Published online Aug 15, 1999. doi: 10.3748/wjg.v5.i4.296
Revised: July 3, 1999
Accepted: July 19, 1999
Published online: August 15, 1999
AIM: To determine whether serum vascular endothelial growth factor (VEGF) levels correlates with the severity of liver cirrhosis and whether portal hypertension impacts on the expression of serum VEGF protein.
METHODS: Fifty-three patients (mean age 56 ± 2 years) with HCV (n = 26), HBV (n = 13), and cryptogenic liver cirrhosis (n = 14) (Child-Pugh-s class A: 24, B: 19 and C: 12) and normal renal function constitute the patient population, who were all diagnosed by clinical, histological and radiological findings. Six healthy people and six patients with acute hepatitis served as controls. Severity of liver disease was evaluated by the CP score. Serum levels of IGF-1 and VEGF were measured by radioimmunoassay and ELISA, respectively. Portal hypertension was assessed using pulsed Doppler ultrasound.
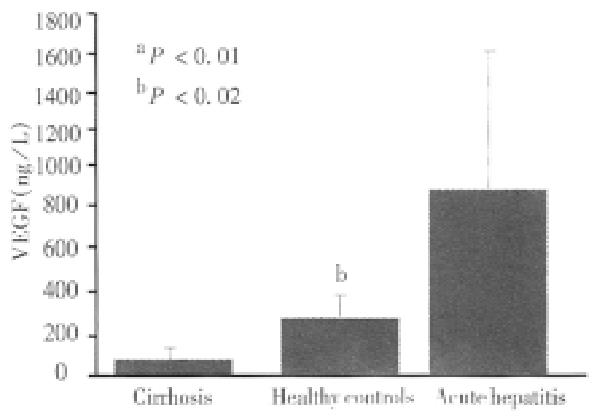
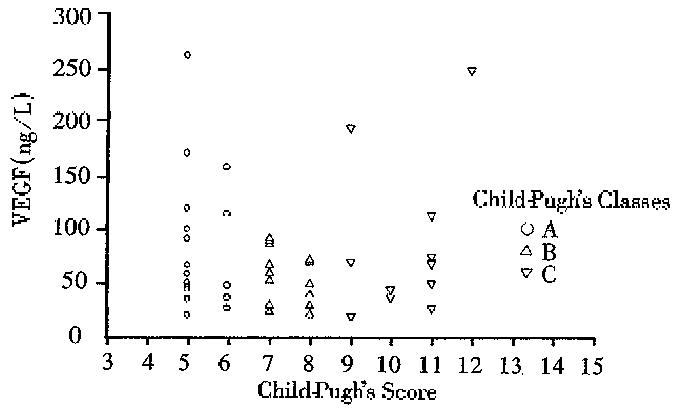
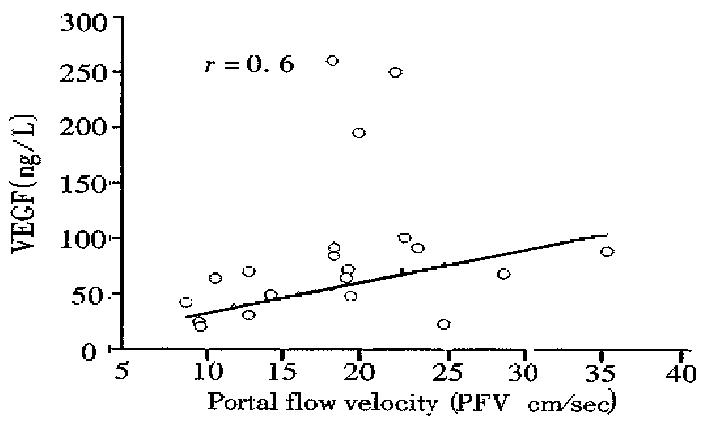
RESULTS: The mean serum VEGF levels in all cirrhotic patients (73 ± 58) were significantly lower than those of healthy controls (360 ± 217, P < 0.01) and acute hepatitis (1123 ± 1261, P < 0.01) respectively. No significant difference in median serum VEGF levels were noted among the different Child-Pugh-s classes (class A: median, 49.4 ng/L, range, 21-260 ng/L, Class B: median 59.9 ng/L; range 21-92, and Class C: median 69; range 20-247 ng/L). A significant correlation was noted between serum VEGF and two accurate parameters of portal hypertension: portal blood flow velocity (r = 06) and spleen size (r = 0.55). No correlation was found between VEGF serum levels and serum albumin, IGF-1, platelets count and aminotrasnferases (r = 0.2, r = 0.1, r = 0.2 and r = 0.2, respectively).
CONCLUSION: Circulating VEGF level in patients with liver cirrhosis could not serve as an indicator of the progression of chronic liver disease but rather, they may reflect increased portal hypertension or decreased hepatic regenerative activity or the combination of both.
- Citation: Nimer A, M P, D G, Y B, G S. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol 1999; 5(4): 296-300
- URL: https://www.wjgnet.com/1007-9327/full/v5/i4/296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i4.296
Angiogenesis or formation of new blood vessels, is a tightly regulated process in which blood vessels supply growing tissue with nutrients, growth factors, hormones, and oxygen to meat the needs of development or repair of injured tissues[1]. Liver growth also depends on angiogenesis. When angiogenesis is reduced, growth and repair decrease significantly[2]. In human subjects with liver cirrhosis, liver regeneration is impaired[3]. This raises questions about angiogenesis as a mediator for the growth of hepatic parenchymal cells and consequently whether angiogenesis reflects the severity of liver disease or not. Vascular endothelial growth factor (VEGF) was closely related to angiogenesis in various human cancers. However, little is known of its circulating levels in liver cirrhosis with or without portal hypertension. The presence or absence of collateral circulation significantly impacts the natural history of portal hypertension in cirrhotic patients. Collateral growth involves two different mechanisms: capillary collateral develops by sprouting (angiogenesis) and muscular collateral develops in situ from preexisting arterioles (arteriogenesis)[4]. The process is mediated also by various angiogenic growth factors such as VEGF, FGFs, IGF-1 and PDGF[5]. The clinical relevance of serum VEGF levels in portal hypertension patients has not been previously described and remains to be clarified.
VEGF is a secreted, 46 kDa dimeric protein active as a direct and specific mitogen for vascular endothelial cells and the only angiogenic factor exhibiting vascular permeability inducing activity. Five distinct human VEGF molecules having 121, 145, 165, 189 and 206 amino acids, respectively, were identified in man, all the result of alternative splicing of the same VEGF gene[1]. VEGF production is known to be upregulated by several substances, including oxygen, steroid hormones, reactive oxygen metabolites and protein kinase C agonists[6]. The level of VEGF gene expression was high in several human cancer cell lines and in tumors of the human gastrointestinal tract, brain, kidney and liver[7-11] as well as in normal tissues. Monacci et al[12] reported by in situ hybridization studies that VEGF gene in the rat liver was located in hepatocytes and a small number of Kupffer cells. However, the function of hepatocytes derived VEGF is not clear. VEGF has been shown to induce the proliferation of hepatic sinusoidal cells by virtue of increased expression of its receptors flt-1 and flk-1 following partial hepatectomy. Furthermore, VEGF gene expression increased in hepatocytes isolated from 70% resected rat liver[13]. We have noted recently that exogenous VEGF 165 administration to partially hepatectomised rats stimulates liver cell proliferation[2]. The aim of the present study was to determine whether serum VEGF levels in patients with liver cirrhosis reflects the degree of liver dysfunction and to determine whether portal hypertension impacts on VEGF serum levels.
The protocol of this study was approved by the Helsinki committees of the Rambam Medical Center and the Israel Ministry of Health. Subjects were briefed on the experimental nature of the study and signed informed consent form. Fifty-three patients (mean age 56±2 years) with HCV (n = 26), HBV (n = 13), and cryptogenic (n = 14) liver cirrhosis and normal renal function constitutes the patient population of this study. All patients were diagnosed by clinical, histological and radiological findings. Twenty-two patients belonged to Child-Pugh-s class A, 19 to Child class B, and 12 to Child class C. Patients with renal failure (creatinine > 106.8 μmol/L and creatinine clearance < 50 mL/min), dysthyroidism, and patients with known hepatocellular carcinoma were excluded. Severity of live r disease was evaluated by the CP score (Child-Pugh-s score)[14] and b y the generation of IGF-1[15]. Portal hypertension was defined as the presence of varices or/and portal vein diameter (PVD) > 13 cm, portal blood flow velocity (PFV) < 15 cm/sec and spleen size > 13 cm[16]. Sera were obtained from 53 patients with liver cirrhosis, six healthy controls and six patients with acute hepatitis. None of the patients with liver cirrhosis had acute illness during the study period.
Serum levels of IGF-1 were measured by radioimmunoassay kit (Incstar Corp, Stillwater, MN, USA) including extraction of octadecasilyl-silica (Sep-Packs). Sensitivity of the assay was 2.0 n mol/L (normal values male: 11-48 nmol/L; female, 9-46 nmol/L, with intra and inter assay variation of less than 8.4% and 10.3%, respectively.
ELISA was employed to determine the level of VEGF. As the concentration of serum VEGF is 15 ng/L-550 ng/L, a sandwich ELISA based on chemiluminescence rather than spectophotometry is required. Black ELISA plates (Corning) were coated with rabbit anti-VEGF IgG purified on protein G Sepharose chromatograph y column and blocked with 0.1% gelatin. Samples to be tested are added, followe d by anti-VEGF monoclonal antibody. Biotinylated rabbit anti-mouse IgG-1 and streptavidin horseradish peroxidase were then used and the presence of horseradish peroxidase was visualized by luminol substrate (Super Signal, Pierce). Photon s released were read in a microplate luminometer, and translated to ng/L according to a titration curve of VEGF included in the plate.
Thirty three patients were examined with duplex Doppler ultrasonography equipped with an ultrasonic system consisting of a mechanical sector scanner (IGE rt 3600) and a pulsed Doppler device with an insonating frequency of 3.5 MHz and a pulse repetition frequency of 2 kHz. A wall filter of 100Hz was used to avoid a false positive diagnosis of portal vein thrombosis, due to an inadequate high pass filter. The study was carried out by the same examiner (DG) to avoid interobserver variability. The examiner was unaware of the grade of cirrhosis so as to prevent from bias in the results. All the patients were kept fasting overnight prior to the sonography. Portal flow measurements were taken in the supine position during expiration. The portal trunk was scanned longitudinally with the sector scanner. We preferred the intercostal approach to keep the angle of insonation below 60°. The portal flow velocity (PFV) in cm/sec was measured directly. Over a given period, by the dedicated software supplied with the Doppler equipment. Each measurement was repeated until good and reproducible spectral patterns and blood flow sounds were obtained. Portal flow velocity and diameter were measured in triplicate and accepted if the coefficient of variation was less than 10%. The arithmetic mean of the three values was analyzed.
Results were expressed as mean ± SD (mean ± SD). Differences among groups were analyzed using Scheffes multiple comparison test. Difference between two groups was assessed by Mann-Whitney test for non-parametric data. The correlation between serum VEGF levels and, IGF-1, albumin, and portal blood flow were evaluated using simple regression analysis and Spearman correlation coefficient analysis. P < 0.05 was considered to be significant.
The clinical, radiological and laboratory data of our patients with liver cirrhosis are shown in Table 1. The mean serum VEGF levels in all cirrhotic patients were significantly lower than those of healthy controls (73 ng/L ± 58 ng/L versus 360 ng/L ± 217 ng/L, P < 0.01) and lower than patients with acute hepatitis (1123 ng/L ± 1261 ng/L, P < 0.01) respectively (Figure 1). No significant difference in the median serum VEGF levels were noted among different Child Pugh classes. The median serum VEGF levels of class A were 494 ng/L, ranging from 21 ng/L to 260 ng/L; Class B 59.9 ng/L, ranging from 21 ng/L to 92 ng/L; and Class C 69 ng/L ranging from 20 ng/L to 247 ng/L (Figure 2).
| Child-Pugh's A n = 22 | Child-Pugh's B n = 19 | Child Pugh's C n = 12 | |
| Age (yrs) | 56.3 ± 12 | 49.0 ± 12.9 | 53.3 ± 14 |
| Gender (m/f) | 9/13 | 13/6 | 10/2 |
| Albumin (g/dl) | 4.1 ± 0.3 | 3.3 ± 0.4 | 2.7 ± 0.3 |
| Bilirubin (mg%) | 1.1 ± 0.3 | 1.8 ± 1.0 | 3.8 ± 1.1 |
| PT (%) | 81 ± 13.5 | 66.3 ± 14.4 | 51.7 ± 9.4 |
| AST (U) | 66 ± 38 | 62 ± 45 | 94 ± 76 |
| ALT (U) | 67 ± 50 | 44 ± 42 | 58 ± 59 |
| VEGF (ng/L) | 78 ± 13.5 | 63 ± 9.4 | 87 ± 20 |
| IGF-1 (nmol/L) | 16.7 ± 1.4 | 13.3 ± 1.06 | 10.9 ± 1.1 |
| PFV (cm/sec) | 19.2 ± 2.7 | 17.2 ± 8.3 | 17.2 ± 5.3 |
| Pv diameter (mm) | 14.5 ± 2.3 | 11.7 ± 3.5 | 13 ± 2.5 |
| Spleen size (cm) | 15.1 ± 2.4 | 16.2 ± 1.9 | 15.4 ± 2.7 |
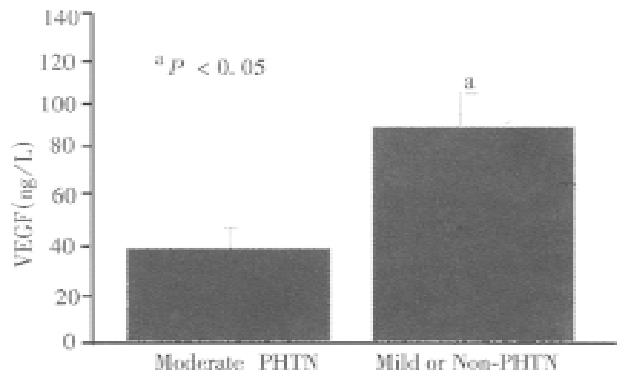
The mean serum VEGF levels were significantly different between patients with mild or non-portal hypertension and patients with moderate to severe portal hyper tension (mean 39.8 ng/L ± 16.5 ng/L, median 37.7 ng/L range 20-69.8 ng/L vs. mean 80.8 ng/L ± 5.7 ng/L, median 67.4 ng/L, range 20-260 ng/L, P < 0.005, respectively, Figure 3). A significant correlation was found between VEGF and two accurate parameters of portal hypertension, portal blood flow velocity (r = 0.6) (Figure 4) and spleen size (r = 0.55). However, weak correlation was found between VEGF and portal vein diameter (r = 0.3) and no significant correlation was noted between VEGF serum levels and serum albumin, IGF-1, and aminotransferases (r = 0.2, 0.1, and 0.2, respectively), suggesting that VEGF reflect neither the hepatic synthetic function in patients with liver cirrhosis nor inflammatory activity. Decreased VEGF levels were not associated with both portal vein thrombosis seen in two of our patients and platelet count (r = 0.2).
Vascular endothelial growth factor (VEGF) is closely related to angiogenesis in various human cancers[8-11]. However, little is known of its circulating levels in liver cirrhosis. We examined the circulating VEGF levels in chronic liver disease to assess their clinical significance. The results of this study clearly indicate that serum VEGF levels decreased which did not correlate with the disease severity in patients with liver cirrhosis. Moreover, the serum levels were down-regulated in the presence of portal hypertension and may be associated with the grade of hepatocyte regeneration.
Decreased serum VEGF levels in our patients with cirrhosis are in accordance with the study of Kraft A et al[17], which showed that ascitic fluid from patients with cirrhosis contained only a median of 303 ng/L (range 116-676 ng/L) of VEGF, corresponding to the low serum levels in our series. Our study is in agreement with the study by Chow et al[18] that VEGF expression did not correlate with the biochemical liver profile, and clinical stage of cirrhosis. That VEGF did not correlate with IGF-1, an accurate marker of synthetic function[15], suggests that expression of serum VEGF may characterize a progression toward lower proliferation rate in cirrhosis in vivo[3]. In a recent report, as well as in our study, patients with acute hepatitis demonstrated a significantly higher level of VEGF than the control group. This high level was not accompanied by serum aminotransferase, suggesting that the serum VEGF is not the result of damaged hepatocytes but rather a high regenerative capacity[19].
VEGF production is known to be down-regulated by several substances including angiostatin, and endostatin[20]. Thus, the low serum VEGF in these patients may be related to the increased activity of these inhibitors. Unfortunately, these inhibitors were not measured in our study subjects. On the other hand, acute phase proteins are upregulated in liver cirrhosis[21]. VEGF induces capillary hypermeability, which may contribute to acute inflammatory changes[1]. However, in spite of the necroinflammatory changes observed in some patients with increased aminotransferases, the serum VEGF levels remained low. More over, no correlation between VEGF and aminotransferases levels was noted in the study population.
Lower VEGF serum levels in the patients with cirrhosis may reflect the lower platelet count. However, no correlation between serum VEGF and platelets count was found in our patients. According to that postulation, the VEGF levels were expected to be elevated since activation of platelets during serum preparation increased the VEGF content by 8-10 times[22]. Finally, the serum VEGF in the liver was not associated with sonographic portal vein thrombosis, which also activates platelets. Low circulating VEGF levels in our patients with cirrhosis also indicate that VEGF was derived from neither the large burden of tumor cells superimposed on cirrhosis, nor platelets activated by the vascular invasion of HCC cells since HCC was excluded from our patients.
The clinical relevance of serum VEGF levels in portal hypertension patients has not been previously described and remains to be clarified. Previous experimental studies have suggested that the paracrine system of endothelin and other growth factors may participate in the regulation of hepatic hemodynamics in cirrhosis[23]. The present study assessed the relationship between increased port al pressure (portal blood flow velocity < 15) and serum VEGF levels in human cirrhosis. The results indicate that expression of VEGF in the serum is down-regul ated in the presence of moderate to severe portal hypertension. The reasons for this remain unclear. The expression and localization of PDGF, bFGF, EGF, and TGF-α in gastric coronary vein of cirrhotic and non-cirrhotic patients was investigated using immunohistochemical technique by Yang et al[24]. The strongly positive immunostaining rates were 93%, 89%, 70% and 68%, respectively in cirrhotic patients with portal hypertension whereas the immunostaining was negative in non cirrhotic patients. These results suggested that gastric coronary vein could produce angiogenic growth factor during cirrhosis, the growth factor might act on the vascular function and/or structure via autocrine-paracrine mechanism and not via endocrine mechanism.
The potential usefulness of duplex sonography in the diagnosis of portal hypertension has been assessed previously by Iwao et al[16]. A portal vein diameter greater than 13 cm or a portal vein flow velocity less than 15 cm/sec indicated portal hypertension with a sensitivity and specificity of over 80%. That VEGF correlates well with the portal blood flow velocity indicates that VEGF is down-regulated in patients with portal hypertension. However, patients with portal blood flow velocity above 15 cm/sec (no or mild portal hypertension) still had lower VEGF serum levels as compared to controls. This finding supports the notion that decreased VEGF serum levels are related to not only increased portal hypertension but also reduced regenerative capacity. This has an important clinical implication since the clinical outcome of these patients may be related to liver regenerative capacity.
In contrast to VEGF, Hsu et al[25] found that serum bFGF, another angiogenic factor, was significantly increased in patients with liver cirrhosis and HCC when compared with those with chronic hepatitis or normal subjects. However, as for VEGF, none of the liver function test findings were correlated with the levels of basic FGF. These results suggest that angiogenic factors play different roles in liver cirrhosis. The reason for the difference in circulating levels between VEGF and bFGF remains unclear.
Circulating VEGF levels in patients with liver cirrhosis can not serve as an indicator of the progression of chronic liver disease but rather, they may reflect increased portal hypertension or decreased regenerative capacity of the cirrhotic liver or combination of both. Further studies are warranted to determine the clinical value of soluble VEGF as a prognostic factor, and a surrogate indicator of angiogenesis.
Edited by MA Jing-Yun
| 1. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 2. | Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Nakano K, Chijiiwa K, Kameoka N. Impaired hepatic ketogenesis and regeneration after partial hepatectomy in cirrhotic rats. Eur Surg Res. 1994;26:257-265. [PubMed] |
| 4. | Sumanovski LT, Battegay E, Stumm M, van der Kooij M, Sieber CC. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044-1049. [PubMed] |
| 5. | Tanoue K, Tarnawski AS, Santos AM, Hanke S, Sugimachi K, Sarfeh IJ. Reduced expression of basic fibroblast growth factor and its receptor mRNAs and proteins in portal hypertensive esophageal mucosa: a mechanism responsible for muscularis mucosae thinning and variceal rupture. Surgery. 1996;119:424-430. [PubMed] |
| 6. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3222] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 7. | Kondo S, Asano M, Suzuki H. Significance of vascular endothelial growth factor/vascular permeability factor for solid tumor growth, and its inhibition by the antibody. Biochem Biophys Res Commun. 1993;194:1234-1241. [PubMed] |
| 8. | Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727-4735. [PubMed] |
| 9. | Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1588] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 10. | Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger DR. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255-1262. [PubMed] |
| 11. | Suzuki K, Hayashi N, Miyamoto Y, Yamamoto M, Ohkawa K, Ito Y, Sasaki Y, Yamaguchi Y, Nakase H, Noda K. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996;56:3004-3009. [PubMed] |
| 12. | Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993;264:C995-1002. [PubMed] |
| 13. | Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene. 1994;9:2683-2690. [PubMed] |
| 14. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 15. | Assy N, Hochberg Z, Amit T, Shen-Orr Z, Enat R, Baruch Y. Growth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosis. J Hepatol. 1997;27:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Iwao T, Toyonaga A, Oho K, Tayama C, Masumoto H, Sakai T, Sato M, Tanikawa K. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012-1017. [PubMed] |
| 17. | Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, Unger C, Marmé D, Gastl G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187. [PubMed] |
| 18. | Chow NH, Hsu PI, Lin XZ, Yang HB, Chan SH, Cheng KS, Huang SM, Su IJ. Expression of vascular endothelial growth factor in normal liver and hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. 1997;28:698-703. [PubMed] |
| 19. | Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41-45. [PubMed] |
| 20. | Raymond E. [Tumor angiogenesis inhibitors: media and scientific aspects]. Presse Med. 1998;27:1221-1224. [PubMed] |
| 21. | Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187-2190. [PubMed] |
| 23. | Poo JL, Jiménez W, María Muñoz R, Bosch-Marcé M, Bordas N, Morales-Ruiz M, Pérez M, Deulofeu R, Solé M, Arroyo V. Chronic blockade of endothelin receptors in cirrhotic rats: hepatic and hemodynamic effects. Gastroenterology. 1999;116:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Yang Z, Tian L, Peng L, Qiu F. Immunohistochemical analysis of growth factor expression and localization in gastric coronary vein of cirrhotic patients. J Tongji Med Univ. 1996;16:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Hsu PI, Chow NH, Lai KH, Yang HB, Chan SH, Lin XZ, Cheng JS, Huang JS, Ger LP, Huang SM. Implications of serum basic fibroblast growth factor levels in chronic liver diseases and hepatocellular carcinoma. Anticancer Res. 1997;17:2803-2809. [PubMed] |