significantly higher hepatic TG and CHO level than A2 (all P < 0.05), and C2 had a markedly lower hepatic TG and CHO content as compared with B2 (both P < 0.05, Table 3).
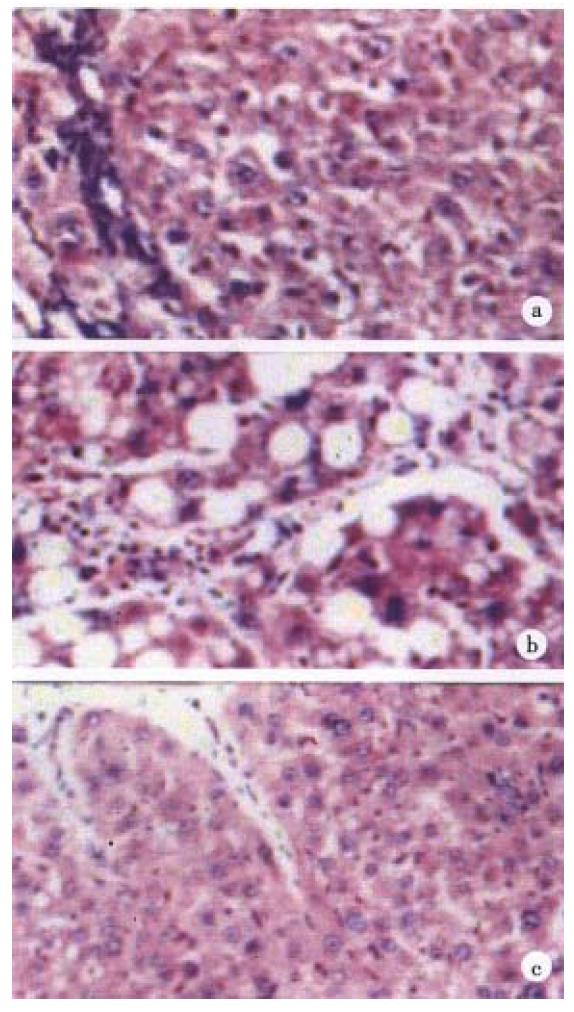
Semi-quantitative assessment of hepatic steatosis revealed that group A1 was negative, A2 +, both B1 and B2 ++++, and both C1 and C2++ (Figure 1).
TPN has been widely applied to patients who are temporarily or permanently unable to intake sufficient nutrition enterally. It, however, can cause some metabolic disorders, especially in long-term users. Of them, hepatic steatosis is the most serious and frequently encountered disorder. The TPN-associated hepatic steatosis may be related to the high concentration of dextrose or glucose in its regimen, and impaired TG secretion by the liver. TPN with the use of glucose or lipid emulsion as the sole energy source usually leads to a more severe hepatic steatosis than that using both glucose and lipid in combination, which was also observed in our preliminary study (data not shown). In cirrhotics, the liver tends to utilize less glucose and more lipid to produce energy. Hence, the glucose-lipid ratio of TPN should be adjusted in cirrhotic patients with, ideally, 55%-60% of energy provided by glucose and 40%-45% provided by lipid.
To date, the effect of lipid emulsion itself on liver function is controversial. It is commonly thought that LCT predisposes to liver function damage. But, Fan et al[8] reported that the MCT/LCT clearance rate was not impaired in cirrhotic livers, and hence believed that MCT/LCT was suitable for TPN in patients with liver cirrhosis. Our study showed that TPN using MCT/L CT could lead to hepatic steatosis in both normal rats (group B1) and cirrhotic rats (group B2). In addition, ALB in group B2 was significantly lowered. It was postulated that hepatic steatosis caused by TPN further damaged the compromise d liver function, and consequently influenced the synthesis of ALB. Regardless o f the causes of hepatic steatosis, it is certainly deleterious to liver function, especially in patients with liver cirrhosis and impaired liver function. There fore, it is necessary to treat the hepatic steatosis in long-term TPN users, and to supplement ALB simultaneously in those with liver cirrhosis and impaired liver function.
Many factors contribute to the development of TPN-associated hepatic steatosis. Absolute or relative deficiency of carnitine is an important factor. Long-term TPN can lead to a reduced level of carnitine, and chronic liver disease itself can also cause deficiency of carnitine. Rudman et al[9] determined serum carnitine level in 273 hospitalized patients and 12 normal subjects, and found that serum carnitine level in cirrhotic patients was only 25% of that in non-cirrhotic patients as well as in normal subjects; and autopsy study revealed t hat the content of carnitine in liver, kidney, muscle and brain of cirrhotics w as only 1/3 to 1/4 that of noncirrhotic patients.
Carnitine plays an important role in β-oxidation of free fatty acid and tricarboxylic acid cycle in mitochondria. In a carnitine deficiency, β-oxidation of free fatty acids would be inhibited, which leads to the liqid infiltration of hepatocytes as well as the insufficient production of ATP and consequent liver failure. Supplementation of carnitine probably can help prevent the TPN-associated hepatic steatosis, and promote oxidation of fat and recovery of liver function. The present study demonstrated that carnitine supplementation could minimize TPN-associated hepatic steatosis in both normal and cirrhotic rats. In addition, group C2 tended to have a lower ALT and higher ALB in comparison with group B2. It suggested that carnitine supplementation during TPN was helpful in reducing the severity of hepatic steatosis and subsequently protecting liver function. It is reasonable to speculate that carnitine supplementation in long term TPN is of therapeutic value, particularly for patients with impaired liver function. Further studies are still, however, needed to fully evaluate its clinical usefulness.
Dr. Li-Jian Liang, Male, Professor of hepatobiliary surgery, having 96 papers published.
Edited by MA Jing-Yun
| 1. | Bowyer BA, Fleming CR, Ilstrup D, Nelson J, Reek S, Burnes J. Plasma carnitine levels in patients receiving home parenteral nutrition. Am J Clin Nutr. 1986;43:85-91. [PubMed] |
| 2. | Hahn P, Allardyce DB, Frohlich J. Plasma carnitine levels during total parenteral nutrition of adult surgical patients. Am J Clin Nutr. 1982;36:569-572. [PubMed] |
| 3. | Moukarzel AA, Dahlstrom KA, Buchman AL, Ament ME. Carnitine status of children receiving long-term total parenteral nutrition: a longitudinal prospective study. J Pediatr. 1992;120:759-762. [PubMed] |
| 4. | Bonner CM, DeBrie KL, Hug G, Landrigan E, Taylor BJ. Effects of parenteral L-carnitine supplementation on fat metabolism and nutrition in premature neonates. J Pediatr. 1995;126:287-292. [PubMed] |
| 5. | Wu MC, Yang GS. The establishment of liver cirrhosis animal model in rats. Zhonghua Shiyan Waike Zazhi. 1984;1:145-147. |
| 6. | Tao RC, Yoshimura NN, Chinn IB, Wolfe AM. Determination of intravenous non-protein energy and nitrogen requirements in growing rats. J Nutr. 1979;109:904-915. [PubMed] |
| 7. | Ruwart MJ, Rush BD, Snyder KF, Peters KM, Appelman HD, Henley KS. 16,16-Dimethyl prostaglandin E2 delays collagen formation in nutritional injury in rat liver. Hepatology. 1998;8:61-64. [PubMed] |
| 8. | Fan ST, Wong J. Metabolic clearance of a fat emulsion containing medium-chain triglycerides in cirrhotic patients. JPEN J Parenter Enteral Nutr. 1992;16:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rudman D, Sewell CW, Ansley JD. Deficiency of carnitine in cachectic cirrhotic patients. J Clin Invest. 1977;60:716-723. [PubMed] |