Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.317
Revised: April 16, 1998
Accepted: May 13, 1998
Published online: August 15, 1998
AIM: To study the reversing effect of Chinese drug tanshinone on malignant phenotype of cancer cells.
METHODS: Human hepatocarcinoma cell line (SMMC-7721) was treated in vitro with 0.5 mg/L tanshinone for 4 days, and variation in cell differentiation wasdetected.
RESULTS: The morphology of cancer cells was tended toward well differentiation and cell growth was markedly inhibited. BrdU uptake assay and immunohistochemical stain of PCNA showed that the BrdU labeling rate and PCNA positive rate were lower than the controls, but no difference was found statistically as compared with all transretinoic acid. Flow cytometric assay demonstrated that S phase cells decreased and G0/G1 phase cells increased. Expression of c-myc oncogene protein decreased but the c-fos oncogene protein markedly increased.
CONCLUSION: Tanshinone could reverse the inducing differentiation in human hepatocarcinoma cells (SMMC-7721). It may become a new prospective inducer of cell differentiation to treat cancers.
- Citation: Yuan SL, Huang RM, Wang XJ, Song Y, Huang GQ. Reversing effect of Tanshinone on malignant phenotypes of human hepatocarcinoma cell line. World J Gastroenterol 1998; 4(4): 317-319
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.317
Study on reversion of cancer cells is an important field of tumor molecular biology at present. The application of all transretinoic acids to treating leukemia is a successful example of differentiation inducing therapy. But attention was also been paid to screening highly effective and low toxicant inducer of differentiation to treat the solid tumors. Tanshinone is an alcohol extract from the root of the traditional Chinese herbal medicine-Salvia Miltiorrhiza Bunge. Previous studies in vitro have shown that tanshinone possessed cytotoxic effect on cancer cells and induced differentiation of cervical carcinoma cells[1,2]. Zhang ZW, et al reported that all transretinoic acid can reverse malignant phenotypes of human hepatocarcinoma cells, and promote carcinoma cells to differentiate toward normal cells[3]. We used transretinoic acids as positive control to study the reversing effect of tanshinone on malignant phenotypes of human hepatocarcinoma cells in cellular morphology, cellular properties of growth and proliferation, cell cycle and expression of oncogene.
Human hepatocarcinoma cell line (SMMC-7721) was derived from Shanghai Institute of Cancer Research [4]. Cells were cultured in RPMR1640 medium that contained 15% inactivated bovine serum and 100 mg/L ampicillin, 100 mg/L streptomycin at 37 °C in a 5% CO2 incubator.
Tanshinone II A (Tan) was provided by the Chinese Institute for Drug and Biological Assay (pure product). It was dissolved in DMSO (final concentration is 0.22% V/V). The solution was filtered through a 0.22 μm microporefilter, then stored at 4 °C for use. SMMC-7721 cells were seeded, and treated with 0.5 mg/L Tan or 0.5 mg/L ATRA. The control cells were added equal amount of DMSO for negative control experiment. The cells were treated with drugs or DMSO for 4 days continuouslly. The usage of all transretinoic acid (ATRA, Sigma’s product) was equal to tanshione.
5-Bro-mo-2’-deoxyuridine labeling and detection kit II is a product of Boehringer Mannheim Company, Germany. Anti-proliferation cell nuclear antigen (PCNA) monoclonal antibody PC10, c-myc and c-fos monoclonal antibody were purchased from Denmark Dake Company and S-P kit from American Maxim Company.
The treated cells were observed under inverse and light microscopy after HE stain. On the other hand, the cells were made into ultra-slice by routine method, and observed under transmission electron microscopy.
The cells treated with drugs and control cells were harvested by trypsinization and the total number of cells was counted by trypan blue exclusion daily for 4 days.
BrdU labeling The cells treated with drugs for 4 days were continuously cultured with BrdU labelling culture liquid (final concentration of 10 μmol/L) for 30 min, centrifuged and washed with PBS, added 5% albumin PBS to make the cell smears. The diluted 1:10 BrdU monoclonal antibody (mouse IgG1) on the smears was added with 1:10 anti-mouse-IgAP, and developing dye (NBT, X-phosphate).
Result observation The distracts of more positive cells were chosen under low power light microscopy. The positive cells of 2000 cells were counted under oil immersion lens. The labeling rate and positive rate were calculated.
LSAB method was used for immunohistochemical staining of PCNA according to SP-kit instruction. PC10 was diluted 1:200. Human tonsil tissues served as positive control and PBS substituted PC10 for negative control. Observation of result is similar to above.
The sample preparation and measurement followed the method described in reference[5]. The cells were harvested, counted and fixed. The cell concentration was adjusted to 105/mL. According to the routine method, using FACS-420 FCM, cell frequency distribution of each phase in cell cycle was measured, and by combined with immunohistochemical method, cell c-myc, c-fos gene and their protein expression were detected. The results were shown with scanning figure and date.
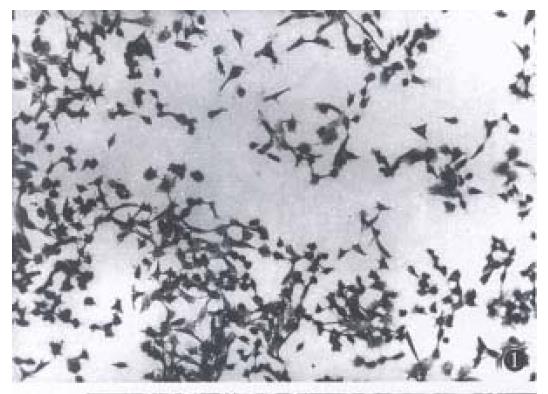
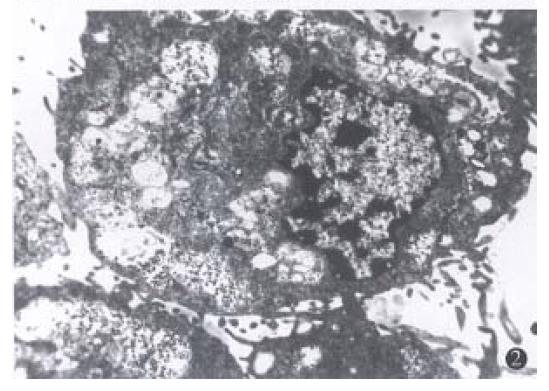
Light and electron microscopy observation. In control group, the cells arranged in aggregation, the cellular shape was polygon or spindle, with different volumes, more tumor giant cells, large karyon and more nucleus. The karyon/cytoplasm ratio rose, with few organelle. In Tan or ATRA group, the cellular arrangement was scattered, the cells became thin and long, the cellular volume became accordant, with less tumor giant-cells (Figure 1). The karyon became small and the nucleus became scarce. The karyon/cytoplasm ratio fell,the well-differentiated organelle such as microfilament and Golgi complex occurred. Morphology of the cells tended towards well-differentiation as compared with the control cells (Figure 2).
Cellular growth was measured by growth curve. The cells treated with Tan and ATRA were markedly inhibited in logarithmic growth phase (2-4 days after treatmentwith drugs). On day 4 after treatment with drugs, the inhibition rates of growth were 58.1% and 52.2%. No cells died by trypan blue exclusion.
The BrdU labeling rate and PCNA positive rate of the Tan and ATRA group were lower than those of control group (Table 1), and there was significant difference statistically (P < 0.01). But, there was no significant difference between PCNA positive rate of Tan group and ATRA group (P > 0.05).
The results are shown in Table 2. Tan or ATRA increased the number of cells in G0/G1 phase, but decreased the number of cells in S phase. Tan markedly inhibited the expression of cellular c-myc oncogene protein, but enhanced the expression of cellular c-fos oncogene protein, while ATRA inhibited the c-myc gene expression, but did not enhance the c-fox gene expression.
Human hepatocarcinoma cells were treated with non-cytotoxic dose of tanshinone. After treatment, cellular morphology and structure tended toward well-differentiation, and cellular growth and proliferation were markedly inhibited. Trypan blue exclusion demonstrated that the inhibiting effects of Tan and ATRA on the cells were not caused by cytotoxicity, but by the effect of inducing differentiation.
BrdU labeling rate and PCNA positive rate are reliable indexes for cellular proliferative ability. BrdU uptake test has many advantages compared with 3H-thymidine uptake test. BrdU was a kind of analogue of thymidine. It can incorporate the multiplicating cells, and label the cells of S phase[6,7]. BrdU monoclonal-antibody-ligase was used as an indicator. This method can measure the cells including BrdU, get the number of multiplicating cells of S phase, and show the cellular DNA synthesis ability. PCNA was an auxiliary protein of DNA polymerase δ, directly participate in DNA duplication in the course of cellular multiplication. Its expression or content represents the multiplication activity of cells. Measured positive cells are the cells of late G1 phase or early S phase[8]. We found that BrdU labeling rate and PCNA positive rate of SMMC-7721 treated with Tan or ATRA markedly decreased. This showed that the cellular number of S phase decreased. This result is consistent with analysis of FCM (the cells were prevented in the G0/G1 phase of cell cycle). Previous studies have showed that the expression of c-myc or c-fos oncogene was closely related to cellular multiplication and differentiation. The amplification and over-expression of c-myc gene were associated with malignancy and tumorigenicity of cells, but its low-expression was related to cellular morphologic changes and differentiation[9]. The result of FCM showed that via the inhibition or enhancement of expression of cellular gene, Tan and ATRA inhibited the ability of DNA polymerase δ and PCNA protein expression, inhibited the cells to enter S phase and DNA synthesis, thus inhibiting the cellular growth, multiplication, and promoted cellular differentiation.
Tanshinone, acting as an effective component of a traditional Chinese medicine to induce cellular differentiation, has many advantages. It has low toxicity and side effects, can induce tumor cells to differentiate, at the same time, exerting other pharmacological effect. So tanshinone is considered to be a new prospective, high effective and low toxicant inducer of differentiation for clinical application.
Project supported by the Science Fund of Chinese Ministry of Public Health, No.1994-1-240 and China Medical Board of New York, No.9408.
| 1. | Wu WL, Chang WL, Chen CF. Cytotoxic activities of tanshinones against human carcinoma cell lines. Am J Chin Med. 1991;19:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Yuan SL, Huang GQ, Wang XJ, Zhou HY, Jiang YH. The differentiation-in-ducing effect of tanshinone and retinoic acid on human cervical carcinoma cell line in vitro.. Chin J Oncol. 1995;17:424-426. |
| 3. | Ai ZW. [Reversing effect of retinoic acid on some phenotypes of human hepatocarcinoma cell line]. Zhonghua Zhongliu Zazhi. 1991;13:9-12. [PubMed] |
| 4. | Dong RC. Establishment and basic observation of cell biological property of human hepatocarcinoma cell line SMMC-7721. Acad J Mil Med Univ. 1980;1:5-6. |
| 5. | Barlogie B, Raber MN, Schumann J, Johnson TS, Drewinko B, Swartzendruber DE, Göhde W, Andreeff M, Freireich EJ. Flow cytometry in clinical cancer research. Cancer Res. 1983;43:3982-3997. [PubMed] |
| 6. | Del Bino G, Silvestrini R, Costa A, Veneroni S, Giardini R. Morphological and clinical significance of cell kinetics in non-Hodgkin's lymphomas. Basic Appl Histochem. 1986;30:197-202. [PubMed] |
| 7. | Connolly KM, Bogdanffy MS. Evaluation of proliferating cell nuclear antigen (PCNA) as an endogenous marker of cell proliferation in rat liver: a dual-stain comparison with 5-bromo-2'-deoxyuridine. J Histochem Cytochem. 1993;41:1-6. [PubMed] |
| 8. | Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta.. Nature. 1987;326:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1328] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 9. | Adesson LC. Gene expression during normal and malignant differentiation. New York: Academy Press. 1985;257-262. |