Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.303
Revised: February 24, 1998
Accepted: April 8, 1998
Published online: August 15, 1998
AIM: To determine whether Hb3 and its fragment F(ab’)2 have practical value in radioimmunoimaging of colorectal cancer.
METHODS: Intact Hb3 was purified by hydroxylapatite chromatography. The fragment F(ab’)2 was prepared by cold digestion and purified as intact Hb3. Hb3 and its fragment F(ab’)2 were labeled with 99mTc by direct labeling method using SnCl2 as reducing agent. The radioactive doses ranged from 15 to 40 mCi. The imaging was accomplished by single photon emission computered tomograph (SPECT) with imaging time ranging from 2.5 to 48 h. In this study, 10 patients were selected. Among them, 7 were administered with intact Hb3, and 3 with F(ab’)2 fragment. All the patients were diagnosed as having colorectal adenocarcinoma.
RESULTS: After purification, intact Hb3 and its fragment F(ab’)2 were fit for radioimmunoimaging. The percentage of labeling of 99mTc to Hb3 or F(ab’)2 was 80.6%-91.5%. Among the 10 patients, 3 of 7 patients administered with intact Hb3 had positive scans, the other 4 had negative scans, and 2 of 3 patients administered with F(ab’)2 had positive scans, the other 1 had negative scans.
CONCLUSION: The results showed that both intact Hb3 and its F(ab’)2 have some practical value in radioimmunoimaging of colorectal cancer, and the effects of imaging with F(ab’)2 was better than that with intact Hb3.
- Citation: Hu JY, Su JZ, Pi ZM, Zhu JG, Zhou GH, Sun QB. Radioimmunoimaging of colorectal cancer using 99mTc-labeled monoclonal antibody. World J Gastroenterol 1998; 4(4): 303-306
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.303
Colorectal cancer is a common malignant disease. In recent years, there have been many reports about radioimmunoimaging used to diagnose the colorectal cancer, but antibodies used were mainly the anti-CEA monoclonal antibodies. Hb3 is an anti-colorectal cancer monoclonal antibody produced by our laboratory, its sensitivity and specificity are superior to that of anti-CEA[1]. The positive rate of radioimmunoimaging with 131I labeled Hb3 in nude mice model of colorectal cancer was 92.4% (13/14)[2]. This result shows that Hb3 may be useful in clinical diagnosis of colorectal cancer. Based on the preparation of intact Hb3 and its fragments, clinical radioimmunoimaging has been done in 10 patients, the results showed that Hb3 is valuable in clinical practice.
The Hb3 ascites was prepared routinely and collected under aseptic condition. Hb3 was purified by hydroxylapatite chromatography (Sigma)[3]. In the process of purification, aseptic condition and pyrogen free condition were maintained.
Cold digestion was performed by modified Ballou method. Briefly, intact Hb3 solution(5 μg/L-10 μg/L), was adjusted to pH 4.0-4.2 with 0.3N acetate buffer, and pepsin (Sigma) in 0.3N acetate buffer (pH 4.0) was added to a final ratio of 1:10 (pepsin: Hb3, Wt/ Wt). The digestion was allowed to proceed for 24-34 h at 4 °C. The reaction was terminated by adding 1M NaOH to bring the pH to 7.8-8.0. The F(ab’)2 fragment was purified using hydroxylapatite chromatography, and the yield of F(ab’)2 was calculated (Wt/Wt).
The purity of Hb3 and its fragment F(ab’)2 were detected by SDS-PAGE using Multiphor II Electrophoresis System (Pharmacia). The concentration of stacking gel was 3%, and resolving gel 7.5%. The constant current was 50 mA, the temperature 15 °C, and the running time 2 h. After electrophoresis, the gel was stained immediately using Coomassie Blue and dried in vacuum condition and reserved in room temperature. Before electrophoresis, 2-mercaptoethanol was added to the sample solution, and the samples then boiled in water bath for 3 min.
The modified Paik’s direct labeling method was used[5]. Briefly, monoclonal antibody Hb3 and its F(ab’)2 (2 mg-8 mg) were adjusted to pH 4.0-4.2 with 0.3N acetate buffer (pH 4.0), then added with freshly prepared SnCl2/HCl and mixed, the antibody-SnCl2 mixture was incubated for 15-30 min at room temperature, then freshly prepared 15 mCi-40 mCi 99mTc was added, reaction of antibody with technium was maintained for 0.5-1.0 h at room temperature.
The free-form and combined 99mTc was detected with G-50 chromatography (0.9 cm × 40 cm, Sephadex), and the radioactivity of free-form 99mTc (FT), combined 99mTC (CT), and G-50 column absorbed 99mTc (AT) was measured with radioactivity meter. The percentage of labeling and radiochemical purity of antibody were calculated (because the labeled antibody was used directly without further purification, percentage of labeling was equal to radiochemical purity). The immunoreactivity of antibody before and after labeling and the fraction of immunoreactivity in G-50 chromatography were detected. Percentage of labeling = radiochemical purity = CT / CT + FT + AT
Wells of plate were coated with 50 μg colorectal cancer crude antigen. The antibodies to be detected were Hb3 and its fragment F(ab’)2. The anti-Ig was rabbit anti-mouse IgG + IgM + IgA conjugated with horseradish peroxidase (diluted 1:1000). The substrate was ABTS. OD405 value was obtained using Microplate Autoreader (EL309, BIO-TEK).
Ten patients (6 males, 4 females, aged from 36 to 74 years, averaging 54.2) were selected (Table 1). Seven patients suffered from rectal cancer, and one of them was complicated with liver metastasis. Three patients had colonic cancer. All of the patients proved to have adenocarcinoma by histopathology and the results of immunohistochemistry of biospies with Hb3 were positive. The amount of antibodies used were 2 mg-8 mg, and the radioactivity of 99mTc used was 15 mCi-40 mCi.
| No. | Sex | Age (year) | Clinical diagnosis | Tumor size(cm) | Pathological diagnosis | Immunohisto chemistry | Quantity of antib ody(mg) | Radioa ctivity of 99m Tc(mCi) | Imaging |
| 1 | Male | 74 | Rectal carcinoma | 3.0 × 5.0 | Adenocarcinoma | +++ | 3.0 | 23 | + |
| 2 | Female | 47 | Rectal carcinoma | 1.8 × 2.0 | Papilloadenocarcinoma | ++ | 5.0 | 20 | - |
| 3 | Male | 35 | Rectal carcinoma | 3.0 × 4.5 | Adenocarcinoma | ++ | 7.5 | 19 | - |
| 4a | Male | 67 | Rectal carcinoma | 3.5 × 4.5 | Low differentiated adenocarcinoma | +++ | 7.5 | 16 | + |
| 5 | Female | 54 | Colonic carcinoma | 4.0 × 5.0 | Adenocarcinoma | ++ | 8.0 | 15 | - |
| 6 | Female | 55 | Rectal carcinoma | 2.5 × 3.5 | Adenocarcinoma stage II | ++ | 5.0 | 19 | + |
| 7 | Male | 61 | Colonic carcinoma | 2.5 × 3.5 | Adenocarcinoma | + | 5.0 | 40 | - |
| 8b | Male | 38 | Rectal carcinoma | 3.5 × 5.0 | Adenocarcinoma | ++ | 5.0 | 38 | + |
| 9b | Male | 65 | Colonic carcinoma | 2.5 × 3.0 | Adenocarcinoma stage II | ++ | 2.0 | 15 | - |
| 10b | Female | 44 | Rectal carcinoma | 2.5 × 3.0 | Adenocarcinoma stage II | ++ | 5.0 | 18 | + |
Radioimmunoimaging was performed with GE STARCAM 3000/4000 SPECT system. 99mTc labeled antibodies were diluted in 100 mL 0.9% NaCl solution, then injected into the patients iv. The anterior, posterior, left, and right side planar scanings were taken 3, 6, 24 and 48 h later. Tomography was performed if the planar imaging is questionable. Thirty-two or 64 angles were recorded in each tomogram.
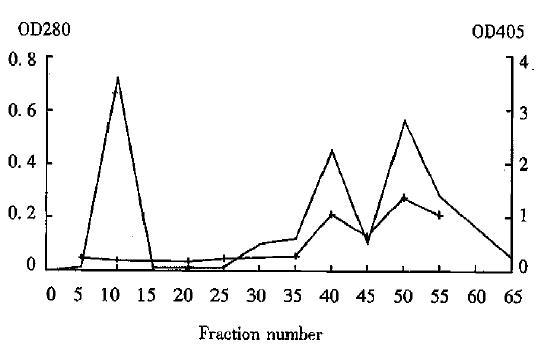
The hydroxylapatite chromatogram of Hb3 ascite showed three peaks. The third peak was IgM and possessed immunoreactivity[3]. After digestion, the chromatogram showed three peaks as well. The first was low molecular weight peptides, the second was F(ab’)2, and the third was intact IgM. Among them, the second and the third peaks possessed immunoreactivity (Figure 1). After digestion, the F(ab’)2 yield was 25. 0%-36.6%, and the immunoreactivity of F(ab’)2 was lower than that of intact Hb3.
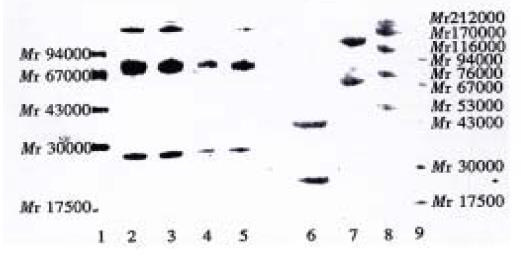
As showed in Figure 2, there were two bands in the lane of intact Hb3 denaturated and broken, their molecular weights were about Mr 72000 and Mr 23000, and they represented H chain and L chain, respectively (lane 2-5, the bands with molecular weight higher than Mr 94000 were contaminated proteins). In the lane of F(ab’)2 (unbroken, lane 7), two bands were found, and their molecular weights were about Mr 130000 and Mr 65000, and represented F(ab’)2 and Fab’ respectively. In the lane of F(ab’)2 (broken, lane 6), there were two bands as well, and their molecular weight were about Mr 43000 and Mr 23000, and represented VH + CH1 and L chain respectively.
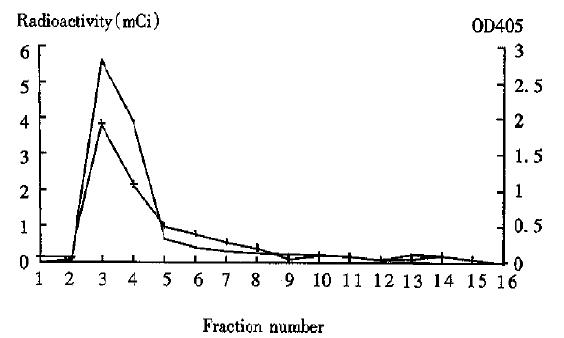
99mTc Labeled Hb3 was analyzed by G-50 chromatography. The percentage of labeling and radiochemical purity was 80.4%-91.5%. In the chromatogram, the fractions of immunoreactivity of labeled antibody overlapped the fractions of radioactivity (Figure 3). After the antibodies were labeled, their immunoreactivity was 90% more than that of unlabeled antibodies.
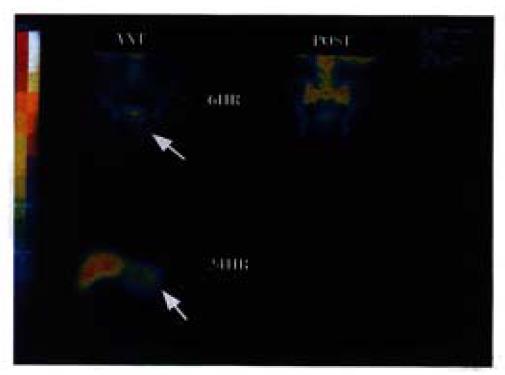
Radiommunoimaging was performed in 10 patients, 5 had positive images, and 5 negative images (Table 1), with no side effects. Patient No. 4 suffered from rectal carcinoma complicated with liver metastasis with positive images at 6 and 24 h. Figure 4 (upper left) shows an unclear abnormal radiation concentration under bladder in 6 h. In 24 h scan (lower left), there was a clear abnormal radiation concentration in the left lobe of liver.
Hydroxylapatite chromatography is simple. The column is short and is convenient for aseptic and pyrogen free treatment. The purity of antibody after purification was fit for clinical usage. As reported, antibody can be digested to F(ab’)2 by cold and warm digestion. In our study we selected cold digestion to prepare F(ab’)2. The yield of F(ab’)2 varied from 25.0% to 36.6%, lower than that prepared by Ballou[4] (cold digestion, 40%-60%), but higher than that prepared by Kurkela[5] (warm digestion, 24% ± 11%). As a matter of fact the prepared fragments contained F(ab’)2 and Fab’ (Figure 2), because pepsin digestion canproduce these two kinds of fragments, and it is difficult for hydroxylapatite chromatography to separate them.
Direct labeling of antibody is to combine the reduced 99mTc with antibody through the disulfied linkage. Its advantage is that the labeling process can be finished in one step, which is beneficial for making a marketable radioimmunoimaging kit. In this study, we selected SnCl2 as reducing agent, and labeled antibody with 99mTc using direct method. The reaction was performed in one step.
Hb3 is an IgM monoclonal antibody against CA-Hb3. The results of immunohistochemistry showed that the positive rate of Hb3 reacting with colorectal carcinoma was high, ant it did not react with normal colorectal tissue [1]. The results of radioimmunoimaging in nude mice model of colorectal carcinoma also showed a higher imaging efficiency[2]. Among the 10 patients in this study, five got positive images. The reasons why the positive rate is not high may be: (1) The effect of antibody molecular weight: The molecular weight of intact Hb3 is Mr 950000, its half-life and optimum imaging time are longer than 48 h, yet the half-life of 99mTc is 6 h, and the imaging is required to be performed within 24 h. In his study, one of the radioimmunoimagings was performed in a transverse colon cancer patient with intact Hb3, the imaging result at 24 h was negative. Fourty-eight hours later, the resected tumor sample was scanned by SPECT. In the circumstance of prolonging the acquisition time (30 min), the radioactivity of tumor was higher than that of the surrounding normal tissues. This evidence showed that the inconsistency of half-life between antibodies and radionuclide was unfavorable to imaging. The molecular weight of F(ab’)2 is 1/7 that of intact Hb3, and its half-life is 26 h, which is more consistent with that of 99mTc, and its imaging efficiency is higher than that of intact Hb3. (2) Effect of free 99mTc: Free 99mTc (5.3%-8.6%, Figure 3) can pass through gastric mucosa accompanying the secretion of gastric juice, and get into the gastrointestinal tract through the peristalsis, resulting in false positiveness[7]. Among the 10 patients in this study, negative images were found in the intestinal tract of four patients. Accumulation of Tc-Sn colloids (absorbed by column G50, 3.2%-11.0%, Figure 3) can also influence tumor imaging. (3) The effect of HAMA (human anti-mouse antibody): Antibody Hb3 is mouse-derived, it can cause HAMA after being injected into human bodies. This reaction will interfere with the combination of antibody with antigen, and then influence the imaging. In this experiment, the duration from antibody injection to imaging was short, so HAMA was not the major influencing factor.
In conclusion, we consider that Hb3 is a valuable monoclonal antibody in radioimmunoimaging of colorectal carcinoma. The imaging effect of F(ab’)2 is better than that of intact Hb3. Further study is needed to optimize the labeling condition, increase the labeling rate of Hb3 with 99mTc, and finally improve the diagnostic value of radioimmunoimaging.
Project supported by the National “Eighth Five-Year Plan” Program, No.85-722-18-02.
| 1. | Sun QB, Ho JJI, Kim YS. Human colonic cancer associated antigens detected by three monoclonal antibodies. Chin Med J. 1986;99:63-74. |
| 2. | Zhang J, Wang CL, Sun QB, Pan AY, Chen SL, Liu CG et al. Radio immunoimaging study with 131I-labeled anti-colorectal carcinoma mono-clonal antibody in nude mouse model. Bull Human Med Univ. 1990;15:235-238. |
| 3. | Stanker LH, Vanderlaan M, Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985;76:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Ballou B, Reiland J, Levine G, Knowles B, Hakala TR. Tumor location using F(ab')2 mu from a monoclonal IgM antibody: pharmacokinetics. J Nucl Med. 1985;26:283-292. [PubMed] |
| 5. | Paik CH, Phan LN, Hong JJ, Sahami MS, Heald SC, Reba RC, Steigman J, Eckelman WC. The labeling of high affinity sites of antibodies with 99mTc. Int J Nucl Med Biol. 1985;12:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kurkela R, Vuolas L, Vihko P. Preparation of F(ab')2 fragments from monoclonal mouse IgG1 suitable for use in radioimaging. J Immunol Methods. 1988;110:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Rhodes BA. Direct labeling of proteins with 99mTc. Int J Rad Appl Instrum B. 1991;18:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Murray JL, Rosenblum MG, Zhang HZ, Podoloff DA, Kasi LP, Curley SA, Chan JC, Roh M, Hohn DC, Brewer H. Comparative tumor localization of whole immunoglobulin G anticarcinoembryonic antigen monoclonal antibodies IMMU-4 and IMMU-4 F(ab')2 in colorectal cancer patients. Cancer. 1994;73:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |