Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.329
Revised: October 10, 1998
Accepted: November 26, 1998
Published online: August 15, 1998
AIM: To investigate the effect of endotoxin on liver fibrosis and further define the role of hepatocytes in production of fibronectin in primary livercell culture by endotoxin.
METHODS: After isolation and seeding of hepatocytes, the obtained cells were added to various doses (0, 5, 10, 15 and 20 mg/L) of LPS treated culture media. The cells were collected and counted at various periods (0, 12, 24, 48, 72, 96, 120 h). The concentrations of fibronectin were tested by electrophoresis.
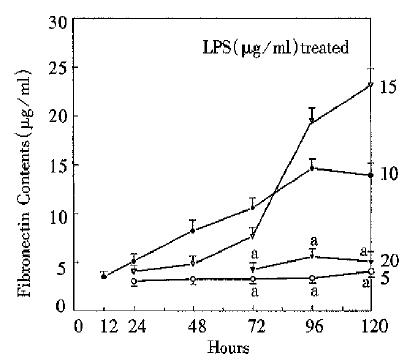
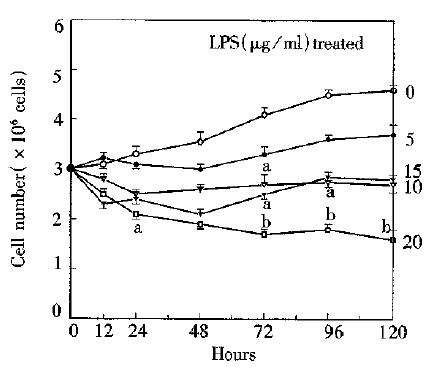
RESULTS: The fibronectin levels tended to increase with prolongation of culture time. There was a sharp increase after 72 h in 10 or 15 LPS treated group. The peak level of fibronectin was above 20 mg/L. However, cell proliferation was inhibited during the course. Cell number of untreated control group (4.6 ± 0.1 × 106) was about three fold that of 20 LPS treated group (1.6 ± 0. 2 × 106) at 120 h.
CONCLUSION: Hepatocytes have a potent ability to produce fibronectin stimulated by endotoxin, suggesting that hepatocytes might participate in the process of liver fibrosis.
- Citation: Jia JB, Han DW, Xu RL, Gao F, Zhao LF, Zhao YC, Yan JP, Ma XH. Effect of endotoxin on fibronectin synthesis of rat primary cultured hepatocytes. World J Gastroenterol 1998; 4(4): 329-331
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/329.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.329
Endotoxin may contribute to the pathogenesis of several disease processes, including thermal injuries, bowel diseases, pancreatitis and septic syndrome[1]. Previous experiments proved that gut-derived endotoxemia was a hallmark of hepatic failure[2]. As an essential presupposition of chronic hepatic failure, liver fibrosis was also directly associated with gut-derived endotoxemia[3]. Besides Ito cells, Kupffer cells, endothelial cells and biliary epithelial cells, some experiments in vivo or in vitro indicated that liver parenchymal cells participated in the process of liver fibrosis[4]. Fibronectin, a component of extracellular matrix, arises at the early stage of liver fibrosis. In this study, we observed the alteration of cell population and fibronectin secreted by primarily cultured rat hepatocytes in response to lipopolysaccharide (LPS).
Hepatocytes were isolated from Wistar rats (250 g-300 g in weight, fasted overnight) by the collagenase method of Gressner[5]. The liver was preperfused with D-Hank’s solution containing 0.2% glucose for 10 min at a flow rate of 10 mL/min to wash out the blood. Then it was perfused with D-Hank’s solution containing 0.05% collagenase (Collagenase type I, Sigma) for another 10 min. After removal, the liver was cut into tiny pieces. Passing through a stainless steel mesh (mesh size: 100 μm), hepatocytes were purified by centrifugation (50 ×g, 1 min × 2, 4 °C). The viability of the final parenchymal cell suspension, checked by trypan blue exclusion, was between 90% and 95%. Cell recovery was about 2 × 108 cells/liver and contamination with non-parenchymal cells was less than 1%. Cells were seeded at a density of 30 × 106 cells/10 cm culture dish (Coring, N. Y. ) and cultured at 3 7 °C in a humidified atmosphere of 5% CO2 and 95% air in 10 mL RPMI-1640 (Gibco) containing 10% fetal bovine serum, 0.02 U/mL insulin, penicillin (100 IU/mL) and streptomycin (100 μg/mL). The medium was changed 3 h after plating, during which non-adherent and non-vital cells were removed. Lipopolysacchar ide (Escherichia coli, 0111B4, Sigma) was added to hepatocyte culture media at various concentrations of 0, 5, 10, 15 and 20 mg/L. Finally, the number of cells per dish were counted at 0, 12, 24, 48, 72, 96 and 120 h. The morphology of hepatocytes was observed under microscope.
Rocket electrophoresis was employed to determine the fibronectin concentration in the culture medium of hepatocytes. Gelatin was made from 0.05 M pH 8.2 sodium barbiturate-HCl buffer containing 2% agarose and 1% fibronectin antibody (Shanghai Biologic Products Research Institute). Time of electrophoresis was limited within 6 h. Voltage and current intensity were controlled respectively at 80 v and 100 mA. The final numerical value was determined by linear regression according to peak-shift length of fibronectin standard ladder concentrations.
The results were expressed as mean ± SD. Variance analysis and q test were used to determine the statistical significance by SPSS/ PC + 4.0 general software. When the P value was less than 0.05, the result was considered statistically significant.
The present experiment demonstrated the effect of LPS on cellular fibronectin. As shown in Figure 1, in 10 and 15 mg/L LPS treated groups, fibronectin tended to increase progressively, especially after 72 h. However, the fibronectin concentrations of 5 and 20 mg/L LPS treated groups varied slightly, but were significantly different from those of 10 and 15mg/L groups (P < 0.05). It should be noted that fibronectin concentration of 0 mg/L LPS treated group could not be tested by the present means. So did it in 5 and 10 mg/L LPS treated group before 24 h, 15 mg/L group before 12 h, and 20 mg/L group before 72 h.
As shown in Figure 2, proliferation of cultured hepatocytes was remarkably inhibited in all LPS treated groups, especially in 20 mg/L group. Hepatocyte population of untreated control group increased progressively. After 72 h, multiplication rate in all groups was accelerated. Hepatocyte population of 10, 15, or 20 mg/L was significantly less than that of control group. Population of control group (4.6 ± 0.1 × 106) was approximately three-fold that of 20 mg/L LPS treated group at 120 h.
In morphological study, appearance of hepatocytes exhibited a slight difference if they were cultured at various concentrations of LPS. Almost in every culturedish, binuclear and even trinuclear cells could be observed, which indicated active proliferation in the culture. The higher the LPS levels were, the more hepatocyte lesions exhibited. These pathologic changes included cloudy swelling, hydropic degeneration, and pyknosis or karyolysis. After 72 h, there were cell debris and new products (without defined characteristics) around vital cells.
Fibronectin is a high molecular weight glycoprotein and increases firstly among extracellular matrix proteins during liver fibrosis. The increased serum fibronectin level is now considered as an important and susceptible marker of fibrosis and necrosis in chronically injuried liver[6]. Normally, it is very difficult to detect fibronectin because of its low concentration. Thus enhanced fibronectin may serve as an indicator of pathophysiological process. Fibronectin in the liver mainly exists in the portal, septal and perisinusoidal matrix. Most of fibronectin is secreted from mesenchymal cells including Kupffer cell, Ito cell and endothelial cells. In some pathological processes, hepatocytes may secrete fibronectin[7]. Until now, no study has shown the direct relation between endotoxin and hepatocytes in the liver fibrosis process. In this study, hepatocytes culture in vitro was used to observe this relationship. In 10 and 15 mg/L LPS treated groups, fibronectin concentrations tended to increase progressively during the whole culture process, especially after 72 h. It may indicate that it is endotoxin that stimulates fibronectin synthesis of hepatocytes. In 5 and 20 mg/L LPS treated group, however, synthesis was not stimulated. Therefore, there may be response limits in which endotoxin acts as a stimulator of fibronectin synthesis. As for control group, hepatocytes did not secrete fibronectin because there was no stimulator.
This study demonstrated that the proliferation of hepatocytes in primary culture was inhibited by endotoxin. There was no positive correlation between fibronectin secretion and hepatocyte proliferation in the first 72 h. Then, the increase of hepatocyte population coincided with that of fibronectin concentration, indicating that there may be refractory period for hepatocytes to secrete fibronectin or proliferate. In terms of morphology, it may be relevant to degeneration, production of uncertain new protein and nuclear mitoses. Previous studies confirmed that biological behavior of hepatocytes was influenced by some mediators secreted by Kupffer cells, such as tumor necrosis factor, transforming growth factor, etc. In our study, it is presumed that there may be a mechanism to modulate the function of liver cells by some factors secreted from hepatocytes (still unknown in this study) if they are stimlulated by endotoxin. According to Roeb’s opinion[8], besides playing a controvertible role as initiator and regulator of fibrogenesis, fibronectin acts as a scaffold for other matrix proteins. Enhanced fibronectin secretion of hepatocytes in the culture media containing LPS suggests that endotoxin plays a crucial role in the liver fibrosis. Therefore, hepatocytes may take part in the liver fibrosis which is stimulated by endotoxin
Project supported by the Science and Technology Development Foundation of Shanxi Provincial Education Commission (No. 9725) and the Scientific Foundation of Ministry of Public Health, No.2179412.
| 1. | Manthous CA, Hall JB, Samsel RW. Endotoxin in human disease. Part 1: Biochemistry, assay, and possible role in diverse disease states. Chest. 1993;104:1572-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Han DW. Advance on hepatic failure mechanism research. Chin J Hepatol. 1995;3:134-137. |
| 3. | Jia JB, Han DW, Xu RL, Chen XM, Zhao YC, Yan JP et al. Role of gut-derived endotoxemia in the pathogenesis of hepatic fibrosis. Chin J Pathophysiol. 1998;in press. |
| 4. | Friedman SL. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990;10:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 5. | Gressner AM, Pfeiffer T. Preventive effects of acute inflammation on liver cell necrosis and inhibition of heparan sulphate synthesis in hepatocytes. J Clin Chem Clin Biochem. 1986;24:821-829. [PubMed] |
| 6. | Soresi M, Di Martino D, Montalto G, Carroccio A, Ruggeri MI, Bascone F, Ippolito S, Notarbartolo A. [Plasma fibronectin in chronic liver diseases]. Recenti Prog Med. 1993;84:602-607. [PubMed] |
| 7. | Koukoulis GK, Shen J, Virtanen I, Gould VE. Immunolocalization of cellular fibronectins in the normal liver, cirrhosis, and hepatocellular carcinoma. Ultrastruct Pathol. 1995;19:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Roeb E, Matern S. [Fibronectin--a key substance in pathogenesis of liver cirrhosis]. Leber Magen Darm. 1993;23:239-242. [PubMed] |