Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.103068
Revised: December 17, 2024
Accepted: January 20, 2025
Published online: March 7, 2025
Processing time: 97 Days and 1.4 Hours
Wedged hepatic venous pressure (WHVP) is a crucial variable for accurately assessing the hepatic venous pressure gradient (HVPG) and is vital for the diagnosis and prognostic evaluation of patients with portal hypertension (PH).
To investigate the anatomical characteristics of balloon-occluded hepatic venous angiography in patients with PH and analyze the relationship between the WHVP and portal venous pressure (PVP).
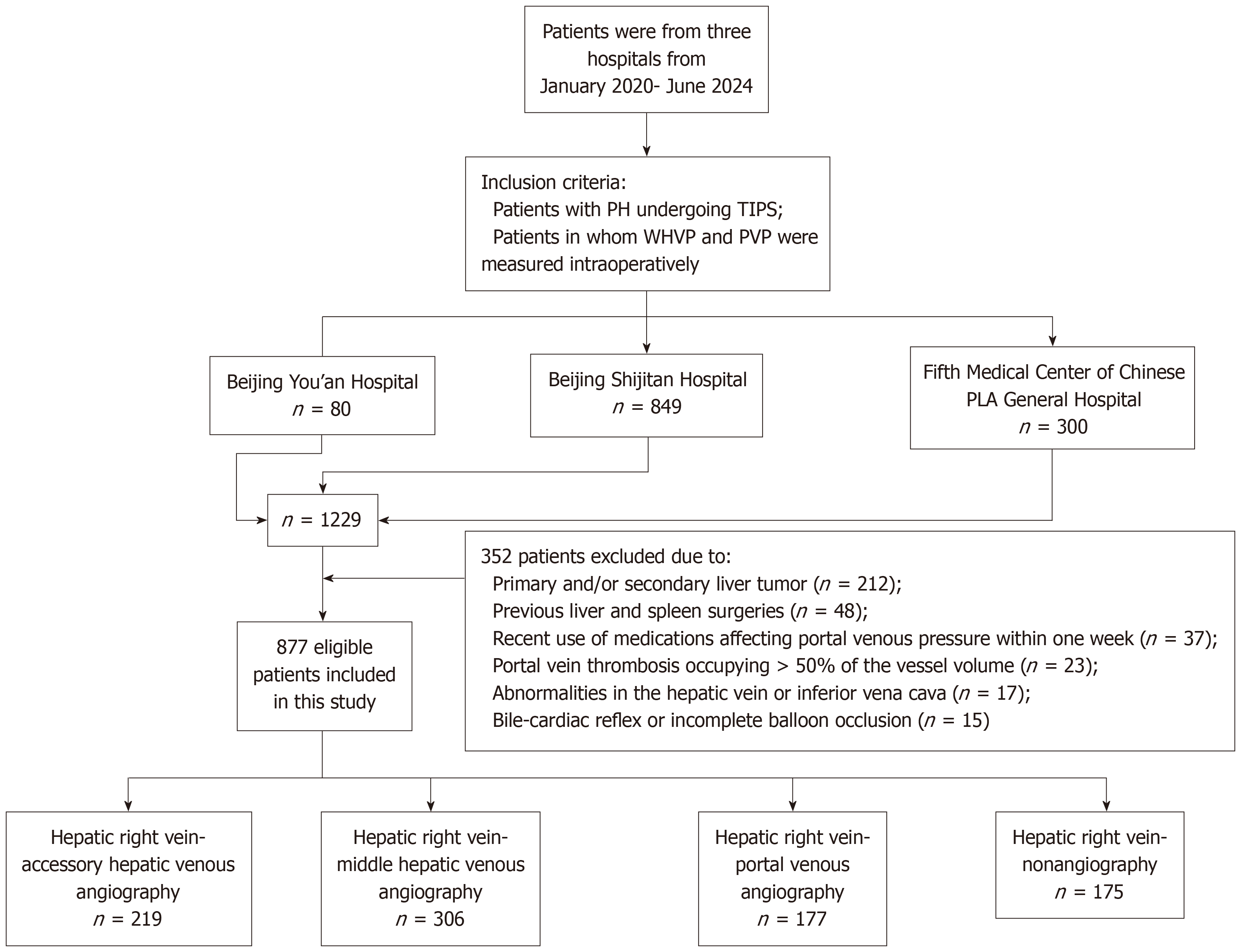
This retrospective study included 877 patients with PH who met the inclusion criteria from January 2020 to June 2024. Routine and innovative hepatic venous angiography was performed during transjugular intrahepatic portosystemic shunt procedures to measure hepatic venous and PVPs. All patients' angiographic images were collected for analysis. The associations between WHVP and PVP in each group were analyzed via linear regression analysis, and a predictive model was established.
The 877 patients had a mean age of 52.6 ± 13.0 years, with 582 males and 295 females. Patients were categorized into four groups on the basis of their anatomical structure. All groups showed strong correlations between WHVP and PVP. The regression coefficient between the WHVP and PVP in the hepatic right vein-portal venous angiography group was 0.884 (P < 0.05); in the hepatic right vein-accessory hepatic venous angiography group, it was 0.721 (P < 0.05); in the hepatic right vein-middle hepatic venous angiography group, it was 0.344 (P < 0.05); and in the hepatic right vein-nonangiography group, it was 0.293 (P < 0.05).
The presence and anatomical classification of hepatic venous collaterals are key factors influencing the relationship between WHVP with and PVP. Based on the different anatomical classifications of hepatic veins, WHVP can be used to estimate PVP, improving the accuracy of PVP prediction.
Core Tip: This multicenter study explored the relationship between the wedged hepatic venous pressure (WHVP) and portal venous pressure (PVP) in patients with portal hypertension patients. By analyzing anatomical characteristics through hepatic venous angiography, significant correlations were identified. An innovative finding revealed that WHVP, based on the anatomical classification of the hepatic vein, can be used to effectively estimate PVP, enhancing predictive accuracy. The presence and classification of hepatic venous collaterals play pivotal roles in this association, offering a promising approach for precise PVP prediction and improving patient prognosis in the management of portal hypertension.
- Citation: Ye QX, Meng MM, Wu YF, Dong CB, Zhang Y, Liu BW, Lv YF, You SL, Lv S, Ding HG, Han Y, Yang YP, Zhu B, Liu FQ. Multicenter analysis on the correlation between the anatomical characteristics of hepatic veins and hepatic venous wedge pressure. World J Gastroenterol 2025; 31(9): 103068
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/103068.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.103068
Portal hypertension (PH) is a clinical syndrome often associated with complications such as ascites, gastroesophageal variceal bleeding, hepatic encephalopathy, and portal hypertensive gastropathy in patients with liver diseases[1]. Clinically, the hepatic venous pressure gradient (HVPG) is considered the gold standard for diagnosing PH, with PH indicated when the HVPG exceeds 5 mmHg[2,3]. However, several recent studies have suggested that the overall correlation between the HVPG and the portal pressure gradient (PPG) is poor when there is hepatic blood circulation shunting, as the HVPG tends to underestimate the PPG in most patients[4].
The core variable determining whether the HVPG accurately represents PPG is the wedged hepatic venous pressure (WHVP)[1]. The WHVP reflects the pressure in the hepatic sinusoids. In normal individuals, the free hepatic venous pressure (FHVP) and WHVP are similar because blood flow from the obstructed hepatic vein is dispersed through small vascular channels in the surrounding sinusoidal space, dissipating most of the pressure and preventing a significant increase in WHVP[5]. Compared with PVP, preoperative measurements of the FHVP and WHVP can provide better insights into the location and severity of portal vein blockage in patients with PH, aiding surgeons in treatment planning[6,7].
The correlation between WHVP and PVP is closely related to underlying liver disease. For example, Thalheimer reported a strong correlation between WHVP and directly measured PVP in patients with alcoholic liver disease and hepatitis B virus, suggesting that WHVP is a reliable alternative measurement[8]. However, in acute PH caused by hepatic sinusoidal obstruction syndrome, WHVP is less accurate in estimating PVP compared to cases of viral or alcohol-related cirrhosis, resulting in an overestimation of PVP[9]. In decompensated nonalcoholic fatty liver disease-related cirrhosis, WHVP predicts PVP less accurately than alcoholic or hepatitis C virus-related cirrhosis does, resulting in an underestimation of PVP[10].
Despite these insights, no large studies have examined the correlation between WHVP and PVP in situations involving anatomical shunting within the liver veins. Therefore, this study aimed to investigate the multivariate impact of different anatomical structures of hepatic venous shunts on WHVP and compare it with PVP. Using linear regression, we explored the relationship between WHVP and PVP and developed a more accurate prediction model for PVP, addressing the challenge of difficult PVP measurements.
Approval of the research protocol: The research protocol adhered to every provision of the Helsinki Declaration and received approval from the Ethics Committees of three hospitals, namely Beijing Shijitan Hospital (Approval No. 2018/01), Fifth Medical Center of Chinese PLA General Hospital (Approval No. KY-2023-12-82-1), Beijing You’an Hospital (Approval No. LL-2023-042-K).
This retrospective study collected data from patients who underwent transjugular intrahepatic portosystemic shunt (TIPS) surgery for PH at three hospitals, namely Beijing Shijitan Hospital, Fifth Medical Center of Chinese PLA General Hospital, Beijing You’an Hospital, from January 2020 to June 2024. During the TIPS procedures, hepatic vein balloon occlusion was performed, and measurements of WHVP and PVP were recorded. Written informed consent was obtained from all patients involved in the study.
The inclusion criteria were as follows: (1) Patients with PH and underwent TIPS; and (2) Patients who had intraoperative measurements of WHVP and PVP. The exclusion criteria were as follows: (1) Patients with primary and/or secondary liver tumor; (2) Patients with chronic liver failure; (3) Patients with any factors that could alter hepatic hemodynamics, such as previous liver and spleen surgeries and recent use of medications affecting portal venous pressure within one week; (4) Patients with portal vein thrombosis occupying more than 50% of the vessel volume; (5) Patients with abnormalities in the hepatic vein or inferior vena cava; and (6) Patients whom accurate pressure measurements were not possible due to factors such as the bile-cardiac reflex or incomplete balloon occlusion.
WHVP and PVP were measured according to established standards[11,12]. Routine disinfection and draping were performed, followed by local anesthesia and puncture through the right internal jugular vein. A catheter was inserted, passing through the brachiocephalic vein, superior vena cava, and right atrium to reach the inferior vena cava and then the hepatic vein. A 5-French Fogarty balloon catheter (manufactured by Edwards Life Sciences LLC, United States) was advanced to the terminal part of the hepatic vein for angiography, aiming to observe the overall anatomical structure of the hepatic vein and reconfirm the position of the balloon. The balloon tip of the catheter was positioned approximately 3-5 cm away from the junction of the hepatic vein and the inferior vena cava, and then balloon dilation was commenced. By injecting 2 mL of normal saline into the balloon, the balloon was gradually inflated. During this process, the pressure exerted by the balloon on the vessel wall as well as the time needed to be strictly controlled. Subsequently, the balloon was inflated to occlude the hepatic vein. While the balloon was dilating and occluding the vessel, 5 mL of contrast medium was injected to evaluate the sealing efficacy of the balloon and the condition of the hepatic vein. After that, the catheter was flushed with normal saline to remove the contrast medium. The FHVP could be measured about 15 seconds after the pressure reading stabilized. The balloon was continuously inflated, and the WHVP could be measured at around 45 seconds. Both FHVP and WHVP were measured three times, and the average values were recorded. If the sealing effect was unsatisfactory, the position of the balloon catheter was adjusted, and the measurements were repeated.
After the initial pressure measurement was completed, an innovative angiographic technique identified in our previous research was adopted to measure the WHVP again[13]. After the standard measurement method was finished, the hepatic vein was occluded again by inserting a balloon at the same position as that in the conventional method. The dose of the contrast medium was increased. A high-pressure injector was used to inject a total volume of 15 mL of contrast medium at a stable pressure ranging from 200 to 300 psi, at a rate of 5 mL/second, with continuous fluoroscopy for more than 6 seconds. The WHVP was measured again once the pressure stabilized. Three measurements were taken and the average value was recorded. Meanwhile, the angiographic anatomical structure of the hepatic vein was documented.
A RUPS-100 puncture set (Cook Medical, United States) was used to puncture the liver parenchyma through the hepatic vein or the inferior vena cava into the intrahepatic portal vein. After successful puncture, a pigtail catheter was advanced over a guidewire into the portal vein for angiography. The catheter was retracted to the main trunk of the portal vein, and once stable, the main portal venous pressure was measured (three measurements were taken, and the average value was recorded), and the PVP value was documented.
The data were initially subjected to tests for normality and homogeneity of variance via SPSS Statistics, version 20.0 (IBM). Normally distributed continuous variables are expressed as the mean ± SD, while non-normally distributed continuous variables are presented as medians (interquartile ranges). The nonparametric Mann-Whitney U test was used for comparisons between two groups. Pearson’s correlation analysis was used to analyze the relationship between WHVP and PVP, and data visualization was performed via R (version 4.2.1). Furthermore, linear regression analysis was conducted via SPSS 22.0 to establish relationships and prediction models for WHVP and PVP in the presence of different shunt structures, and predictive equations were derived. GraphPad Prism (version 9.5) software was used for data visualization. P < 0.05 was considered to indicate statistical significance.
A flow chart of the study, from initial retrieval to the final study cohort, is shown in Figure 1. A total of 877 patients (582 males, 295 females) were included in this study. The average age was 52.6 ± 13.0 years (ranging from 14 to 87 years). The etiological classification and main symptoms of PH are shown in Table 1. The hemodynamic pressure measurements were as follows: The average WHVP was 27.3 ± 9.3 mmHg, and the average PVP was 33.7 ± 7.05 mmHg.
| Variable | Statistics |
| Age, year, mean ± SD | 52.6 ± 13.0 |
| Sex | |
| Male | 582 (66.36) |
| Female | 295 (33.63) |
| Etiology | |
| HBV-related cirrhosis | 407 (46.41) |
| Alcoholic cirrhosis | 156 (17.79) |
| Autoimmune cirrhosis | 109 (12.43) |
| Hepatic veno-occlusive disease | 79 (9.01) |
| HCV-related cirrhosis | 46 (5.25) |
| Cirrhosis of unknown cause | 29 (3.31) |
| Drug-induced cirrhosis | 27 (3.08) |
| Arteriovenous fistula and Wilson's disease, etc. | 13 (1.48) |
| Idiopathic portal hypertension | 11 (1.25) |
| Clinical sign | |
| Gastrointestinal bleeding | 453 (51.7) |
| Ascites | 189 (21.6) |
| Gastrointestinal bleeding + ascites | 158 (18.0) |
| Others (hepatorenal syndrome, hepatic congestion, etc.) | 77 (8.8) |
| Child-Pugh | |
| A | 354 (40.4) |
| B | 443 (50.5) |
| C | 80 (9.1) |
| Group | |
| Hepatic right vein-middle hepatic venous angiography group (group A) | 306 (34.9) |
| Hepatic right vein-accessory hepatic venous angiography group (group B) | 219 (25.0) |
| Hepatic right vein-portal venous angiography group (group C) | 177 (20.2) |
| Hepatic right vein-nonangiography group (group D) | 175 (19.9) |
After routine injection of 5 mL of contrast medium following hepatic venous balloon occlusion, the collateral display rate was only 25.5%. Specifically, the display rate for the right hepatic vein to the middle hepatic vein collaterals was 88.9%, for right hepatic vein to the accessory hepatic vein collaterals was 7.9%, and that for the right hepatic vein to the portal vein it was 3.2%. The remaining 74.5% did not show any collaterals, portal veins, or minor branches of the hepatic veins.
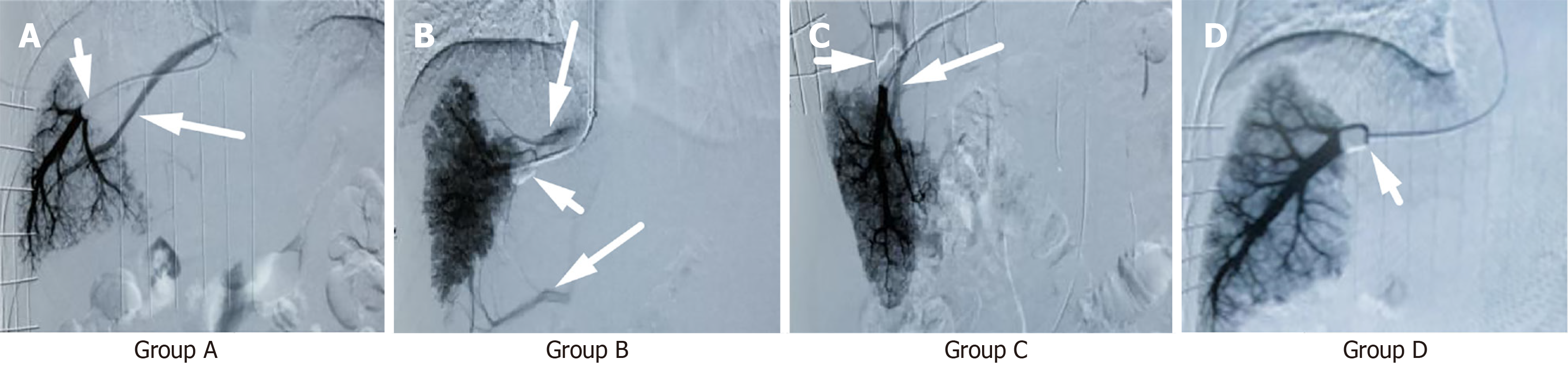
Group A: Hepatic right vein-middle hepatic venous angiography: During venography, another normal hepatic vein was visualized (Figure 2A).
Group B: Hepatic right vein-accessory hepatic venous angiography group: During venography, simultaneous visualization of one or multiple accessory hepatic veins occurred (Figure 2B).
Group C: Hepatic right vein-portal venous angiography: During venography, visualization of the portal vein occurred (Figure 2C).
Group D: Hepatic right vein nonangiography: During venography, no other veins or portal veins were visualized (Figure 2D).
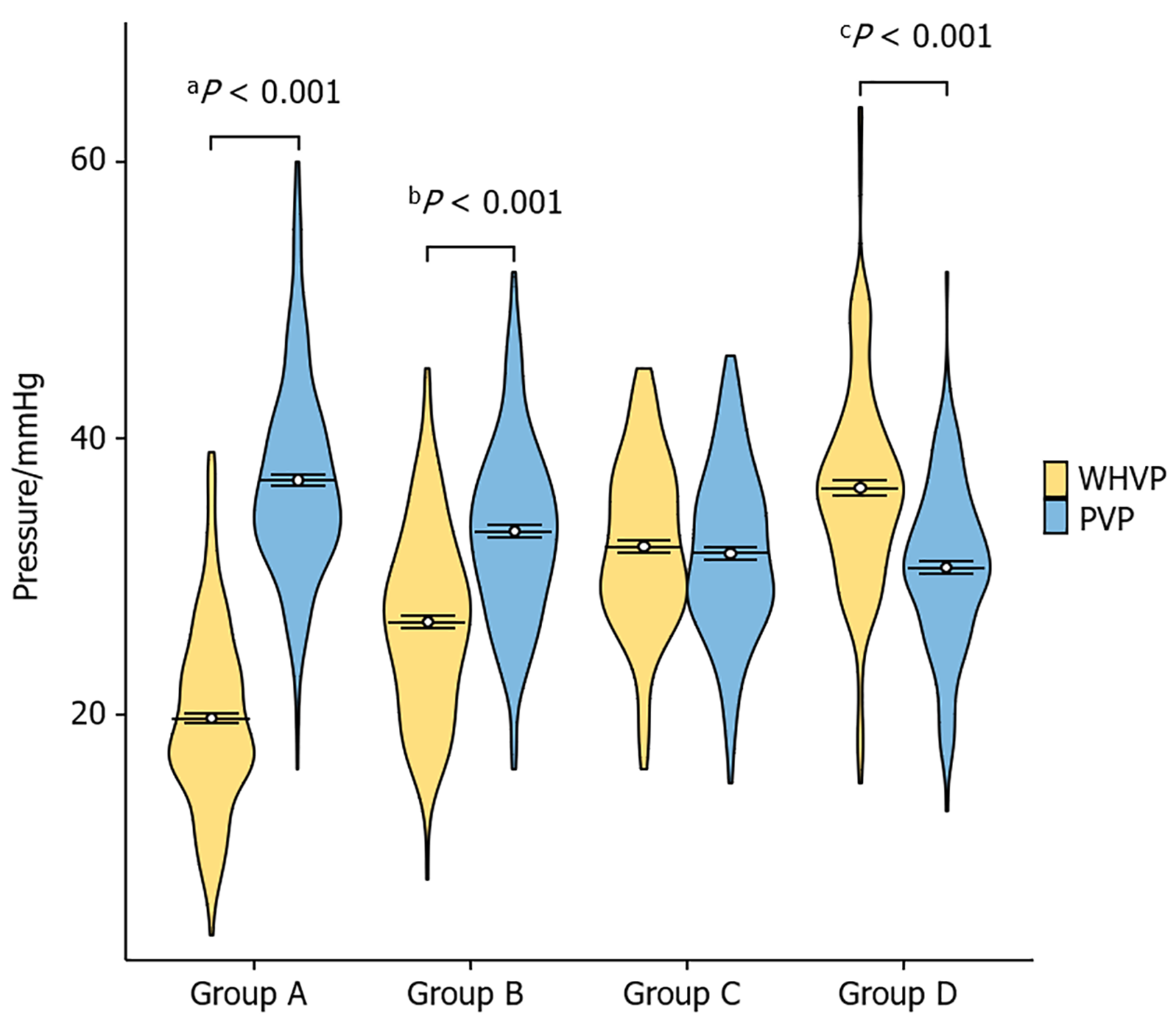
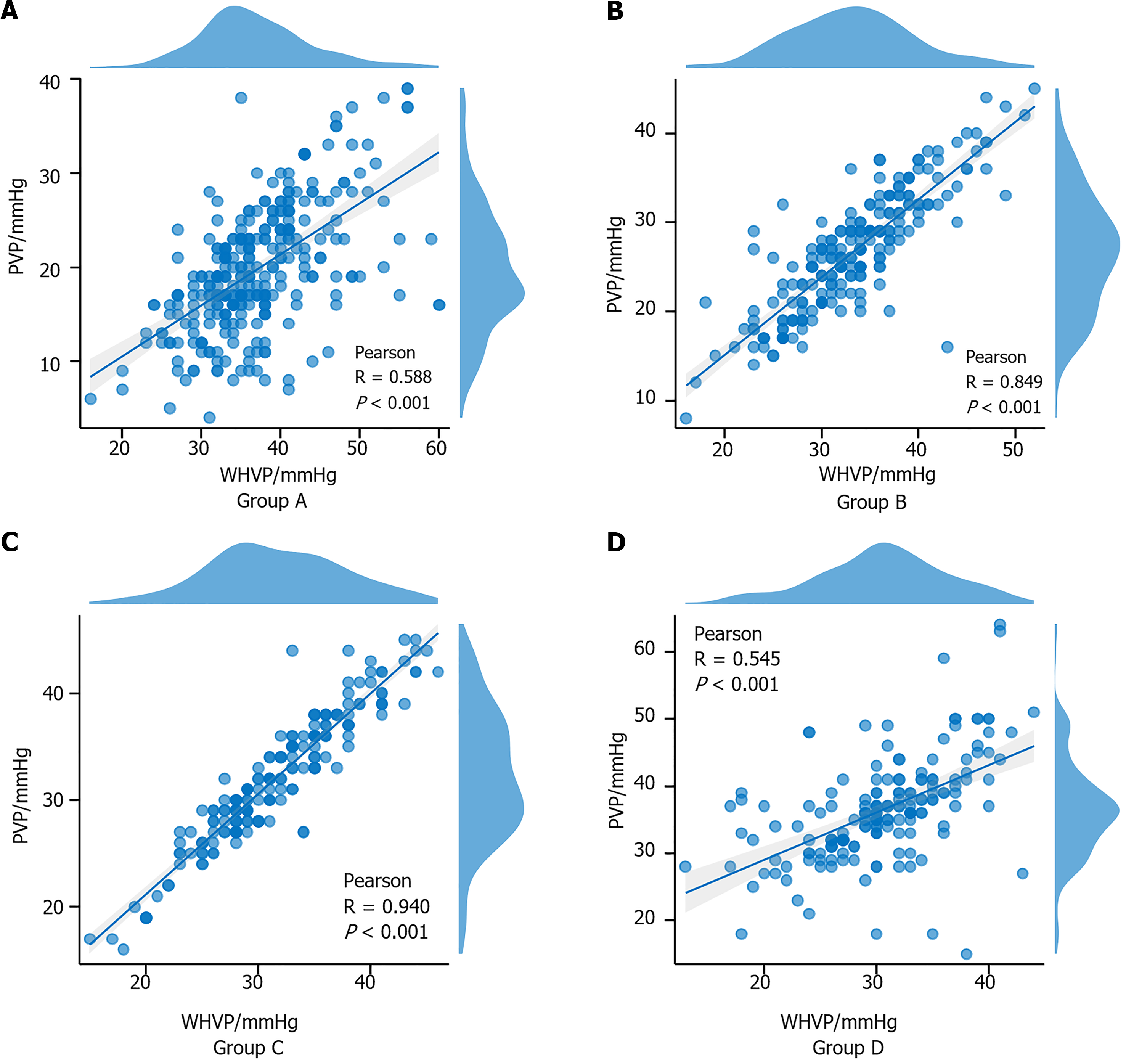
The relationship between WHVP and PVP in each group was calculated via the nonparametric Mann-Whitney U test (Table 2 and Figure 3). Pearson's correlation coefficient between these two pressure values was calculated for each group (Figure 4). In Group A, r = 0.588 (95%CI: 0.5-0.7, P < 0.001); in Group B, r = 0.849 (95%CI: 0.8-0.9, P < 0.001); in Group C, r = 0.940 (95%CI: 0.9-1.0, P < 0.001); and in Group D, r = 0.545 (95%CI: 0.4-0.6, P < 0.001). The absolute value of the correlation coefficient represents the degree of correlation, where 0-0.3 represents weak or no correlation, 0.3-0.5 indicates weak correlation, 0.5-0.8 indicates moderate correlation, and 0.8-1 indicates strong correlation.
| Variable | PVP | WHVP | U value | P value |
| Group A | 36 (32%-41%) | 19 (16%-24%) | 3645 | < 0.001 |
| Group B | 33 (29%-37%) | 27 (22%-31%) | 11590 | < 0.001 |
| Group C | 31 (28%-36%) | 32 (28%-36%) | 16440 | 0.419 |
| Group D | 30 (27%-34%) | 36 (32%-40%) | 22330 | < 0.001 |
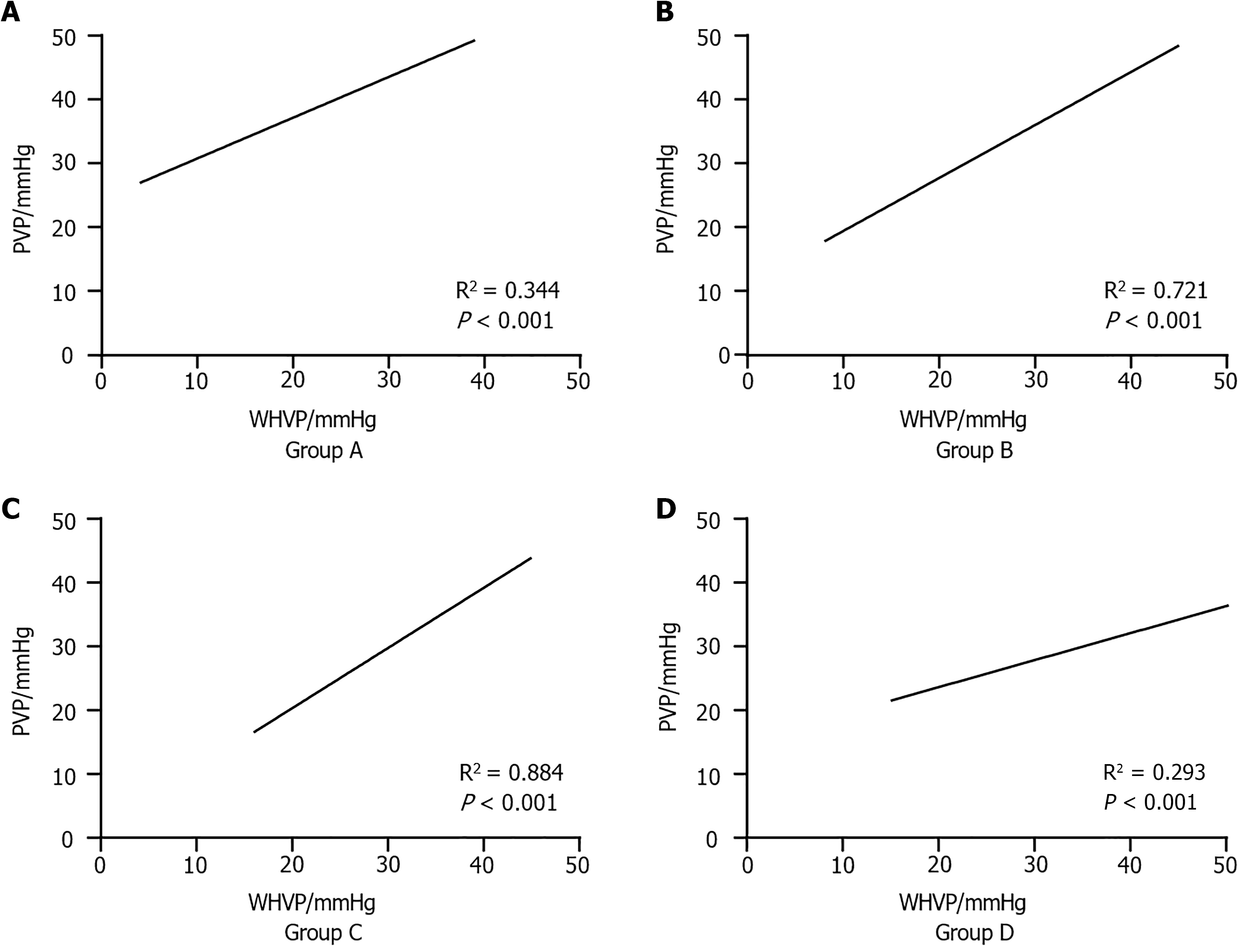
A linear regression model was established with WHVP as the independent variable and PVP as the dependent variable. The model exhibited a good fit, and residual analysis did not reveal any significant outliers or deviations from the model assumptions. All four groups presented a significant positive correlation. Group C exhibited the best regression performance. Additionally, predictive equations for WHVP regarding PVP were derived for each group. The results are presented in Table 3 and Figure 5.
| Group | Adjusted R2 | Constant | Regression equation | P value |
| Group A | 0.344 | 24.359 | Y = 0.6384X + 24.36 | < 0.001 |
| Group B | 0.721 | 11.185 | Y = 0.8266X + 11.18 | < 0.001 |
| Group C | 0.884 | 1.435 | Y = 0.9404X + 1.435 | < 0.001 |
| Group D | 0.293 | 15.172 | Y = 0.4209X + 15.17 | < 0.001 |
For patients with PH, determining whether the HVPG can truly represent the PPG, with WHVP as the core variable, holds significant clinical relevance. Investigating the correlation and predictive models between WHVP and PVP is essential.
In healthy individuals, liver hemodynamics can self-adjust to maintain normal portal venous pressure within a healthy range[14]. The etiology of liver diseases is diverse, including infections, cirrhosis, intrahepatic and extrahepatic bile duct obstruction, drug toxicity, and alcohol-related conditions[15]. These factors lead to pathological changes such as intrahepatic and extrahepatic bile duct obstruction, liver fibrosis, and cirrhosis in patients, resulting in increased portal vein resistance, blocked blood flow, and elevated portal venous pressure[16]. Cirrhosis progresses from an asymptomatic compensatory phase to decompensated, where complications like esophageal variceal bleeding, ascites, hepatic encephalopathy, and jaundice, significantly affect prognosis. These complications are crucial variables for risk-stratifying and mortality[17,18]. As cirrhosis progresses, accurate assessment of portal venous pressure becomes essential for implementing therapeutic measures aimed at reducing portal venous pressure, preventing first-time variceal bleeding, and improving liver reserve function[19,20]. From the perspective of anatomical relationships and hemodynamics of normal liver vasculature, the pressure measured after blocking the hepatic vein is equal to the pressure in the hepatic sinusoids, while direct pressure in the portal vein should be slightly greater than or equal to the hepatic sinusoidal pressure[21]. Therefore, the HVPG is recognized as the gold standard for predicting PPG and serves as a diagnostic tool for PH, predicting liver disease prognosis, assessing drug treatment effectiveness, and predicting the correlation with primary liver cancer[1,22]. While determination of the HVPG does not require advanced technical expertise or sophisticated hospital equipment, accuracy is paramount. Inaccuracy can directly affect disease staging, treatment selection, prognosis assessment, clinical practice, and scientific research[12]. Consequently, the development of minimally invasive and precise diagnostic technologies remains a focal point and challenge in this field.
In the presence of changes in liver hemodynamics, pathophysiology, and anatomical shunting, controversy arises regarding whether the HVPG can still serve as the "gold standard" representative of the PPG. Our research team reported in earlier studies that the overall correlation between the HVPG and PPG in patients with hepatitis B-related cirrhotic PH and autoimmune liver diseases was poor. In most patients, the HVPG cannot accurately represent the PPG[23]. The appearance of hepatic vein collaterals during angiography is a key factor in underestimating HVPG, with earlier collateral appearance leading to more significant underestimation, while the absence of collaterals overestimates the HVPG[5]. When the WHVP is measured, a routine injection of 5 mL of contrast medium after balloon occlusion of the hepatic vein results in a collateral display rate of only 25.5%, primarily revealing collaterals between the right hepatic vein and middle hepatic vein. This fails to fully reflect the hepatic veins and shunting conditions. Since WHVP is the core variable of the HVPG and determines the accuracy of the PVP assessment, in our study involving patients from three hospitals, we employed innovative hepatic vein angiography by increasing the contrast medium dosage from 5 mL to 15 mL, controlling the injection time, and using digital subtraction angiography. We specifically examined the correlation between the WHVP and PVP under different anatomical conditions of the hepatic vein shown by this innovative angiography, establishing a regression prediction model. The results indicate that the correlation and regression of the WHVP and PVP are strongest in patients with right hepatic vein-to-portal vein collaterals; in patients with collaterals between the right hepatic vein and accessory hepatic veins, there is a high correlation and good regression between the WHVP and PVP; even in patients with collaterals between the right hepatic vein and middle hepatic vein or without collaterals, there is a correlation and regression between the WHVP and PVP. All four groups can use a regression model with WHVP to predict PVP, enhancing the accuracy of PVP prediction. These differences are statistically significant (P < 0.05).
An innovative HVPG study on 306 patients (34.9%) revealed intrahepatic venous-venous collateral circulation from the right hepatic vein to the middle hepatic vein. Research suggests that when normal hepatic veins form collaterals with other hepatic veins, there is a relatively large shunt volume. Following balloon occlusion of the hepatic vein, the pressure increase space is minimal as the pressure is diverted and significantly lower than the sinusoidal pressure, leading to significantly lower measured WHVP compared to PVP. On the other hand, 219 patients (25.0%) had intrahepatic venous-venous collateral circulation from the right hepatic vein to the accessory hepatic vein. This scenario involves collateral formation between normal hepatic veins and relatively smaller accessory hepatic veins, resulting in a relatively smaller shunt volume. After balloon occlusion, there is some increase in pressure, but part of the pressure is still diverted, leading to the WHVP being lower than the PVP. In the case of 177 patients (20.2%) in whom the portal vein was visualized, upon balloon occlusion caused the pressure to increase to match the sinusoidal pressure, allowing the contrast agent to reflux into the portal vein, resulting in basic pressure equilibrium where WHVP equaled PVP. In 175 cases (19.9%), no collaterals formed between normal hepatic veins or with other hepatic veins during hepatic vein imaging, and the portal vein did not show contrast. This suggests a significant pressure rise after hepatic vein occlusion due to the absence of decompression channels, resulting in contrast not passing through the sinusoids into the portal vein. This situation mainly occurs in patients with portal vein reflux or significant shunting (splenorenal shunt, gastrorenal shunt, patent umbilical vein, etc.), possibly due to disrupted sinusoidal flow regulation by high-pressure hepatic arteries, leading to the WHVP being higher than the PVP. The presence and different anatomical types of hepatic vein collaterals significantly affect the correlation and regression between WHVP and PVP. This allows for the estimation of PVP based on the WHVP of different anatomical types of hepatic veins, providing a convenient and effective method for assessing PH.
WHVP can directly measure the pressure within the hepatic veins and precisely reflect the pressure status of the hepatic vascular bed, providing a more direct and accurate quantitative assessment of the degree of PH. In contrast, ultrasound and enhanced computed tomography mainly infer the situation of PH through indirect indicators such as observing the morphology of the liver, the diameter of blood vessels, and the blood flow velocity. It is difficult for them to obtain pressure values as accurately as WHVP. The measurement of WHVP can not only reflect the pressure but also, to some extent, embody the overall hemodynamic changes in the liver. However, the measurement of WHVP requires percutaneous puncture of the hepatic vein and placement of a catheter, which is an invasive procedure with numerous risks, such as bleeding, infection, and even life-threatening consequences. Meanwhile, it also has relatively high requirements for the skills of the operating doctors. They need to possess rich experience in interventional operations, proficient catheter manipulation skills, professional angiographic equipment, pressure measurement instruments, and so on. Moreover, there may be operator-dependent variability in obtaining and interpreting WHVP measurement values, which might introduce a certain degree of imprecision into the results.
However, this study has several limitations. Larger clinical studies are needed to further validate the relationship between WHVP and PVP. Additionally, variations in etiology and intragroup hepatic vein anatomical structures may affect pressure measurement consistency, necessitating personalized studies to analyze the correlation between WHVP and PVP in different diseases to establish more reliable predictive models. Meanwhile, although the innovative angiography technique used in this paper has improved the collateral display rate of the hepatic veins, whether the use of contrast agents will have a transient impact on the hemodynamics of patients, thereby interfering with the results of pressure measurements, as well as the total amount of contrast agents, injection doses (per second), and injection pressures that are most suitable for the innovative angiography still require further research and discussion. This study suggests that using different predictive models to evaluate PVP based on different anatomical collateral types of hepatic veins can offer a more accurate, convenient, minimally invasive, and personalized approach, potentially providing new perspectives and strategies for the management, diagnosis, and treatment of liver disease patients. With further research and clinical validation, WHVP could become a simpler and more reliable tool for assessing PVP, offering better guidance for patient prognosis and treatment outcomes.
In conclusion, this study established a predictive model and equation for WHVP and PVP in PH patients through correlation and linear regression analyses. The results revealed a correlation and regression relationship between the WHVP and PVP in PH patients, highlighting the significance of hepatic vein collaterals in influencing this relationship. This allows for the estimation of PVP based on the WHVP of different anatomical types of hepatic veins. Despite these limitations, this model has predictive capabilities and promising clinical applications. Future research should focus on refining and validating this model to increase its practicality and reliability in the diagnosis and treatment of PH.
We are grateful for the active cooperation and assistance of the doctors and nurses from the three hospitals, Capital Medical University Affiliated Beijing Shijitan Hospital, Beijing You’an Hospital and department of Diagnosis and Treatment of Hepatic Vascular Disease Center, the Fifth Medical Center of Chinese PLA General Hospital, during the surgical procedures for obtaining research data.
| 1. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1506] [Article Influence: 502.0] [Reference Citation Analysis (2)] |
| 2. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Vilarinho S, Sari S, Yilmaz G, Stiegler AL, Boggon TJ, Jain D, Akyol G, Dalgic B, Günel M, Lifton RP. Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension. Hepatology. 2016;63:1977-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Wang L, Song QK, Yue ZD, Zhao HW, Fan ZH, Wu YF, Liu FQ, Meng K, Zhang L, Jiang HG, Ding YN, Zhang Y. [Study on the correlation between PPG and HVPG in patients with portal hypertension]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, Gatta A. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Zardi EM, Di Matteo FM, Pacella CM, Sanyal AJ. Invasive and non-invasive techniques for detecting portal hypertension and predicting variceal bleeding in cirrhosis: a review. Ann Med. 2014;46:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis. 2005;37:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Cheng Y, Gu L, Yin X, Wang X, Xiao J, Wang Y, Zhang W, Wang L, Zou X, Zhang M, Zhuge Y, Zhang F. Agreement between Wedged Hepatic Venous Pressure and Portal Pressure in Hepatic Sinusoidal Obstruction Syndrome. J Pers Med. 2022;13:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 10. | Ferrusquía-Acosta J, Bassegoda O, Turco L, Reverter E, Pellone M, Bianchini M, Pérez-Campuzano V, Ripoll E, García-Criado Á, Graupera I, García-Pagán JC, Schepis F, Senzolo M, Hernández-Gea V. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol. 2021;74:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Reiberger T, Schwabl P, Trauner M, Peck-Radosavljevic M, Mandorfer M. Measurement of the Hepatic Venous Pressure Gradient and Transjugular Liver Biopsy. J Vis Exp. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis. 2011;43:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Liu B, Yue Z, Cui T, Zhao H, Wang L, Fan Z, Wu Y, Meng M, Zhang K, Jiang L, Ding H, Zhang Y, Liu F. Innovative angiography: a new approach to discover more hepatic vein collaterals in patients with cirrhotic portal hypertension. BMC Gastroenterol. 2023;23:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Stankovic Z, Jung B, Collins J, Russe MF, Carr J, Euringer W, Stehlin L, Csatari Z, Strohm PC, Langer M, Markl M. Reproducibility study of four-dimensional flow MRI of arterial and portal venous liver hemodynamics: influence of spatio-temporal resolution. Magn Reson Med. 2014;72:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, Acevedo J, Duran-Güell M, Nuñez L, Costa M, Torres M, Horrillo R, Ruiz-Del-Árbol L, Villanueva C, Prado V, Arteaga M, Trebicka J, Angeli P, Merli M, Alessandria C, Aagaard NK, Soriano G, Durand F, Gerbes A, Gustot T, Welzel TM, Salerno F, Bañares R, Vargas V, Albillos A, Silva A, Morales-Ruiz M, Carlos García-Pagán J, Pavesi M, Jalan R, Bernardi M, Moreau R, Páez A, Arroyo V. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology. 2019;157:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (1)] |
| 16. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 862] [Article Influence: 215.5] [Reference Citation Analysis (1)] |
| 17. | Hernández-Gea V, Baiges A, Turon F, Garcia-Pagán JC. Idiopathic Portal Hypertension. Hepatology. 2018;68:2413-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Selicean S, Wang C, Guixé-Muntet S, Stefanescu H, Kawada N, Gracia-Sancho J. Regression of portal hypertension: underlying mechanisms and therapeutic strategies. Hepatol Int. 2021;15:36-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1821] [Article Influence: 260.1] [Reference Citation Analysis (2)] |
| 20. | D'Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 21. | Turco L, Villanueva C, La Mura V, García-Pagán JC, Reiberger T, Genescà J, Groszmann RJ, Sharma BC, Merkel C, Bureau C, Alvarado E, Abraldes JG, Albillos A, Bañares R, Peck-Radosavljevic M, Augustin S, Sarin SK, Bosch J, García-Tsao G. Lowering Portal Pressure Improves Outcomes of Patients With Cirrhosis, With or Without Ascites: A Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:313-327.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 22. | Burroughs AK, Thalheimer U. Hepatic venous pressure gradient in 2010: optimal measurement is key. Hepatology. 2010;51:1894-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Tey TT, Gogna A, Irani FG, Too CW, Lo HG, Tan BS, Tay KH, Lui HF, Chang PE. Application of a standardised protocol for hepatic venous pressure gradient measurement improves quality of readings and facilitates reduction of variceal bleeding in cirrhotics. Singapore Med J. 2016;57:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |