Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.348
Revised: January 20, 1998
Accepted: February 19, 1998
Published online: August 15, 1998
AIM: To investigate clinic effects of Fuzheng Huayu 319 recipe (319 recipe) on liver fibrosis in chronic hepatitis B.
METHODS: Ninety-five patients with chronic hepatitis B were divide into the treated (63 cases) and control (32 cases) group, and orally administrated with 0.5g 319 capsule or 0.5g Dahuang Zhachong pill tid for 3 months, respectively. The liver functions and serological fibrotic markers were observed before and after treatment, 12 cases in the treated group were examined with liver biopsy.
RESULTS: Three hundreds nineteen recipe could remarkably decreased serum ALT level and total bilirubin and significantly improve serum albumin and A/G ratio. Its effects were better than Dahuang Zhachong pill. Before treatment, patients¡äserum monamine oxidase activities, tissue inhibitor of metalloproteinase (TIMP)-1, procollagen type III and laminin were all higher than those of health peoples. These levels decreased remarkably after treatment, and urine hydroxyproline level increased significantly (P<0.001-0.05). Compared with the control, the improvement in treated group was better than that in the control except TIMP-1. According to the scoring system for staging of chronic hepatitis, the fibrotic extents of 7 cases among 12 cases examined by liver biopsy decreased remarkably (1 case decreased by 3 scores, 5 by 2 scores, 1 by 1 score).
CONCLUSION: Fuzheng Huayu 319 recipe had good therapeutic effects on chronic hepatitis B, it could reverse the development of liver fibross to some extent. In general its effects were better than that of Dahuang Zhachong pill.
- Citation: Liu P, Liu C, Xu LM, Hu YY, Xue HM, Liu CH, Zhang ZQ. Effects of Fuzheng Huayu 319 recipe on liver fibrosis in chronic hepatitis B. World J Gastroenterol 1998; 4(4): 348-353
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.348
Our previous study showed that Fuzheng Huayu 319 recipe had good effect on the patients with post-hepatitic cirrhosis[1]. In order to study this recipe actions on chronic liver diseases and liver fibrosis, 95 patients with chronic hepatitis B (CHB) were treated and observed. Among them 63 cases were treated with 319 recipe, 32 with Dahuang Zhachong pill as controls. Patients’ liver functions and serum fibrotic markers were observed and the liver histological changes in 12 cases were examined by biopsy before and after treatment.
Ninety-five in-patients were included, 11 female and 84 male, aged from 20 to 65 years, averaging 37.6. They were clinically diagnosed as CHB according to the revised standard of the sixty Shanghai national virus hepatitis symposium in 1990. Each case had at least one positive serum HBV marker. Among them, there were 63 cases with HBeAg positive, 33 with jaundice, 29 with vascular spider or liver palm, 37 with splenomegaly. 26 cases were examined randomly by laparoscopy or biopsy and the histopathological assessment was in accord with clinic diagnosis.
Ninety-five patients were randomly divided into the treated group (63 cases) and the control group (32 cases). The patients’ ratio of sex, range of age, course of disease and liver functions were paralleled between two groups.
The treated group orally took 1.5 g 319 recipe, tid, composed of Semen Persicase, Radix Salviae, Miltiorrhiae, Cordyceps Sinnensis (Berk) Sace, Pollen Pini, etc. and was purified and made into capsules (0.3 g per capsule, equivalent to 8.17 g raw herbs) by Shanghai Zhonghua Pharmaceutical Factory. The control group orally took 1.5 g Dahuang Zhachong pill, tid, which was one of the traditional typical recipes for supporting healthy energy and eliminating blood stasis effectively, produced by Shanghai First Herb company (lot No.91221A1) and smashed to powder and made into capsules (0.3 g per capsule). Both groups were treated for 3 months, accompanying with the same expectant treatment and observed by double blind method and continuously observed for half a year after the treatment.
After thoroughly medical history inquiring, physical examination and the course of treatment, the blood samples were collected from patients with empty stomach in the morning before and after treatment and the following items were detected such as HBV markers, liver functions, monamine oxidase (MAO) activity and fibrotic markers etc. Also 24 h urine amount was recorded and measured for fibrotic markers. The normal serum samples were collected from healthy donors, whose sex ratio and age range were paralleled to the patients.
MAO activity clorimetric assay, the kit was from Wenzhou Yilikang Biology Technique Company.
Tissue inhibitor of Metalloproteinases-1 (TIMP-1) content ELISA, the kit was from Japan Fuji Medical Industrial Company.
Type-III-procollagen-N-peptide (P-III-P) content ELISA, the kit was from Jingshi Medical Technique Development Institute.
Type IV Collagen (IV-C) content ELISA, the kit was from Japan First Chemical Medicine Company.
Hyaluronic Acid (HA) content radioimmunoassay, the kit was from Shanghai Navy Medical Institute.
Laminin (LM) content Radioimmunoassay, the kit was from Shanghai Navy Medical Institute.
Hydroxyproline (Hyp) content (Jamall’s method)[2].
Laparoscopy and biopsy Twelve cases in the treated group were examined with liver biopsy before and after treatment, among them 6 were also examined with laparoscopy. The patients’ liver, spleen, peritoneum, mesentery, etc. were observed under STORZ fiber laparoscope and photographed. Also under laparoscopy, liver tissues were quickly taken in the point on the diaphragmatic surface of liver left lobe 2.5 cm away from falciform ligament and liver edge. The needle liver biopsy was punctured with 1 second paracentesis.
Liver histology The liver tissue samples were fixed in 10% neutral formalin buffer, and embedded in paraffin regularly, continuously cut to 5 μm slice and stained with HE. The pathologic examination standard was observed according to the grading and staging of the draft “The project of preventing and treating virus hepatitis” in 1995[3]. The inflammation activities were classified into 5 grades (G0-4), fibrosis degres into 5 stages (S0-4).
Rabbit anti-bovine type I collagen (I-C) serum, rabbit anti-human type III collagen (III-C) anti-serum, and rabbit anti-human IV-C serum were from the pathology department, Shanghai Medical University and rabbit anti-human LM serum from Sigma Company.
The paraffin sections of liver samples were deparaffined, gradient dehydrated with alcoholic solution, incubated at 37 °C for 1 h with H2O2 methyl alcohol so the endogenous peroxidase was removed, and digested with 0.2% peptic solution at 37 °C for 1 h, stained with regular PAP method. The negative and blank control were made.
Statistical analysisχ2, t test Ridit and u test methods were used.
ALT activities There were 52 cases were with increased ALT activities in the treated group before treatment, 48 (recovery rate 92.3%) recovered in 14 d-80 d after treatment, the average recovery time was 33.8 d. 24 had higher ALT activities in the control before treatment, 19 (79.2%) recovered after treatment in 14 d-90 d, the average recovery time was 37.0 d.
Serum total bilirubin In the treated group, 30 cases were higher before treatment, 23 (76.7%) became normal after treatment, 6 decreased but 1 increased. In the control, 12 cases were higher before treatment, 8 (66.7%) became normal after treatment, 2 decreased and 2 increased.
Serum albumin In the treated group, the serum albumin (Alb) content was 40.04 ± 5.2 before treatment, and 45.3 g/L ± 5.1 g/L ( P < 0.001) after treatment. Among them 13 cases were less than 35 g/L before treatment, and 11 were normal after treatment. In the control, that was 39.0 ± 4.9 before treatment and 39.7 g/L ± 5.3 g/L after treatment. Six cases were less than 35 g/L before treatment, 2 were normal after treatment. The Alb differences between two groups before and after treatment were 4.9 g/L ± 4.9 g/L, 0.7 g/L ± 2.5 g/L respectively. Significant difference (P < 0.001) was found.
Serum-A/G ratio In the treated group, it was 1. 46 ± 0.43, and 1.72 ± 0.48 respectively before and after treatment (P < 0.001). Twenty-one cases were less than 1.3 before treatment, 13 were normal after treatment. In the control, it was 1.32 ± 0.34 and 1.31 ± 0.34 before and after treatment, 8 cases were less than 1.3, 1 case normal. A/G difference between two groups before and after treatment were 0.26 ± 0.34, -0.01 ± 0.21 respectively and significant difference was found (P < 0.001).
The main symptoms and signs in both groups were improved obviously, such as fatigue, abdominal flatulence, nausea, yellow in eyes and hypochondriac pains etc., but no significant difference was found (Table 1).
| Group | n | Fatigue | Anorexia | Abdominal flatulence | Nausea | Yellow urine | Yellow eye | Hypochondriac pain | |
| Treated | 63 | Before | 34 | 20 | 21 | 13 | 16 | 21 | 21 |
| After | 1 | 1 | 1 | 1 | 3 | 3 | 4 | ||
| Control | 32 | Before | 24 | 10 | 9 | 12 | 6 | 9 | 10 |
| After | 1 | 1 | 1 | 0 | 1 | 2 | 1 |
HBV markers Before treatment there were 52 cases with positive HBsAg and HBeAg. 36 cases in the treated group, 2 or 16 of them had HBsAg or HBeAg negative conversion (5.6% or 41.0%) after treatment respectively. For 16 in the control, 1 was with HBsAg and 6 with HBeAg negative conversion (6.3% or 37.5%). No case had HBsAb negative conversion in both groups. There were 8 cases in the treated group (20.5%) and 1 in the control (6.3%) with positive HBeAb conversion, but no significant difference.
Follow-up observation Half a year after the treatment, ALP of 5 among the 48 recovered cases relapsed in the treated group, and 7 of 19 cases in the control relapsed. The HBsAg and HBeAg negative conversion rates were 2.8% and 38.5% in the treated group, 0 and 25% in the control respectively. For the therapeutic evaluation, 54 cases were effective, 9 not effective, and the effective rate was 85.7% in the treated group. 21 cases were effective, 11 not effective, and the effective rate was 65.6% in the control (Table 2, Table 3).
| Group | MAO(U) | TIMP | P-III-P | IV-C | HA | LN(ng/mL ) | Urine Hyp | |
| Cases | 30 | 24 | 20 | 29 | 44 | 42 | 29 | |
| Before | 64 ± 30 | 184 ± 58 | 1.81 ± 3.9 | 547 ± 345 | 377 ± 293 | 369 ± 73 | 21.6 ± 8.9 | |
| Treated | After | 28 ± 16d | 153 ± 54b | 0.80 ± 1.69a | 386 ± 212b | 210 ± 241d | 208 ± 85d | 24.5 ± 7.8a |
| Difference | 36 ± 27c | 31 ± 44 | 1.01 ± 2.08 | 161 ± 262 | 167 ± 199 | 161 ± 116 | 3.2 ± 7.5 | |
| Cases | 7 | 18 | 14 | 17 | 17 | 17 | 17 | |
| Before | 60 ± 30 | 204 ± 59 | 1.57 ± 1.46 | 404 ± 179 | 356 ± 313 | 382 ± 68 | 20.7 ± 7.3 | |
| Control | After | 47 ± 33 | 139 ± 39d | 1.71 ± 3.14 | 333 ± 150a | 220 ± 218a | 282 ± 103d | 22.6 ± 5.6 |
| Difference | 13 ± 14 | 65 ± 47 | 0.14 ± 2.51 | 72 ± 121 | 136 ± 251 | 99 ± 92 | 2.0 ± 6.5 |
MAO activities Compared with normal people, patients’ serum MAO activities increased obviously before treatment, 22 cases (59.5%) among 37 patients were more than 54 U (normal peoples’x + 2SD the same as following).
TIMP-1 Serum TIMP content increased obviously before treatment, in 16 cases (38.1%) with more than 204 ng/mL (-379 ng/mL) among 42 cases. P-III-P and IV-C Serum P-III-P and IV-C increased obviously before treatment, in 17 cases (50%) with more than 0.55 ng/mL (-24.22 ng/mL) P-III-P in 34 cases, in 45 cases (97.8%) with more than 145 ng/mL (-1511 ng/mL) in 46 cases.
HA Patients’ serum HA content increased obviously before treat ment, in 44 cases (72.1%) with more than 110 ng/mL. In the treated group , there were 31 cases with HA levels more than 110 mg/mL before treatment , after treatment 14 cases recovered, 15 decreased, 1 had no change and 1 increa sed. In the control there were 13 cases with HA levels more than 110 mg/mL before treatment; 5 recovered, 6 decreased, 1 had no change and 1 increased after treatment.
LM The serum LM contents of patients increased significantly compared with the normal (P < 0.001). There are 31 cases among 61 patients with LM contents more than 372 ng/mL (-540 ng/mL). All 21 cases in the treated group with LM more than 372 ng/mL recovered. For 10 cases in the control with more than 372 ng/mL LM, 6 recovered, 3 decreased and 1 increased. After treatment both groups had less MAO, TIMP-1, P-III-P, IV-C, HA and LM contents than before treatment, but the treated group decreased more significantly than the control (P < 0.05).
Urinary Hyp In the treated group urinary Hyp increased signific antly after treatment compared with that before treatment (P < 0.05). It slightly increased in the control, but there was no significant difference.
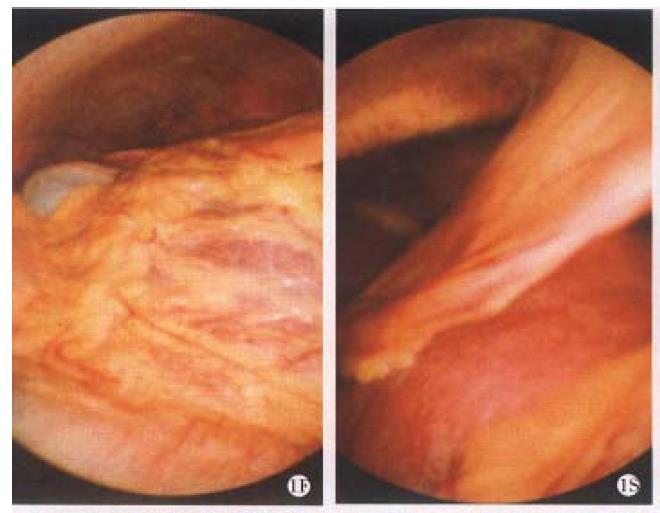
Laparoscopical examination One case was diagnosed as chronic persistent hepatitis (CPH) according to the old classification, the liver right lobe enlarged slightly; the edge was a little bit blunt, the small vessels on the ligamentum teres hepatis proliferated slightly and the spleen swelled mildly before treatment. After treatment the vessel proliferation attenuated. The livers of 4 patients with chronic active hepatitis (CAH) swelled and enlarged to varying degrees before treatment. The liver edges was slightly blunt, the color was pink or red, the most of the surface capsule thickened, the stripe was not clear enough. Among these 4 cases, 1 showed wave wrinkle, 2 had mass ( ± 4 mm diameter), 1 osmosised fibrin, and liver surface of 1 case covered by the greater omentum. After treatment, in 1 case the liver surface was separated from omentum (Figure 1). In another case with early liver cirrhosis, the right and left lobe of front liver swelled, accompanied with blunt edge, dark red color, thick capsule in the liver surface, vague stripe, 4 mm-6 mm diameter mass surrounded by the fibrous bundle, edemaed ligamentum teres hepatis, swollen spleen, and slightly varicosed veins in the great omentum and mensentery etc. After treatment the swelling of the liver lobe attenuated, fibrous bundles on liver surface reduced and the varicose vein on the omentum and mensentory recovered.
After treatment the liver inflammatory grades of 4 cases had decreased (1 decrea sed by 3 scores, 1 by 2 scores, 2 by 1 score), 6 cases had no obvious changes, a nd 2 cases increased by 1 score. Therefore, the improvement of liver inflammatio n was not significant on the whole ( P > 0.05) . But the improvement of the liver fibrotic stages was significant (P < 0.005). 7 cases improved (1 decreased by 3 scores, 5 by 2 scores, 1 by 1 score), 4 cases had no marked change, only 1 case increased by 1 score (Table 4).
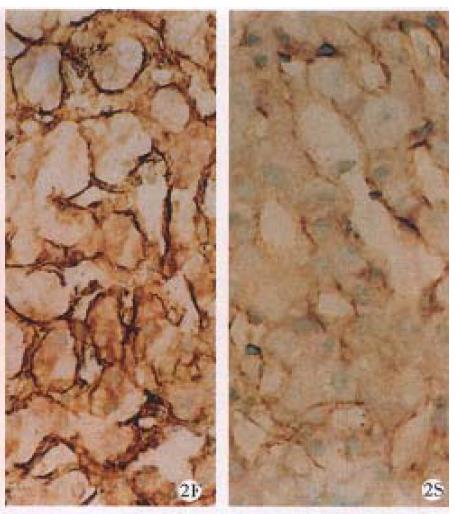
Type I collagen Before treatment, I-C was positively stained along the most liver sinusoid with a ground circular shape. The portal areas became wider, surrounded by hepatocyte necrosis and formed fibrous septum stretched from the portal areas to hepatic lobules. After treatment, these collagen became smaller, thinner or discontinuous. Stained areas decreased in 1/3 of cases, unchanged in 1/2 of cases, increased in only 1 case. The portal areas decreased in 1/2 of cases, the others unchanged. Although positive stained in hepatocellular necrotic areas, 6 of 8 cases positive stained in fibrous septum before treatment showed either negative stain or decreased areas, none in-creased after treatment (Figure 2).
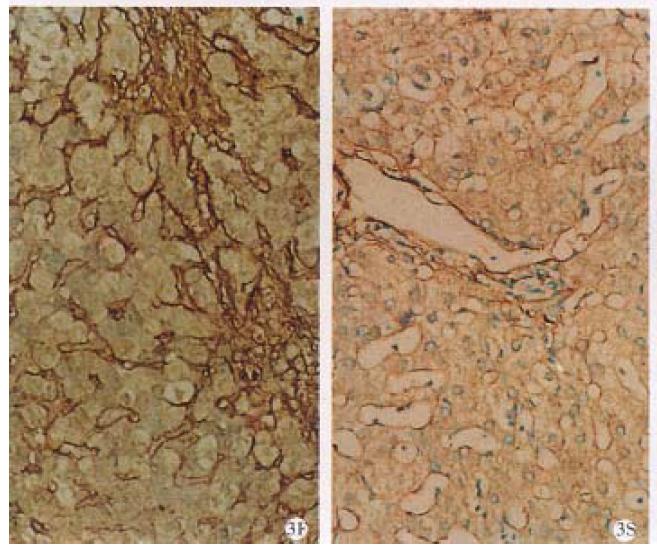
Type III collagen Before treatment, most III-C with ground circular shape expressed around the sinusoids as well as I-C, in widen portal areas, in hepatocyte necrotic areas along limitlate plate and in fibrous septum. After treatment, the positive stains did not change obviously around sinusoid, but decreased, unchanged or increased respectively for 1/3 of cases in the portal areas. Of the 9 cases positive stained before treatment in fibrous septum, 6 cases became negative or decreased, 1 unchanged and 2 increased slightly (Figure 3).
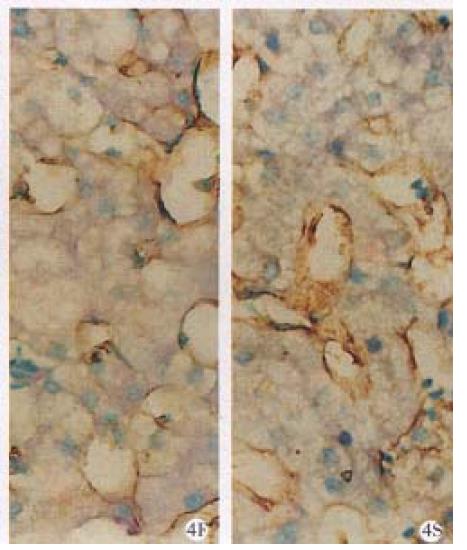
Type IV collagen Before treatment, IV-C around sinusoids expressed as thicker or thinner circles, some of them were thin and continuos. In the portal areas, hepatocellular necrosis areas and fibrous septum, IV-C expressed as I-C and III-C. After treatment, IV-C expression around sinusoid in half of the cases increased, or became grosser or become continuous from discontinous, none decreased. 10 cases were stained positive for IV-C in fibrous septum before treatment, and after treatment, 6 became negative or decreased, 2 unchanged, 2 slightly increased (Figure 4).
Laminin Before treatment, LM was stained slightly positive, scattered around sinusoids and in fibrous septum like thin circle or discontinuous string. But in sinusoids with hepatocyte speacemeal necrosis and in the portal areas, LM expressed strong positive and thick circles. After treatment, among 10 cases with LM positive stain around sinusoids, 2 decreased, 6 unchan ged, 2 slightly increased. The changes in the portal areas were similar to those around sinusoids. Among 9 cases with positive staining in fibrous septum, 4 decreased or became negative, 4 unchanged, 1 increased.
The histological staging for fibrotic degrees was emphasized on the new classifi cation of chronic hepatitis[4]. WANG reported that although most inflamation activities decreased after treatment, fibrosis still existed even developed in the patients observation of twice liver biopsy [5]. It is suggested that it is necessary to treat fibrosis during chronic hepatitis.
319 recipe had good therapeutic effect on post-hepatitic cirrhosis. In this study it had been found that 319 recipe could decrease serum ALT activities and serum bilirubin, improve serum Alb and A/G ratio. The effects on CHB were better than Dahuang Zhachong pill.
The fibrotic degrees could demonstrate pathological development of chronic hepatitis. Because the difficulty and limit of liver biopsy, serum fibrotic markers important for diagnosis and evaluation of drug effects[6]. Serum MAO was first used in clinic[7], later serum P-III-P, LM, IV-C, HA and TI MP-1 were applied[8-12]. But these markers could be influenced by body connective tissue metabolism, and single marker lacked specificity, so recently it is maintained that multi-markers should be combined for practice[6]. In this paper, serum MAO, TIMP, P-III-P, IV-C, HA and LM in patients all increased remarkably compared with normal people. If “x± 2SD” was set as abnormal value, their abnormal ratios were IV-C (97.8%) , HA (72.1%), MAO (59. 4%), LM (50.8%), P-III-P (50.0%), TIMP (38.1%), similar to other reports[10-12]. After the treatment, however, these 6 markers were improved to some extents . Except TIMP, the other 5 markers improvement in the 319 recipe treated group were more obvious than the control, especially the serum MAO and LM. Urinary Hyp excretion increased also after the treatment. It is shown that 319 recipe not only improved patients’ liver functions, but also decreased serum fibrotic markers which pathologically increased in CHB. Its comprehensive effects are better than Dahuang Zhachong pill.
Among 11 cases with fibrotic stage 1-4 in the treated group, 7 cases had obviously decreased fibrotic septum, the enlarged portal areas reduced, and hepatic lobule structure nearly recovered. In 5 cases, fibrotic stages decreased by 2 scores, 1 case by 3 stages and 1 case by 1 stage. Type I, III and IV collagen expressions in portal areas and fibrotic septum in most samples decreased or became negative, but in some cases the changes were not obvious. LM expressions in these places also decreased and were paralleled to pathological staging. All these indicated that 319 recipe could inhibit CBH fibrotic development, improve degradation of proliferated fibrous tissues in liver, and had a significant application on inhibition of liver cirrhosis.
Project supported by the National 8-5 Scientific Program, No. 859190402
| 1. | Liu P, Liu C, Hu YY, Zhu JL, Xue HM, Xu LM. The clinical investigation of Fuzheng Huayu 319 recipe effects on post hepatic cirrhosis. Chin J Inter Med. 1996;16:459-462. |
| 2. | Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 421] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Zai WR, Wang TL, Zhou XJ, Zhang TH. Diagnosis, grading and staging for chronic hepatitis. Chin J Dis. 1996;16:277-281. |
| 4. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1506] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 5. | Wang TL, Liu X, Zhao JB, Dong J, Chen FX, Zhang M et al. Investigation of classification for chronic hepatitis: Pathological analysis about liver biopsy in 316 cases. J Diagn Pathol. 1994;1:19. |
| 6. | Liang KH, Li SB. Hepatology, 1st edition. Beijing: Peoples' Health Press. 1995;570-830. |
| 7. | Liang KH; Itokenyiqi. Diagnostic meaning of monamine oxidation for liver fibrosis. Clin Patholo. 1973;21:143. |
| 8. | Rohde H, Vergas L, Hahn E, Timpl R. Radioinmmunoassay for type III col-lagen pepetide and its application to human liver: collagen type IV, laminin and fibronectin. Gut. 1980;21:63-70. |
| 9. | Niemelä O, Risteli L, Sotaniemi EA, Risteli J. Type IV collagen and laminin-related antigens in human serum in alcoholic liver disease. Eur J Clin Invest. 1985;15:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Engström-Laurent A, Laurent UB, Lilja K, Laurent TC. Concentration of sodium hyaluronate in serum. Scand J Clin Lab Invest. 1985;45:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Murawaki Y, Yamamoto H, Kawasaki H, Shima H. Serum tissue inhibitor of metalloproteinases in patients with chronic liver disease and with hepatocellular carcinoma. Clin Chim Acta. 1993;218:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |