Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.103188
Revised: January 3, 2025
Accepted: January 20, 2025
Published online: March 7, 2025
Processing time: 99 Days and 2.5 Hours
Shortening the recipient warm ischemia time (rWIT) has been proven to be effective for improving the short- and long-term outcomes after liver transplan
To investigate whether shortening the rWIT could improve the outcomes of ECD LT.
Rat ECD autologous orthotopic LT were performed with variable rWITs (0, 10, 20, and 30 minutes). Near-infrared fluorescence imaging (FI) was used for the real-time assessment of liver graft ischemia-reperfusion injury after the anhepatic phase. Survival was assessed, and liver function and histological analyses were performed on the third day after transplantation.
The FI curve growth rate and postoperative three-day survival rate significantly increased, and the liver function and Suzuki score of the liver grafts significantly improved when the rWIT was ≤ 10 minutes (P < 0.05).
The post-transplant outcomes were significantly better with a shorter rWIT (10 minutes or less) than with a longer rWIT, which could be a strategy for expanding the liver donor pool.
Core Tip: Shortening the recipient warm ischemia time (rWIT) can eliminate the negative impact of an extended cold ischemia time. Therefore, this study assessed the effect of shortening the rWIT on the outcomes of liver transplantation using extended-criteria donor (ECD) liver grafts in rats. The fluorescence imaging curve growth rate and postoperative 3-day survival rate significantly increased, and the liver function and Suzuki score of the liver grafts improved when the rWIT was ≤ 10 minutes. Therefore, using ECD liver grafts while shortening the rWIT could be a strategy for expanding the liver donor pool in humans.
- Citation: Yu JW, Xiang LB, Dong XJ, Yang CX, Wang L, Liu XY, Song YH, Bai XJ, Xiao JW, Ren L, Xu QH, Yang GH, Lv Y, Lu Q. Shortening the recipient warm ischemia time could be a strategy for expanding the liver donor pool. World J Gastroenterol 2025; 31(9): 103188
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/103188.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.103188
With advancements across multiple disciplines, liver transplantation (LT) is a routine life-saving therapy for end-stage liver disease[1,2]. However, liver graft shortage is a major issue that limits the further development of LT[3]. Expanding the liver donor pool can reduce the mortality rate of patients on the waiting list for LT[4-6]. Using liver grafts from extended-criteria donors (ECDs) can alleviate the shortage of liver grafts. However, ECD liver grafts are often less desirable because of the greater susceptibility to ischemia-reperfusion injury (IRI) and poor post-transplant prognosis[7]. The total ischemia time for liver grafts during LT consists of the warm ischemia time (WIT) and cold ischemia time (CIT). Unlike cold ischemia, warm ischemia of the donor liver is more harmful to hepatic cells, biliary epithelial cells, and vascular endothelial cells owing to the high energy demands starting when the liver core temperature reaches 20 °C. In LT from brain-dead donors, warm ischemia only occurs during donor liver implantation, called recipient WIT (rWIT), and is defined as the time between the end of cold preservation for the liver graft and the reperfusion of liver blood flow. It is well known that a prolonged rWIT is a risk factor for worse LT outcomes, and liver grafts from ECDs are more likely to cause primary non-function, primary poor function, and biliary ischemia, even if the rWIT is an “acceptable length”[8].Currently, the rWIT, known as “the time for liver implantation”, ranges between 30 and 50 minutes based on the surgical implantation technique[9-11]. However, the rWIT has considerably decreased with the emergence of novel liver implantation techniques[12-15]. Zhang et al[16] reported a fast liver graft implantation technique using magnetic devices, with a median rWIT of only 10.3 minutes. Another study indicated that shortening the rWIT improves short- and long-term outcomes after LT and offsets the negative impact of prolonged CIT[17]. Based on these studies, we hypothesized that shortening the rWIT may improve the prognosis of LTs from ECDs, which could help expand the donor liver pool. Therefore, this study assessed the effect of shortening the rWIT on the outcomes of LT using ECD liver grafts in rats.
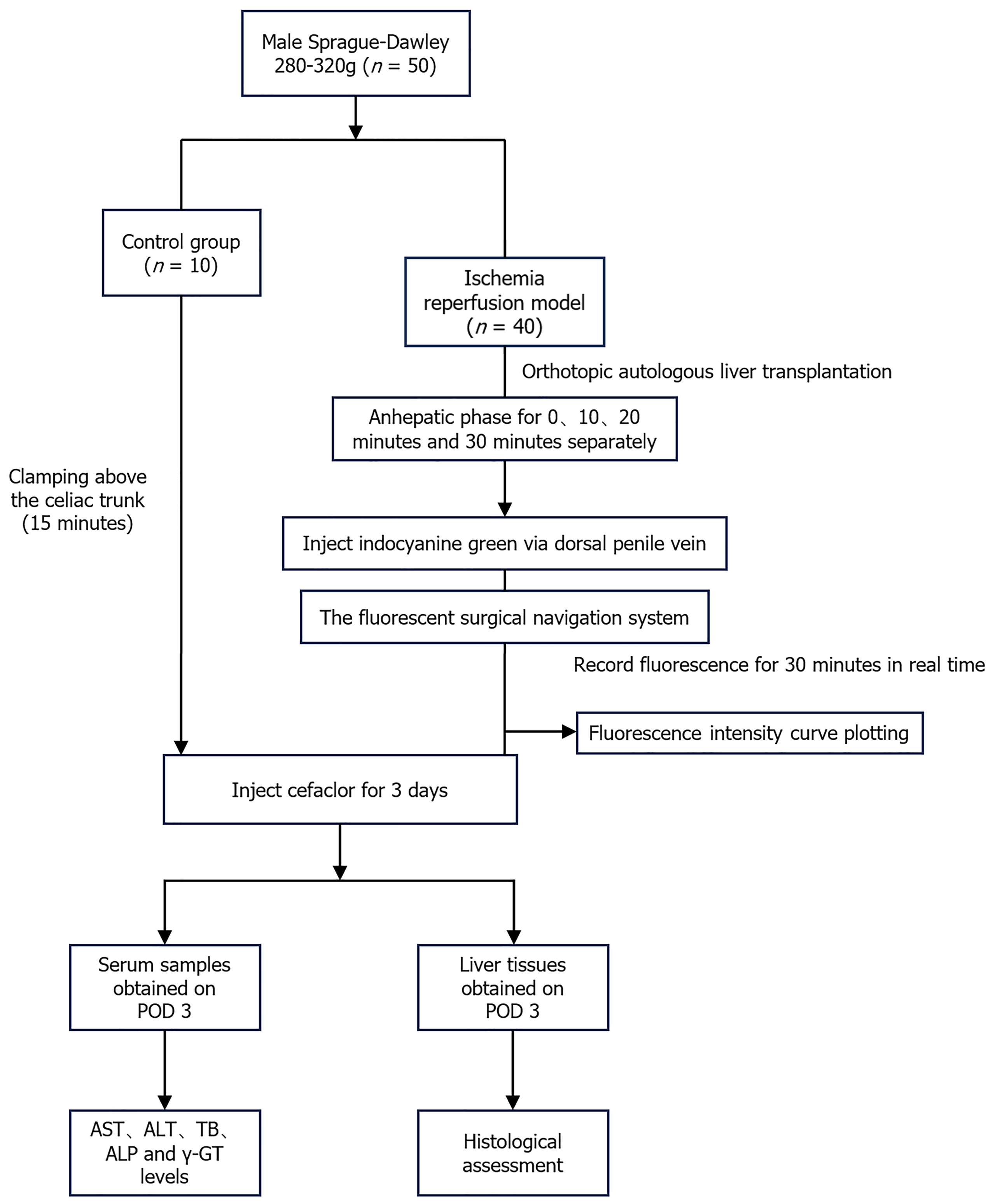
Sprague-Dawley rats (male, weighing 250–300 g) were provided by the Experimental Animal Center of Xi'an Jiaotong University. The Ethics Committee of Animal Experiments at Xi’an Jiaotong University approved this study. All experiments complied with the Animal Research: Reporting of In Vivo Experiments guidelines and were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978). All rats were randomly assigned to one of five groups based on the rWIT duration (Figure 1): Control group [n = 10, rats only received ECD modeling without autologous orthotopic LT (aOLT)], 0-minute group (n = 10, rWIT = 0 minute), 10-minute group (n = 10, rWIT = 10 minutes), 20-minute group (n = 10, rWIT = 20 minutes), and 30-minute group (n = 10, rWIT = 30 minutes).
Figure 2 outlines the surgical procedure of the rat ECD aOLT, which included the following.
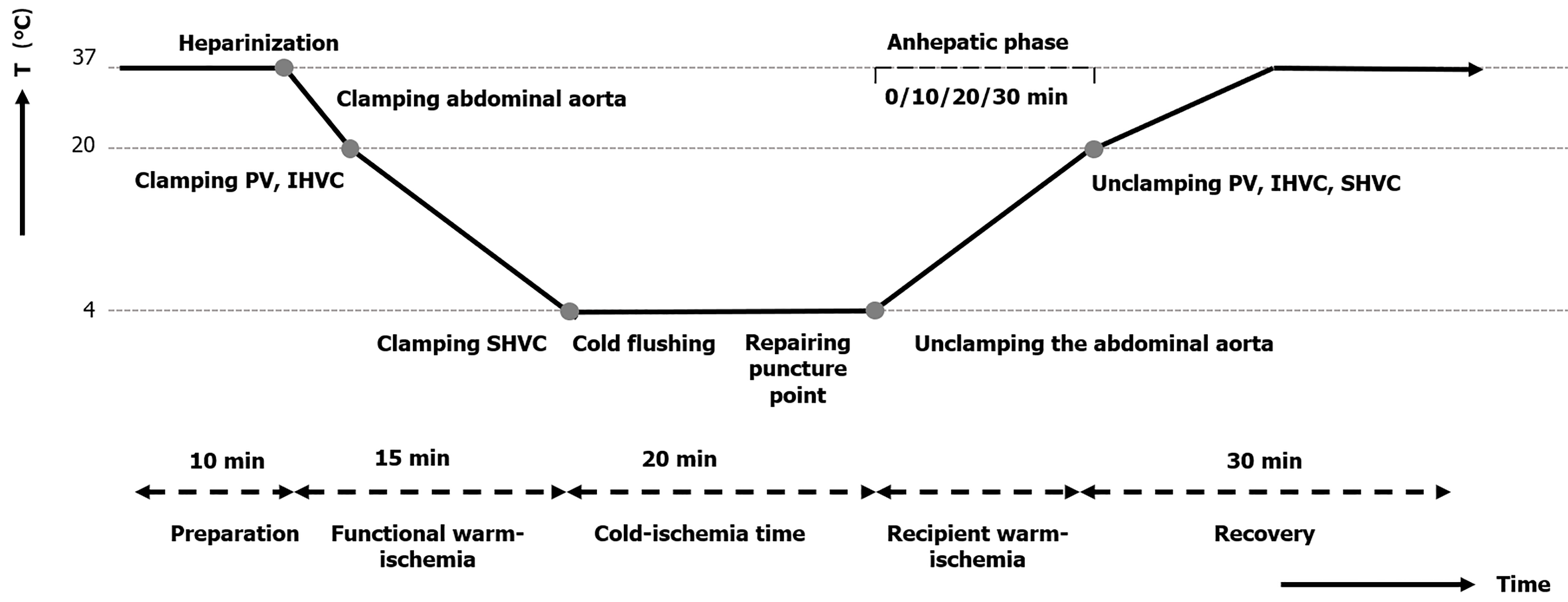
Induced anesthesia of the rats was performed with 5% isoflurane at a flow rate of 1.5 L/min in an organic glass box, and anesthesia was maintained with 2 vol% isoflurane at a flow rate of 0.6-0.8 L/min in a conical mask. A 4-cm midline abdomen incision was performed, and the falciform ligament and connective tissues around the hepatic artery, portal vein (PV), suprahepatic vena cava (SHVC), infrahepatic vena cava (IHVC), and abdominal aorta were also dissected. Then, 50 units of heparin diluted in 2 mL of physiological saline solution was injected through the dorsal penile vein to prevent thrombosis.
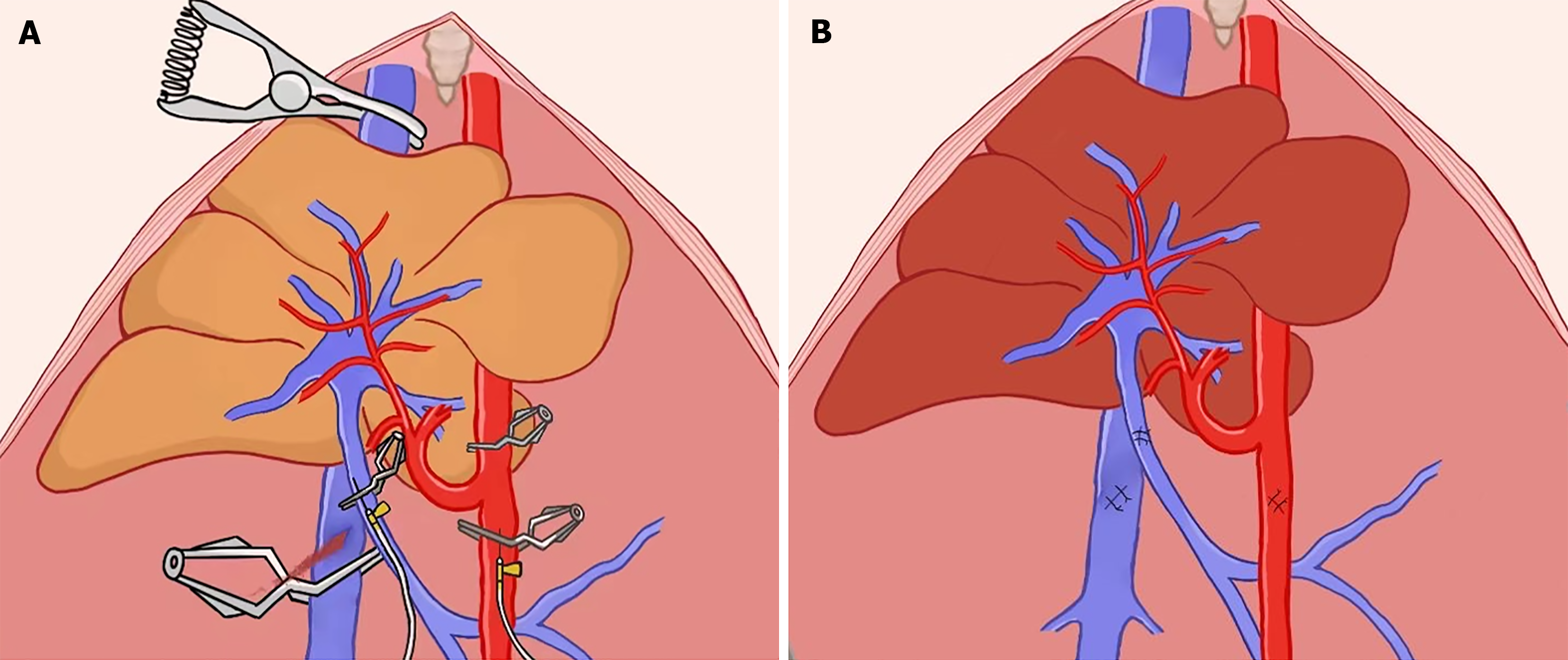
The abdominal aorta was separated and clamped above the celiac trunk to halt the blood flow to the PV and hepatic artery accordingly. Next, the PV, SHVC, and IHVC were sequentially clamped such that the liver was in functional warm ischemia. A rat ECD model was established by extending the functional WIT (fWIT) to 15 minutes. The fWIT was defined as the duration between abdominal aortic clamping and cold flushing of the liver graft. Finally, the abdominal aorta and PV were punctured with a transfixion pin and fixed using another clamp, which was in preparation for subsequent liver flushing, as previously described[18].
The liver was flushed through the PV and hepatic artery with cold Lactated Ringer’s solution containing heparin (12.5 U/mL at 4 °C) using an infusion pump (2.5 mL/min) as previously described[18] (Figure 3). Similarly, the liver surface was rinsed with 4 °C Lactated Ringer’s solution to lower the liver temperature to 4 °C. The puncture holes of the abdominal aorta, PV, and IHVC were microscopically repaired using 9-0 Prolene sutures after liver cold flushing was completed. The hepatic artery was then clamped, and indocyanine green (ICG) solution was administered through the dorsal penile vein at 0.5 mg/kg before unclamping the abdominal aorta.
The abdominal aorta clamp was released after administering protamine (0.5 mg) through the dorsal penile vein to neutralize the heparin. At this time, the hepatic inflow remained clamped, marking the beginning of the recipient warm ischemia phase. rWIT was defined as the duration between the abdominal aorta unclamping and hepatic inflow unclamping. The rWIT duration varied based on the group and ranged from 0 to 30 minutes.
After recipient warm ischemia, the abdominal cavity was flushed with warm physiological saline solution for rapid rewarming. The time when the recipient warm ischemia ended was set as the 0 time point, and real-time recording of fluorescence changes in rat liver was performed for 30 minutes. Finally, the abdominal closure in two layers was performed with a continuous 3-0 nonabsorbable surgical suture.
All rats were placed in a clean cage after the operation and were administered cefuroxime at 16 mg/kg and buprenorphine at 0.05 mg/kg intraperitoneally every 12 hours for three days. The survival rate in each group was calculated, and the surviving rats in each group were sacrificed on the third day after transplantation to obtain liver tissue and blood samples.
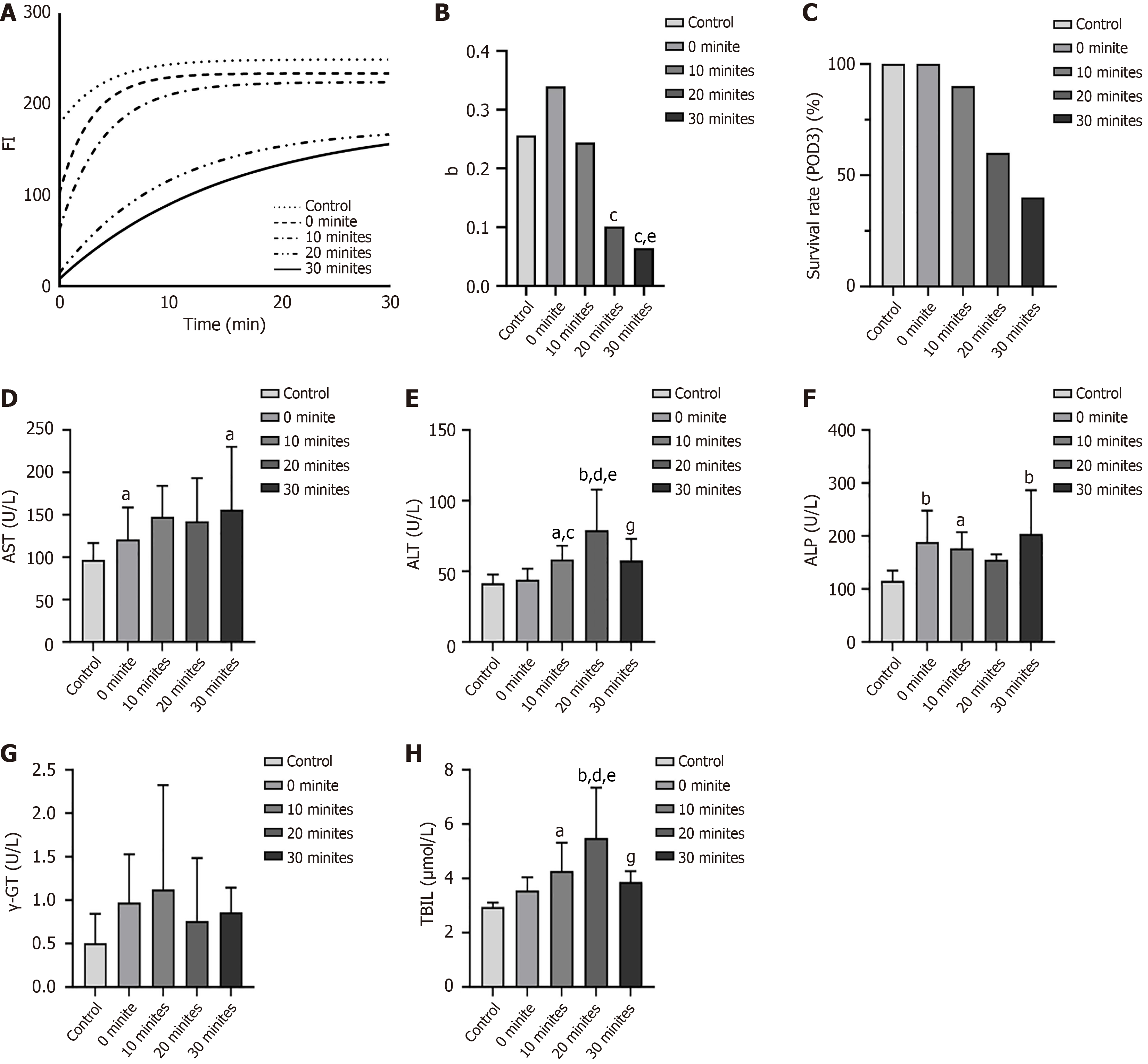
Liver IRI was assessed using Near-infrared (NIR) fluorescence imaging (FI) with ICG, as described previously[19]. The fluorescence images of each group were processed and analyzed using ImageJ software (United States National Institutes of Health, Bethesda, MD, United States), and the curve growth rate (b) was calculated and compared among the groups.
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (γ-GT), and total bilirubin (TBIL) levels were measured on the third day after transplantation.
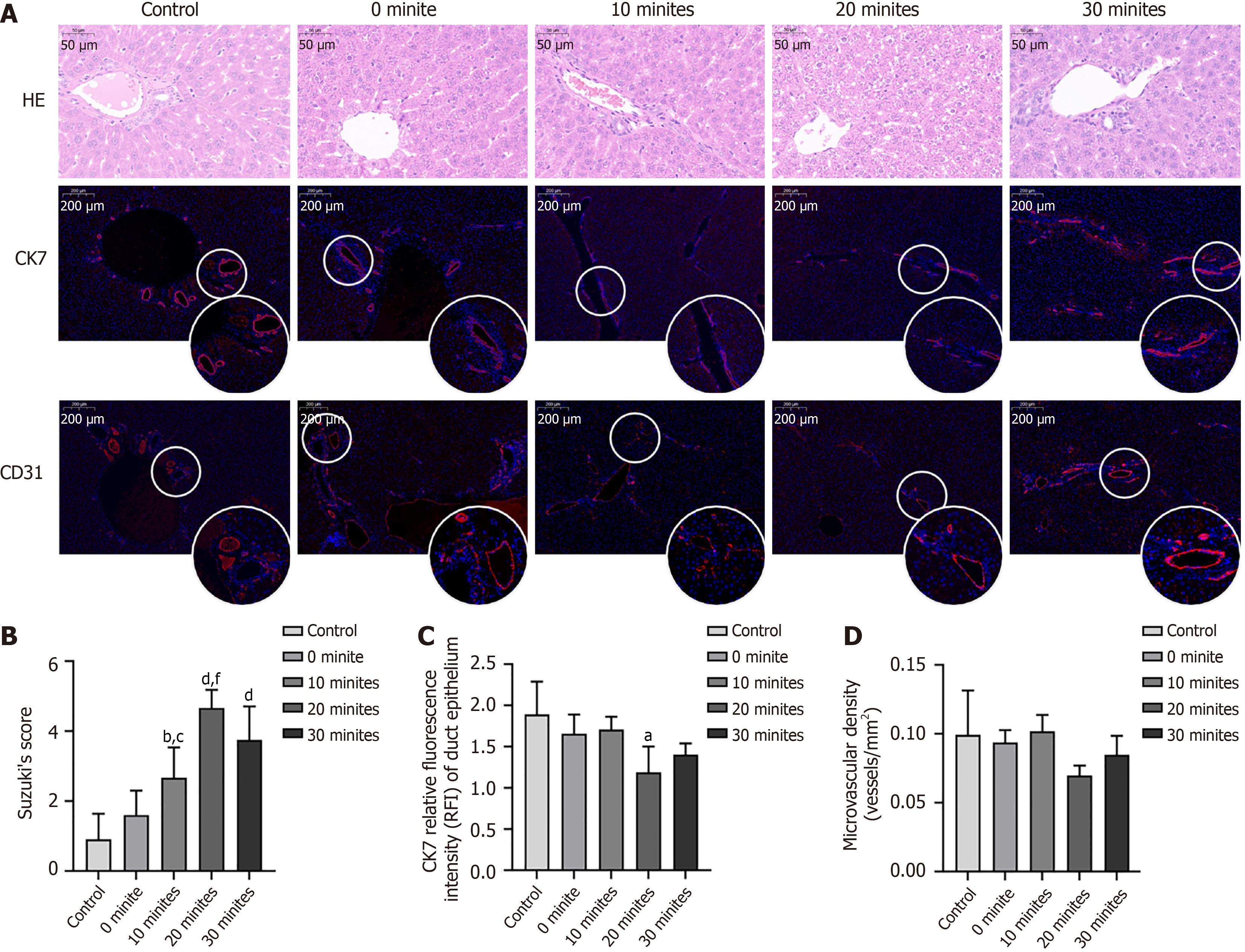
Liver tissues were fixed overnight in 10% buffered formalin and then embedded in paraffin. After cutting 4-μm thick liver sections and staining with hematoxylin and eosin, microscopic scanning of the tissue sections was performed using PRECICE 500B (Unic Technologies, Beijing, China). For immunofluorescence, the liver slides were incubated with primary antibodies overnight, and then the appropriate horse radish peroxidase-labeled secondary antibody was applied before counter-staining of the nucleus with CY3-TSA. The slides were photographed using a confocal microscope, and arbitrary fluorescence intensity units were measured using Image J software.
Statistical analyses were conducted using SPSS version 20.0 (SPSS Inc., Armonk, NY, United States). Normally distributed metric data were presented in the form of mean ± SE and analyzed using an unpaired, two-tailed t-test. Non-normally distributed metric data were presented in the form of medians and analyzed using the Wilcoxon rank-sum test. Enumeration data were presented as n (%) and were analyzed using the χ2 test. Statistical significances were set at P values of < 0.05.
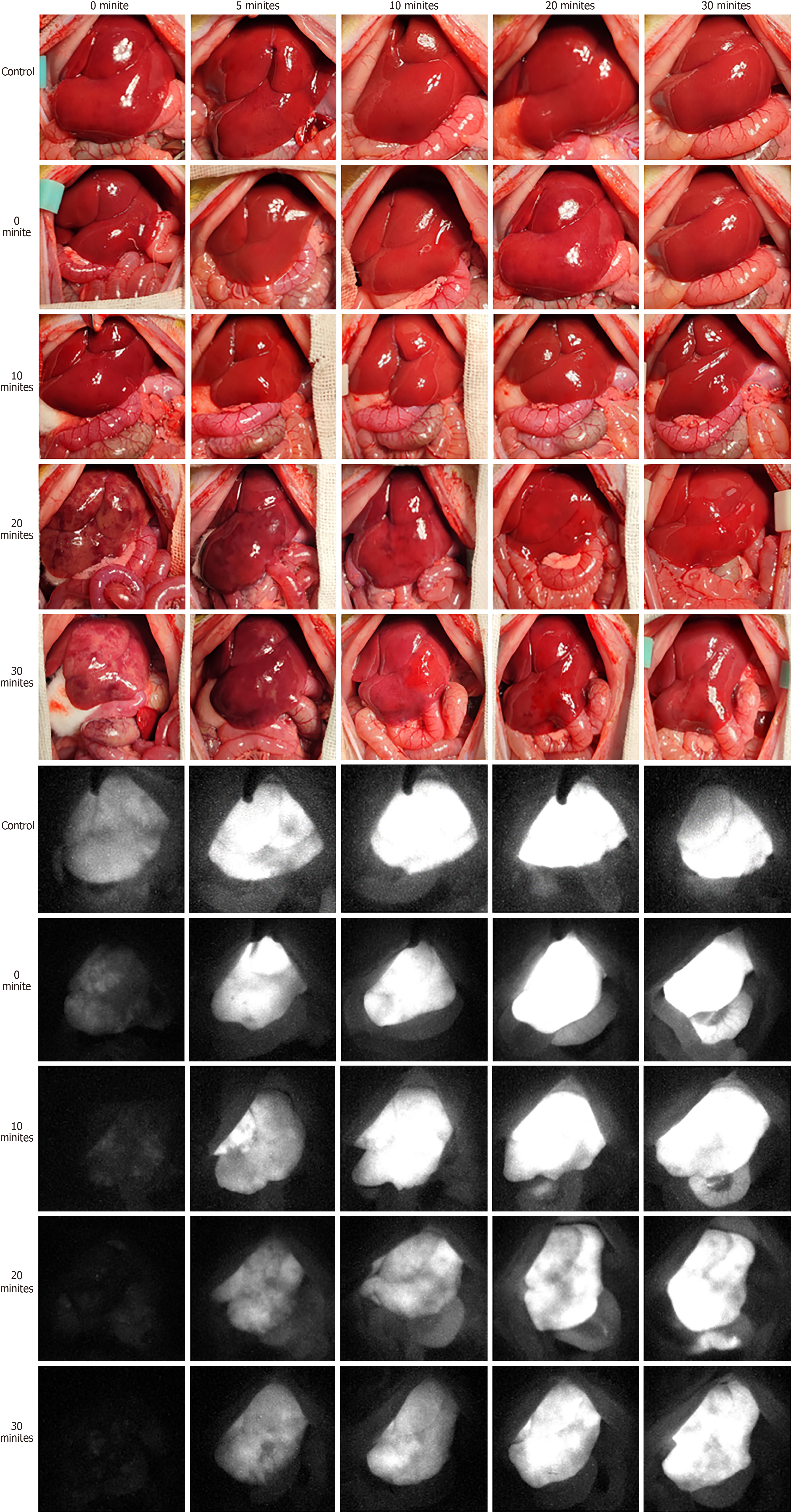
All procedures were performed successfully in all groups. Figure 4 presents the optical and NIR FI images for the 0-, 5-, 10-, 20-, and 30-minute rWIT groups. When the recipient warm ischemia ended, the liver was filled with blood and turned red over time in the control, 0-minute, and 10-minute groups. However, the liver graft perfusion was disordered when the rWIT was ≥ 20 minutes, even though the liver blood flow had been restored for 30 minutes. NIR FI confirmed this phenomenon. On the third day after transplantation, all rats survived in the control and 0-minute groups; nine rats (90%) survived in the 10-minute group, but only six (60%) and four (40%) rats survived in the 20- and 30-minute groups, respectively (Figure 5C). However, these rats all died from liver failure.
Figure 5A and B present the FI and growth rate (b) curves, respectively. The liver graft FI curves increased faster in the 0- and 10-minute groups than in the 20- and 30-minute groups but were comparable to that of the control group. In addition, the FI curve peaked approximately 15 minutes after the anhepatic phase ended in the control, 10-minute, and 20-minute groups, whereas it peaked after almost 30 minutes in the 20- and 30-minute groups. The FI growth rate (b) was higher in the control, 0-minute, and 10-minute groups than in the 20- and 30-minute groups.
Figure 5D–H present the serum AST, ALT, ALP, γ-GT, and TBIL levels. The average serum AST level three days after transplantation was significantly lower in the control group than in the 0- and 10-minute groups (Figure 5D, P < 0.05). The average serum ALT level gradually increased from 0 to 20 minutes of rWIT but decreased after 30 minutes (Figure 5E, P < 0.05). The average serum ALP level was lower in the control group than in the other groups. However, the rWIT duration did not affect the serum ALP level (Figure 5F, P > 0.05). The average serum γ-GT level also did not differ among the groups (Figure 5G, P > 0.05). The TBIL levels followed a similar pattern to the ALT levels (Figure 5H), gradually increasing from 0 to 20 minutes of rWIT but decreasing after 30 minutes (P < 0.05).
Figure 6A presents the representative hematoxylin and eosin and immunofluorescence staining images of the liver grafts. The Suzuki scores of the liver grafts gradually increased as the rWIT increased but decreased when the rWIT was 30 minutes (Figure 6B). The numbers of CK7+ cells in the liver graft were significantly lower in the 20-minute group than in the control group (P < 0.05, Figure 6C) but comparable between the control, 0-minute, 10-minute, and 30-minute groups
Several strategies have been introduced to improve IRI and thus expand the pool of available DCD liver grafts, all of which are based on machine perfusion technology, mainly because machine perfusion can reduce the post-LT transaminase peak, increase organ utilization and potentially be a platform for testing the effects of therapeutic agents directly on the liver graft[20-24]. However, these strategies only focus on liver graft injury during organ preservation and transportation. In fact, liver graft injury occurs during organ preservation and during organ implantation, currently defined as recipient warm ischemia injury. Several studies have indicated that shortening the rWIT improves the IRI of the liver graft to within the acceptance criteria[9,17,25]. Previous clinical retrospective studies have found that shorter graft revascularization was a protective factor in LT, particularly in the setting of ECD liver grafts[9]. Al-Kurd et al[17] found that shortening rWIT could mitigate the adverse effects of prolonged CIT. Compared with liver grafts within the acceptance criteria, liver grafts from patients with ECD are susceptible to IRI caused by cold and warm ischemia. Thus, significantly shortening the rWIT may reduce the susceptibility of ECD liver grafts to IRI and improve the outcome, which was confirmed in this study.
In this study, the ECD model was established by extending the fWIT to 15 minutes; most rats died within three days after transplantation even though the rWIT was considered an “acceptable length”[26-28], indicating that the animal model was reliable. However, when the rWIT was shortened to 10 minutes or less, most rats survived aOLT, and the liver function improved as the rWIT gradually shortened. In addition, the FI curve growth rate was higher for rats that underwent aOLT with a short rWIT (10 minutes or less) than for those with an rWIT of 20 minutes or more. NIR FI has been applied intraoperatively for real-time assessment of liver function in open hepatopancreatobiliary surgery[29]. Shan et al[19] also found that NIR FI with ICG could effectively estimate the degree of liver graft IRI. In our study, the FI curves reached the plateau phase after approximately 15 minutes in rats with an rWIT of 10 minutes or less, but it exceeded 30 minutes when the rWIT was 20 minutes or more. This result indicates that the ability of the ECD liver graft to take up ICG was impaired as the rWIT extended. Thus, 10 minutes or less of rWIT seemed to be an “acceptable length” for ECD liver grafts in rats.
Notably, a rWIT of 10 minutes or less seems impossible with the current surgical implantation techniques in clinical LT, mainly because of the time-consuming process of liver graft revascularization. Several novel technologies for fast liver revascularization have emerged in animal LT models[12,14,15], and a new method has already been applied in clinical LT with a median rWIT of 10.3 minutes[16]. Another retrospective study evaluated ischemia-free LT, a novel technique that eliminates the need for recipient warm ischemia, reporting improved prognoses of the recipients using functionally marginal liver grafts[30,31], which is consistent with our study’s results.
Shortening the rWIT improves the prognosis of ECD LT primarily by alleviating liver graft IRI. We found that the ECD liver graft FI curve growth rate significantly decreased when the rWIT was ≥ 20 minutes, meaning that the liver’s ability to take up ICG is impaired because of IRI. In addition, liver function gradually deteriorated with a prolonged rWIT. However, the liver function of the rats in the 30-minute group was better than that in the 20-minute group. Endothelial and biliary epithelial cells are considered more susceptible to IRI than hepatocytes. Unfortunately, the number of biliary duct epithelial cells and microvascular density of the liver graft did not differ between the groups with an rWIT of ≥ 20 minutes and < 20 minutes. The main reason for this abnormal phenomenon is survivor bias; only 60% and 40% of the rats in the 20- and 30-minute groups, respectively, survived.
The shortening of the anhepatic phase in the recipients also explains the protective effect of a shortened rWIT. It is well known that profound metabolic alterations due to blood flow disorders are associated with prolonging the anhepatic phase[32,33], and those alterations harm native organs and the liver graft. Thus, shortening the time of disordered blood flow in the recipient may also minimize damage to the ECD liver graft.
However, some issues need to be addressed to expand the donor liver pool by shortening the rWIT in future clinical practice. First, this study found that ≤ 10 minutes of rWIT appeared to be an “acceptable length” for ECD liver grafts. However, when rWIT is between 10 and 20 minutes, the outcome of LT using ECD liver grafts remains unclear, warranting further studies. Second, shortening rWIT to within 10 minutes still relies on improvements in liver imp
This study has some limitations. First, the abdominal aorta was clamped for 15 minutes during the establishment of the rat ECD liver graft model, which could lead to long-term ischemia of the intestines, kidneys, and lower limbs, potentially triggering adverse outcomes. However, renal or intestinal necrosis or death was not observed in the control group. Second, only some rats survived until postoperative day 3, which may have introduced a survivor bias into the results, particularly in the histological evaluations of the liver grafts. In fact, the Suzuki scores of the liver grafts decreased when the rWIT was 30 minutes. This may also be caused by survivor bias. Furthermore, this study only evaluated the survival rate within three days of LT. The impact of shortened rWIT on the long-term prognosis of ECD LT, especially in ischemic cholangiopathy, remains unclear and requires further study.
Shortening the rWIT improved the IRI of ECD liver grafts in rats and might be a strategy for expanding the donor liver pool in humans.
| 1. | Lucey MR, Furuya KN, Foley DP. Liver Transplantation. N Engl J Med. 2023;389:1888-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 2. | Della Guardia B, Boteon APCS, Matielo CEL, Felga G, Boteon YL. Current and future perspectives on acute-on-chronic liver failure: Challenges of transplantation, machine perfusion, and beyond. World J Gastroenterol. 2022;28:6922-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Feng S, Roll GR, Rouhani FJ, Sanchez Fueyo A. The future of liver transplantation. Hepatology. 2024;80:674-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Goldaracena N, Cullen JM, Kim DS, Ekser B, Halazun KJ. Expanding the donor pool for liver transplantation with marginal donors. Int J Surg. 2020;82S:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 5. | Toniutto P, Zanetto A, Ferrarese A, Burra P. Current challenges and future directions for liver transplantation. Liver Int. 2017;37:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Ivanics T, Abreu P, De Martin E, Sapisochin G. Changing Trends in Liver Transplantation: Challenges and Solutions. Transplantation. 2021;105:743-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Ghinolfi D, Melandro F, Torri F, Martinelli C, Cappello V, Babboni S, Silvestrini B, De Simone P, Basta G, Del Turco S. Extended criteria grafts and emerging therapeutics strategy in liver transplantation. The unstable balance between damage and repair. Transplant Rev (Orlando). 2021;35:100639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Muiesan P, Girlanda R, Jassem W, Melendez HV, O'Grady J, Bowles M, Rela M, Heaton N. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005;242:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Buchholz BM, Gerlach UA, Chandrabalan VV, Hodson J, Gunson BK, Mergental H, Muiesan P, Isaac JR, Roberts KJ, Mirza DF, Perera MTPR. Revascularization Time in Liver Transplantation: Independent Prediction of Inferior Short- and Long-term Outcomes by Prolonged Graft Implantation. Transplantation. 2018;102:2038-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Jochmans I, Fieuws S, Tieken I, Samuel U, Pirenne J. The Impact of Implantation Time During Liver Transplantation on Outcome: A Eurotransplant Cohort Study. Transplant Direct. 2018;4:e356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M, Cao C, Chen T, Lipshutz GS, Holt C, Gordon S, Gornbein J, Amersi F, Ghobrial RM. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905-16; discussion 916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Lu Q, Liu K, Shi AH, Zhang W, Wan Y, Wu RQ, Lv Y, Wang SP. Liver transplantation using magnetic anastomosis in pigs. Sci Rep. 2023;13:20143. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Kruk E, Kalinowski P, Gibiński K, Dudek K, Skalski M, Przybysz M, Zhylko A, Nazarewski Ł, Morawski M, Grąt M. Stapled Anastomosis for Side-to-Side Cavo-Cavostomy in Orthotopic Liver Transplantation. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Lv Y, Wang B, Zhang Y, Jiang A, Li JH, Zhang XF, Li QY, Meng KW, Liu C, Yu L, Pan CE. Novel magnetic rings for rapid vascular reconstruction in canine liver transplantation model. Transplant Proc. 2006;38:3070-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Liu SQ, Lei P, Cui XH, Lv Y, Li JH, Song YL, Zhao G. Sutureless anastomoses using magnetic rings in canine liver transplantation model. J Surg Res. 2013;185:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zhang XG, Liu XM, Wang SP, Lu Q, Shi AH, Li Y, Qian YR, Liu K, Ma F, Wang HH, Li YL, Wu R, Zhang XF, Wang B, Lv Y. Fast Vascular Reconstruction With Magnetic Devices in Liver Transplant: A Novel Surgical Technique. Liver Transpl. 2021;27:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Al-Kurd A, Kitajima T, Delvecchio K, Tayseer Shamaa M, Ivanics T, Yeddula S, Yoshida A, Rizzari M, Collins K, Abouljoud M, Nagai S. Short recipient warm ischemia time improves outcomes in deceased donor liver transplantation. Transpl Int. 2021;34:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Zhu XH, Pan JP, Wu YF, Ding YT. Establishment of a rat liver transplantation model with prolonged biliary warm ischemia time. World J Gastroenterol. 2012;18:7194-7200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Shan L, Chen H, Yang L, Feng Z, Wang Y, Wang R, Zhang N, Wu R, Lv Y, Ma T. Near-infrared fluorescence imaging with indocyanine green for assessment of donor livers in a rat model of ischemia-reperfusion. BMC Gastroenterol. 2022;22:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 856] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 21. | Watson CJE, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K, Gimson AE, Allison M, Upponi S, Brais R, Jochmans I, Butler AJ. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 22. | Yang L, Cao H, Sun D, Hou B, Lin L, Shen ZY, Song HL. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020;381:239-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Ni D, Wei H, Chen W, Bao Q, Rosenkrans ZT, Barnhart TE, Ferreira CA, Wang Y, Yao H, Sun T, Jiang D, Li S, Cao T, Liu Z, Engle JW, Hu P, Lan X, Cai W. Ceria Nanoparticles Meet Hepatic Ischemia-Reperfusion Injury: The Perfect Imperfection. Adv Mater. 2019;31:e1902956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Gillooly AR, Perry J, Martins PN. First Report of siRNA Uptake (for RNA Interference) During Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation. 2019;103:e56-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Sakamoto A, Sakamoto K, Hikida T, Ito C, Iwata M, Shine M, Uraoka M, Nishi Y, Nagaoka T, Honjo M, Tamura K, Funamizu N, Ogawa K, Takada Y. Prolonged warm ischemia time in the recipient is associated with post-transplant biliary stricture following living-donor liver transplantation. Surg Today. 2024;54:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Ma Y, Wang GD, Wu LW, Hu RD. Dynamical changing patterns of histological structure and ultrastructure of liver graft undergoing warm ischemia injury from non-heart-beating donor in rats. World J Gastroenterol. 2006;12:4902-4905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | He XS, Ma Y, Ju WQ, Wu LW, Wu JL, Liang YJ, Hu RD, Chen GH, Huang JF. Dynamic microcirculatory changes in liver graft from non-heart-beating donor with warm ischemia injury in rat. Hepatobiliary Pancreat Dis Int. 2004;3:179-182. [PubMed] |
| 28. | He XS, Ma Y, Wu LW, Ju WQ, Chen GH, Hu RD, Huang JF. Influence of warm ischemia injury on hepatic functional status and survival of liver graft in rats. Hepatobiliary Pancreat Dis Int. 2003;2:504-508. [PubMed] |
| 29. | Narasaki H, Noji T, Wada H, Ebihara Y, Tsuchikawa T, Okamura K, Tanaka E, Shichinohe T, Hirano S. Intraoperative Real-Time Assessment of Liver Function with Near-Infrared Fluorescence Imaging. Eur Surg Res. 2017;58:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wang S, Lin X, Tang Y, Liang Y, Zhang M, Xie Z, Guo Y, Dong Y, Zhao Q, Guo Z, Wang D, He X, Ju W, Chen M. Ischemia-free liver transplantation improves the prognosis of recipients using functionally marginal liver grafts. Clin Mol Hepatol. 2024;30:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Huang C, Chen Z, Wang T, He X, Chen M, Ju W. A marginal liver graft with hyperbilirubinemia transplanted successfully by ischemia-free liver transplantation. Ann Transl Med. 2021;9:425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Carmichael FJ, Lindop MJ, Farman JV. Anesthesia for hepatic transplantation: cardiovascular and metabolic alterations and their management. Anesth Analg. 1985;64:108-116. [PubMed] |
| 33. | Shangraw RE, Winter R, Hromco J, Robinson ST, Gallaher EJ. Amelioration of lactic acidosis with dichloroacetate during liver transplantation in humans. Anesthesiology. 1994;81:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |