Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.340
Revised: April 22, 1998
Accepted: May 30, 1998
Published online: August 15, 1998
AIM: To investigate the copper-chelating therapeutic effect in Wilson disease (WD) with different clinical phenotypes and polymorphisms of ATP7B gene.
METHODS: One hundred and twenty-two WD patients with different clinicalphenotypes were given DMPS intravenously and Gandou copper-chelating tablet orally for one month. The therapeutic effect was judged by modified Goldstein mothod. Exon 18 of ATP7B gene extracted from the DNA of patients and 20 healthy volunteers was amplified with PCR mutation and polymorphism were screened with SSCP technique.
RESULTS: Four kinds of abnormal migration bands in PCR-SSCP were observed in 37 WD patients, mutation frequencies of three different disease phenotypes, and curative effect between mutation group and non-mutation group showed no statistically significant difference (P > 0.05), but the total effectiveness rates in patients with Wilson type or pseudosclerosis type were significantly higher than those of patients with hepatic type ( χ2 = 6.17, P < 0.05).
CONCLUSION: Most WD patients are compound heterozygotes, the patients with different clinical phenotypes have different response to copper-chelating therapy. Specific mutation, at least in part, plays a role in influencing the disease phenotypes and therapeutic effect.
- Citation: Ren MS, Hu WB, Zhang Z, Ju SW, Fan YX, Wang GQ, Yang RM. Copper-chelating therapeutic effect in Wilson disease with different clinical phenotypes and polymorphisms of ATP7B gene. World J Gastroenterol 1998; 4(4): 340-342
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.340
Wilson disease (WD) occurs most frequently in adolescence and characterized by failure to incorporate copper into ceruloplasmin in the liver, and failure to excrete copper from the liver into bile[1]. This results in toxic accumulation of copper in the liver, and also in kidney, brain and cornea. The induced liver cirrhosis and/or progressive neurological damage are fatal if not treated with copper chelating agents promptly. WD is divided into different clinical phenotypes according to the age at onset and clinical presentations. The therapeutic effect and its prognosis depend on the clinical phenotypes. With the cloning of WD gene (ATP7B) and elucidation of its molecule structure, it is possible to explore the relationships between clinical therapeutic effect and phenotypes or mutations. In this report, we observed the effect of copper-chelating therapy in WD with different phenotypes and polymorphisms of ATP7Bgene.
From March 1995 to July 1996, 122 inpatients with WD were chosen for this study. There were 74 men and 48 women with a mean age of 19 ± 6.5 years and an average disease course of 2.5 ± 1.5 years. They had clinically definite WD by criteria that there are typical extrapyramidal system and/or liver symptoms; the concentration of caeruloplasmin < 200 mg/L and urinary copper excretion >100 μg/24 h[2]. According to clinical symptoms, we divided all WD patients into Wilson type (74 cases), pseudosclerosis type (36 cases) and hepatic type (12 cases)[3]. The severity degree of sickness were graded by modified Goldstein method[4]. They included 21 patients with grade I, 45 grade II, 41 grade III and 15 grade IV. Twenty healthy volunteers consisting of 11 men and 9 women with a mean age of 20 ± 4.5 years were chosen as control.
All WD patients were given DMPS (unithiol) intravenously, at a dose of 20 mg/kg daily. Meanwhile, Gandou copper-chelating tablets were given orally, 10 tablets, three times daily, with one month as acourse of treatment. They had low-copper diet throughout the study. Before and after treatment, typing, grading and therapeutic effect judgment were accomplished independently by two experienced neurologists in our institute.
White blood cells were first separated from 10mL peripheral blood anti-coagulated with 3 mL ACD, then genomic DNA was extracted as described by salting-out method[5]. The primer sets for exon 18 mutation analysis were: 5’-ACCTGTTGCCAACACTAGCAT-3’ and 5’-TCCCAGCACCCACAGCC-3’[6]. Genomic DNA of 800 ng was dissolved in 25 µL PCR buffer solution containing 20 µmol/L TrisCl (pH 8.5), 50 mmol/L KCl, 2 mmol/L MgCl2, 200 µmol/L dATP, dGTP, dCTP, dTTP,0.5 µmol/L each primer and 1 unit Taq DNA polymerase (Promega Corporation). The cDNA was amplified in a DNA thermal cycler (MJ research, U.S.A.). The cycle of denaturation at 93 °C for 45 s, renaturation at 58 °C for 45 s and extension at 70 °C for 90 s was repeated for 35 times, with the last step extended to 10 min. Nondenatured 8% polyacrylamide gel (acrylamide/bis-acrylamide cross linker with a ratio of 49:1, and glycerol content 5%) was poured between two glass plates of DYY-III vertical electrophoresis gel tank apparatus with cooling compartment, placed at least 2 h in room temperature for the gel to set, the wells were added with 1 × TBE buffer using a Pasteur pipette or pipette tip. After electrophoresis for 30-60 min in advance,5 µl-8 µl PCR product was added with same amount of SSCP gel loading buffer (containing 96% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol FF). All samples were denatured at 98 °C for 5 min, and then quickly chilled on ice for electrophoresis. Fragments were resolved by electrophoresis (30 W, 15 °C-20 °C) for 6-8 h. Silver staining was performed and photograph was taken for further analysis according to the method reported by Yu Long[7].
Marked effectiveness. After one month of copper-chelating treatment, conditions were improved remarkably by two grades.
Improvement. There were some improvement in clinical condition, conditions were one grade better than before treatment.
Inefficiency or exacerbation. Conditions had no obvious changes or became worse.
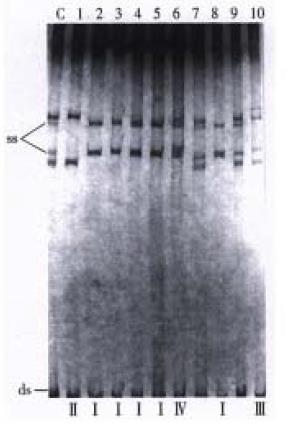
The exon 18 of 122 WD patients was analyzed. Four kinds of abnormal migration bands in PCR-SSCP were observed in 37 WD patients, which were named by us as type I, II, III and IV temporarily (Figure 1). It was found that type I, II and III had both abnormal band shifts and similar band shift as control group, which indicated that most WD patients were compound heterozygotes, i.e., they carry two different mutations. According to the relationship of ssDNA conformation with molecular electrophoresis shift, mutation frequencies of Wilson type, pseudosclerosis type and hepatic type WD were 29.7%, 30.6% and 33.3%, respectively. No statistically significant differences were found by Chi-square test (P > 0.05, Table 1).
| Phenotype | Cases | Length of PCR product (bp) | Abnormal SSCP | Mutation frequency (%) | ||||
| I | II | III | IV | Total | ||||
| Wilson | 74 | 204 | 17 | 4 | 1 | 0 | 22 | 29.7 |
| Pseudosclerosis | 36 | 204 | 9 | 2 | 0 | 0 | 11 | 30.6 |
| Hepatic | 12 | 204 | 3 | 0 | 0 | 1 | 4 | 33.3 |
No statistically significant difference was found by Ridit analysis in notable effectiveness between three different phenotypes (P > 0.05), while the total effectiveness of Wilson type or pseudosclerosis type was much better than that of hepatic type WD ( χ2 = 6.17, P < 0.05). Comparison of total and notable effectiveness between mutation group and non-mutation group showed no significant difference (P > 0.05, Table 2).
WD is certainly preventable and to some extent is curable, if it is diagnosed and treated in early stage. Recently, we have achieved satisfactory therapeutic effects in treating WD patients with copperchelating agents DMPS, DMSA and Gandou copperchelating tablet[8-10]. The gene for WD, predicted to encode a coppertransporting membrane protein, has now been cloned, making it possible to explore the relationship between therapeutic effect and different phenotypes or mutations. It has been proved that mutation of WD gene could disrupt ATPase function so that copper was detained in tissues and cells, leading to the occurrence of different clinical phenotypes. Up to now, Thomas et al[11] has identified 23 disease-specific mutations including 59 monosomic mutations in 58 WD patients, which explained the diversity of the disease phenotypes, but he did not sum up the relationship between mutation and clinical phenotypes due to the limited number of cases. These mutation sites involved largely functional regions of ATPase (exon 13-18), but occasionally detected in copperbinding regions. It was reported that the frequencies of missense mutation detected in exon 14 and 18 were 28% and 10%, so that they were usually chosen as important sites of genetic screening[12]. PCR-SSCP technique is of great value in direct mutation identification and polymorphism analysis, and SSCP analysis of PCR products was made by denaturing ds DNA and fractionating the strands on a non-denaturing polyacrylamide gel. Under the appropriate condition, the electrophoretic mobility of the DNA was dependent not only on its length and molecular weight, but also on its conformation. This secondary structure was determined by the balance between destabilizing thermal forces and weak stabilizing forces such as intra-strand base pairings and stacking, which were in turn dependent on the primary structure of the DNA strand, not only did the complementary strands migrate as separate bands on the gel, but small differences in sequence also altered the mobility of these strands. Aberrant bands were then sequenced to confirm the presence of mutation or polymorphism [13]. In this study, 37 WD patients had aberrant migration bands in PCR-SSCP, indicating strongly that the exon 18 of ATP7B gene may be one of the regions with a higher mutation frequency, although the exact mutation sites and its trait could be identified by further sequential analysis. At same time, comparative results of the therapeutic effect showed that there were no statistically significant differences between the three kinds of clinical phenotypes as well as mutation group and non-mutation group (P > 0.05). In view of the fact that most WD patients who carry two different mutations are compound heterozygotes and total effectiveness of Wilson type or pseudosclerosis type WD are much better than that of hepatic type WD, we consider that specific mutation, at least in part, plays a role in influencing the disease phenotype and curative effect.
Project supported by the National Natural Science Foundation of Anhui Province, No.97412001.
| 1. | Ren MS, Fan YX, Yang RM, Han YZ, Wu GJ, Xin YR et al. A comparative study of biliary elements and clinical phenotypes in Wilson disease. China Natl J New Gastroenterol. 1997;3:260-262. |
| 2. | Shi YQ. Practical neurology. 2th ed. Shanghai: Shanghai Science and Technol-ogy Press. 1995;771-772. |
| 3. | Walshe JM. Wilson's disease. In: Vinken PJ, Brruyn GW, Klawans HL, eds. Handbook of clinical neurology, extrapyramidal disorder. Amsterdam: Elsevier. 1986;223-224. |
| 4. | Yang RM. Hepatolenticular degeneration. 1st ed. Hefei: Anhui Science and Technology Press. 1995;204-205. |
| 5. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13387] [Cited by in RCA: 14458] [Article Influence: 390.8] [Reference Citation Analysis (0)] |
| 6. | Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1311] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Yu L, Gong LQ, Deng Y, Shi SL, Zhao SY. A simple and easy preparation method of silver staining PAG glue film for PCR-SSCP and STR analysis. Chin J Med Genet. 1996;13:177. |
| 8. | Ren MS, Yang RM, Zhang B, Xu SH. Comparison of therapeutic effects be-tween unithiol, succimer and penicillamine on hepatolenticular degeneration. Chin J New Drugs Clin Remedies. 1998;17:23-25. |
| 9. | Ren MS, Yang RM. Clinical curative effects of dimercaptosuccinic acid (DMSA) on hepatolenticular degeneration and the impact of DMSA on bil-iary trace elements. Chin Med J. 1997;110:694-697. |
| 10. | Ren MS, Zhang B, Yang RM. [Clinical study of integrated traditional and Western medicine therapy on hepatolenticular degeneration]. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:136-138. [PubMed] |
| 11. | Thomas GR, Forbes JR, Roberts EA, Walshe JM, Cox DW. The Wilson disease gene: spectrum of mutations and their consequences. Nat Genet. 1995;9:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 346] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Fan Y, Yang R, Yu L, Wu M, Shi S, Ren M, Han Y, Hu J, Zhao S. Identification of a novel missense mutation in Wilson's disease gene. Chin Med J (Engl). 1997;110:887-890. [PubMed] |
| 13. | Griffin HG, Griffin AM. PCR technology: current innovation. Florida: CRC Press. 1995;165-166. |