Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.100607
Revised: January 12, 2025
Accepted: January 18, 2025
Published online: March 7, 2025
Processing time: 181 Days and 5.2 Hours
Inflammatory bowel disease (IBD), encompassing Crohn’s disease and ulcerative colitis, manifests as a chronic, recurrent, and refractory intestinal inflammatory condition significantly impacting patients’ quality of life. Despite ongoing research, its etiology and pathogenesis remain incompletely understood. Recent advancements in medical research highlight the critical role of drug combination therapies in managing IBD. This paper employs the strengths, weaknesses, opportunities, and threats framework to evaluate the four strategic elements (strengths, weaknesses, opportunities, and threats) pertaining to combination therapies for IBD. Among the strengths, the paper underscores the efficacy of multi-targeted strategies, the advancement of personalized medicine, and the mitigation of drug resistance. Nonetheless, the analysis identifies significant weaknesses, including the prohibitive cost of treatment, issues with patient compliance, and the necessity for comprehensive long-term safety data. The paper also delineates opportunities to augment therapeutic success through the incorpo
Core Tip: Ongoing advancements in medical research have solidified drug combination therapy as pivotal in the management of inflammatory bowel disease (IBD). This editorial evaluates the current landscape of IBD combination therapy using the strengths, weaknesses, opportunities, and threats analysis and proposes pathways to enhance the next generation of treatment strategies.
- Citation: Yan JW, Nie M, Zhang H, Liu YM, Tang FS. Strengths, weaknesses, opportunities, and threats analysis of combination therapy for inflammatory bowel disease. World J Gastroenterol 2025; 31(9): 100607
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/100607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.100607
This letter to the editor provides an analytical perspective on a recent publication by Lowell et al[1], which appeared in the World Journal of Gastroenterology. Inflammatory bowel disease (IBD), comprising Crohn’s disease and ulcerative colitis, represents a chronic, recurrent, and challenging inflammatory condition of the intestinal tract. Despite extensive research by both domestic and international scholars into the pathogenesis, clinical diagnosis, and treatment of IBD in recent years, the exact etiology remains elusive, and a definitive cure has yet to be found[2,3]. The inflammatory response triggered by dysregulated mucosal immune system activity plays a pivotal role in the development of IBD, influenced by a complex interplay of environmental factors, genetic predisposition, infections, and immune dysregulation[4]. Moreover, approximately half of IBD patients experience extraintestinal manifestations[5].
Due to the multifaceted pathogenesis and diverse clinical presentations of IBD, relying solely on first-line medications often fails to achieve optimal therapeutic outcomes. Consequently, with ongoing advancements in medical research, combination therapy has emerged as a pivotal strategy for IBD treatment[6]. In recent years, dual biological therapy, combining two biological agents or one biological agent with a small-molecule agent, has shown promising development in treating IBD patients[7]. Similarly, combination therapy, which typically combines immunosuppressive agents like mercaptopurine, azathioprine, or methotrexate with biological agents [usually tumor necrosis factors (TNF)], has been employed for several years[8,9].
Currently, prevalent clinical practices often involve pairing anti-TNF drugs with vedolizumab or ustekinumab with vedolizumab, demonstrating superior efficacy and safety compared to monotherapy[10-13]. Despite years of investigation, the comprehensive risks and benefits associated with drug combination therapies in IBD patients remain incompletely understood. Therefore, this paper employs the strengths, weaknesses, opportunities, and threats (SWOT) analysis to evaluate the current landscape of combination therapy for IBD and outlines pathways to enhance the efficacy and safety of the next generation of therapeutic approaches.
SWOT analysis, initially proposed by Professor Werrick at the University of San Francisco, serves as a comprehensive tool for evaluating projects, corporate strategies, or environmental analyses. It aids decision-makers in assessing both external and internal factors across four dimensions: Strengths, weaknesses, opportunities, and threats[14,15].
In recent years, with significant advancements in medical research, drug combination therapy has emerged as a crucial strategy for treating patients with moderate to severe IBD[5]. Currently, common clinical practices involve combining anti-TNF drugs with vedolizumab, or ustekinumab with vedolizumab, whose studies indicate offer improved efficacy and safety compared to single-agent therapies. The effectiveness of drug combination therapy in treating IBD patients hinges largely on two factors: The evolution of combination therapies themselves and the influence of the external environment.
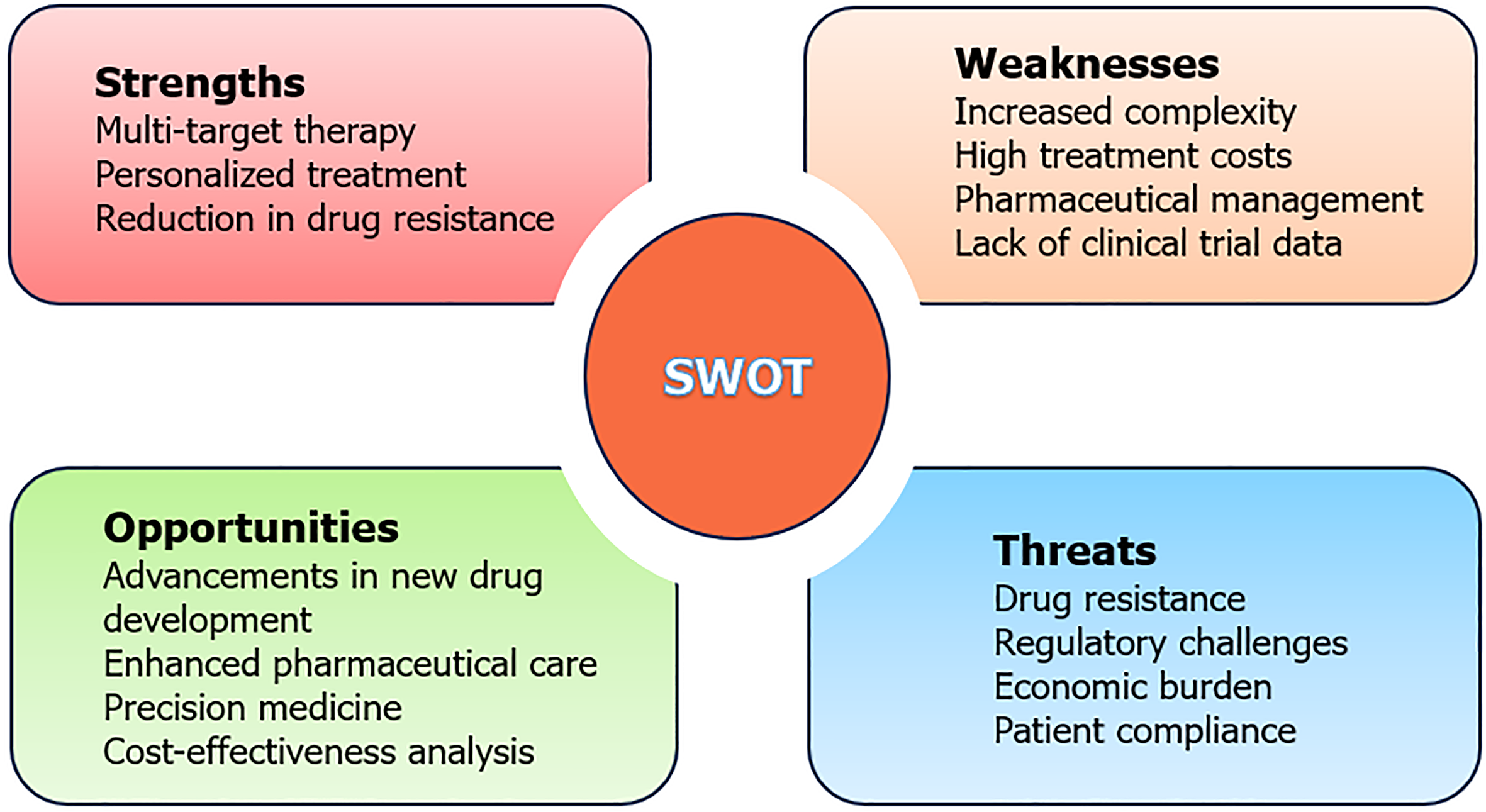
This paper conducts a detailed examination of the internal strengths, weaknesses, external opportunities, and threats associated with IBD combination therapy (Figure 1). It employs a systematic analysis to assess the current landscape of combined drug therapy for IBD and proposes recommendations for advancing the next generation of these therapies[14].
Multi-target therapy: Combination drug therapy is a potent strategy for controlling inflammatory responses in patients with IBD, achieving this by simultaneously engaging multiple pathological pathways and thereby diminishing the risk of recurrence. For example, the research by Triantafillidis et al[6] has shown that anti-TNF biologics mitigate inflammation by binding to TNF-α. Ustekinumab exerts its therapeutic effect by inhibiting the pro-inflammatory cytokines interleukin-12 and interleukin-23. Vedolizumab works by binding to the α4β7 integrin, preventing T cells from infiltrating tissues and thereby reducing inflammation. Tofacitinib, a small-molecule Janus kinase inhibitor, interferes with cellular signaling pathways. Each of these agents acts through a unique mechanism, and in concert, they act synergistically to address diverse inflammatory pathways. This multi-pronged approach results in a more efficacious attenuation of inflammation, leading to enhanced clinical and laboratory markers of patient improvement.
Personalized treatment: Tailored to individual conditions and genetic profiles, combination therapy optimizes drug selection, enhances therapeutic outcomes, and minimizes side effects. For instance, ustekinumab combined with vedolizumab and vedolizumab combined with anti-TNF were the most commonly used combination therapies for Crohn’s disease. For ulcerative colitis, the most frequently used combination therapies were vedolizumab plus anti-TNF and vedolizumab plus tofacitinib[10].
Reduction in drug resistance: Utilizing multiple drugs in combination lowers the likelihood of developing resistance to any single drug[16].
Increased complexity: Managing drug combination therapies involves handling multiple medications simultaneously, necessitating careful monitoring by pharmacists for drug interactions, dosage adjustments, and potential side effects[6,17].
High treatment costs: Implementing drug combination therapies significantly raises patient treatment expenses, potentially limiting their widespread adoption in clinical settings. For instance, a retrospective study by Goessens et al[18] highlighted higher treatment costs due to increased adverse reactions and hospitalizations among patients receiving combined biological agents and small molecules.
Pharmaceutical management: Complex treatment protocols pose challenges for patient adherence. Additionally, the limited availability of high-quality evidence supporting the use of combined biological agents in IBD necessitates pharmacists to utilize robust professional knowledge to evaluate treatment regimen suitability, provide medication counseling, and monitor treatment efficacy and safety[19].
Lack of clinical trial data: Comprehensive data supporting the safety and efficacy of IBD drug combination therapies, which involve diverse biological agents, remains scarce. For instance, while initial treatments combining immunomodulators and infliximab have shown short-term benefits, long-term use may increase risks of infections and malignancies such as lymphoproliferative diseases or non-melanoma skin cancers[20-22]. Therefore, further extensive clinical trials are crucial to validate the efficacy and safety of combination therapies.
Advancements in new drug development: Continued research into the pathogenesis of IBD, coupled with advancements in medical technology, has led to the development of safer and more effective drugs. This expansion provides a broader range of options for IBD drug combination therapy. Several promising candidates, such as anti-interleukin-23 monoclonal antibodies, Janus kinase inhibitors, and sphingosine-1-phosphate receptor modulators, are currently progressing through phase II and III clinical trials[23].
Enhanced pharmaceutical care: Strengthening patient education about IBD and its treatment options can significantly improve medication adherence. Educating patients about the benefits of drug combination therapy can enhance their understanding and compliance[19,24].
Precision medicine: The emergence of genomics and biomarkers enables personalized treatment approaches in IBD. Genetic testing and biomarker analysis allow for the formulation of tailored combination treatment plans. Current biomarkers used for IBD diagnosis include serological (C-reactive protein, erythrocyte sedimentation rate, and nitric oxide), fecal (calprotectin, lactoferrin, and myeloperoxidase), and urine biomarkers[25]. Genetic testing can also distinguish between Crohn’s disease and ulcerative colitis, providing insights into personalized treatment strategies. For instance, Stankovic et al[26] discovered an association between Crohn’s disease and certain variants of the nucleotide-binding oligomerization domain containing 2 gene, as well as Toll-like receptor 4 ASP299Gly, TNF-α G-308A, interleukin-6 G-174C, and interleukin 1 receptor antagonist (IL-1RN) VNTR A2 variants. In contrast, ulcerative colitis is specifically linked to the IL-1RN VNTR A2 variant. Ongoing research into inflammatory pathways promises to identify more sensitive and specific markers, further refining personalized treatment for IBD patients.
Cost-effectiveness analysis: With the decline in drug prices and the enhancement of treatment outcomes, combination therapies, such as the concomitant use of anti-TNF-α agents with immunosuppressants, hold promise for improved long-term cost-effectiveness. For instance, in the treatment of Crohn’s disease, the integration of anti-TNFα agents (e.g., infliximab) with immunosuppressants has been shown to significantly bolster treatment efficacy, diminish relapse rates, and decrease the need for hospitalization compared to immunosuppressants monotherapy. This approach ultimately results in a greater number of quality-adjusted life years[27]. Although the immediate high cost of these medications may currently mitigate the cost-effectiveness of combination therapy, the emergence of biosimilars and the potential for further price reductions could enhance the long-term economic viability of such treatments, making them a more feasible option from an economic standpoint.
Drug resistance: Despite the advantages of drug combination therapy in reducing resistance risks, prolonged use of medications may still lead to resistance development.
Regulatory challenges: The complexities and time-consuming nature of drug approval processes pose significant challenges to the marketing of new drugs and combination therapies. While biologics offer unprecedented benefits for IBD treatment, they also raise quality and safety concerns, potentially delaying their introduction to the market[28].
Economic burden: IBD imposes a growing financial burden on healthcare systems globally, particularly in high-income countries. This burden is driven by the introduction of expensive new drugs and the need for intensive disease management and surveillance. The prevalence of IBD is also rising in low- and middle-income countries, exacerbating economic challenges. The high cost of treatment limits the widespread adoption of drug combination therapies in clinical practice, especially in regions with limited healthcare resources. Addressing cost-effectiveness and feasibility is crucial for broader implementation[29,30].
Patient compliance: The complexity of drug combination therapies and the long-term nature of IBD treatment can compromise patient adherence. Educating and supporting patients in medication management is essential to ensure treatment effectiveness.
Personalized treatment: As genomics and biomarker research continues to advance, precision medicine is becoming increasingly feasible in treating IBD. Genetic testing and biomarker analysis can aid in diagnosing and distinguishing between Crohn’s disease and ulcerative colitis. Optimal biomarker concentrations can also indicate inflammation severity and monitor treatment response[25,26]. This approach facilitates the development of personalized combination treatment plans tailored to individual patients, thereby enhancing treatment efficacy and reducing side effects[31,32]. Personalized medicine represents a pivotal direction for future IBD treatment, ensuring that each patient receives the most suitable therapeutic approach.
Combination therapy with biological agents: Studies indicate that combining biological agents improves efficacy and safety in refractory IBD cases[10-13]. For example, the study by Yang et al[33] highlighted the significant clinical and endoscopic improvements achieved through the combination of vedolizumab and ustekinumab. The continuous advancement of medical research and the emergence of novel biological agents are poised to broaden the spectrum of combination therapies, thereby refining treatment outcomes further.
Emerging drugs and treatments: Continuous advancements in new drugs and treatments are diversifying the landscape of IBD therapy. For example, olamkicept, an inhibitor of the interleukin-6 trans-signaling pathway, has shown promising efficacy and safety profiles in patients with moderate to severe ulcerative colitis[34]. These innovative treatments are anticipated to broaden the therapeutic options available to individuals living with IBD.
Large-scale clinical trials: Due to the complexity of drug combination therapy and the limited high-quality data supporting its use in IBD patients[6], there is a critical need for more large-scale, randomized, double-blind, mono
Utilization of biomarkers and artificial intelligence: Biomarkers and artificial intelligence (AI) offer promising avenues to identify the most suitable patient populations for combination therapy. Biomarkers aid in diagnosing IBD and assessing inflammation severity, with some showing considerable potential in clinical diagnostics[25]. AI can analyze multiple biomarkers to monitor disease progression and response to treatment, thereby enhancing treatment precision and effectiveness[6,36]. Future research should focus on optimizing treatment protocols, minimizing side effects, and improving patient quality of life through these technologies.
International collaboration: The global burden of IBD is escalating, with varying disease prevalence and patterns across regions. International collaboration in research and clinical trials is crucial to developing effective strategies for IBD prevention and treatment[37]. Collaborative efforts facilitate the advancement and adoption of a new generation of combination therapies[31], enabling researchers to share data and insights across borders. Strengthening international cooperation and conducting multi-center studies are pivotal in confirming the efficacy and applicability of these therapies worldwide.
Long-term follow-up and clinical data: While biologic agents have demonstrated significant therapeutic benefits in treating IBD, concerns about treatment quality and patient safety persist[28]. For example, the initial use of immunomodulator regimens and the long-term administration of biologics such as infliximab may pose potential risks, including susceptibility to infections and an increased likelihood of malignancies[20-22]. Long-term follow-up studies, along with comprehensive clinical trial data, are essential for assessing the safety and efficacy of next-generation combination therapies. This is especially important for IBD, a chronic and relapsing condition, where the true measure of treatment success is contingent upon long-term outcomes.
The value of long-term follow-up lies not only in its ability to provide a nuanced understanding of treatments’ longitudinal effects on patients’ health, specifically regarding disease control and the maintenance of remission, but also in its capacity to discern efficacy trends across varied patient cohorts. This insight is instrumental in crafting personalized treatment approaches. As clinical evidence accumulates, potential adverse effects and challenges such as drug resistance can be more readily identified, paving the way for refined treatment protocols and improved efficacy. The ongoing evaluation of the safety and utility of biologics and immunomodulators is especially critical, as it ultimately contributes to the enhancement of treatment safety and overall patient outcomes.
Our SWOT analysis underscores the substantial benefits of combination therapy for IBD, notably its ability to provide multi-targeted therapy, personalized treatment strategies, and a reduction in drug resistance. The amalgamation of extensive clinical trials, biomarker research, AI, international partnerships, and the accumulation of long-term clinical data is poised to bolster the effectiveness, accessibility, and safety of combination therapies in the management of IBD. Looking to the future, overcoming existing challenges, capitalizing on emerging opportunities, and progressing collaborative treatment methodologies will rely on the concerted efforts of physicians, patients, pharmacists, and policymakers. Through personalized treatment planning, healthcare professionals can select the most impactful medications and adjust dosages to align with individual genetic makeup and health status, thereby maximizing efficacy while minimizing adverse effects. Continuous surveillance of drug performance and resistance, coupled with timely interventions, is key to sustained treatment success. Furthermore, the application of AI shows promise in refining the precision of drug choice and dosage modifications, particularly for immunosuppressants and biological therapies. Interdisciplinary collaboration, involving gastroenterologists, immunologists, and nutritional specialists, will be essential in formulating more rigorous and individualized treatment protocols. To deepen patients’ comprehension and involvement in their treatment, it is imperative for healthcare providers to enhance communication throughout the treatment journey. By clearly articulating the advantages and potential hazards of treatments, healthcare professionals can not only boost patient compliance but also more effectively manage the condition, culminating in a marked enhancement of the global IBD patient community’s quality of life.
| 1. | Lowell JA, Farber MJ, Sultan K. Back to the drawing board: Overview of the next generation of combination therapy for inflammatory bowel disease. World J Gastroenterol. 2024;30:3182-3184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (1)] |
| 2. | Chinese Medical Education Association Inflammatory Bowel Disease Professional Committee. [Consensus on biological agents in treating patients with inflammatory bowel disease in China]. Zhonghua Xiaohuabing Yu Yingxiang Zazhi. 2021;11:244-256. [DOI] [Full Text] |
| 3. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1043] [Article Influence: 94.8] [Reference Citation Analysis (18)] |
| 4. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 629] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 5. | Ashton JJ, Beattie RM. Inflammatory bowel disease: recent developments. Arch Dis Child. 2024;109:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 6. | Triantafillidis JK, Zografos CG, Konstadoulakis MM, Papalois AE. Combination treatment of inflammatory bowel disease: Present status and future perspectives. World J Gastroenterol. 2024;30:2068-2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Reference Citation Analysis (3)] |
| 7. | Eronen H, Kolehmainen S, Koffert J, Koskinen I, Oksanen P, Jussila A, Huhtala H, Sipponen T, Ilus T. Combining biological therapies in patients with inflammatory bowel disease: a Finnish multi-centre study. Scand J Gastroenterol. 2022;57:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Haider M, Lashner B. Dual Targeted Therapy for the Management of Inflammatory Bowel Disease. J Clin Gastroenterol. 2021;55:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Ahmed W, Galati J, Kumar A, Christos PJ, Longman R, Lukin DJ, Scherl E, Battat R. Dual Biologic or Small Molecule Therapy for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:e361-e379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 10. | Mas EB, Calvo XC. Selecting the Best Combined Biological Therapy for Refractory Inflammatory Bowel Disease Patients. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Privitera G, Onali S, Pugliese D, Renna S, Savarino E, Viola A, Ribaldone DG, Buda A, Bezzio C, Fiorino G, Fantini MC, Scaldaferri F, Guidi L, Danese S, Gasbarrini A, Orlando A, Armuzzi A. Dual Targeted Therapy: a possible option for the management of refractory Inflammatory Bowel Disease. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Glassner K, Oglat A, Duran A, Koduru P, Perry C, Wilhite A, Abraham BP. The use of combination biological or small molecule therapy in inflammatory bowel disease: A retrospective cohort study. J Dig Dis. 2020;21:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 13. | Kwapisz L, Raffals LE, Bruining DH, Pardi DS, Tremaine WJ, Kane SV, Papadakis KA, Coelho-Prabhu N, Kisiel JB, Heron V, Faubion WA, Loftus EV Jr. Combination Biologic Therapy in Inflammatory Bowel Disease: Experience From a Tertiary Care Center. Clin Gastroenterol Hepatol. 2021;19:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Hu YR, Zhang YJ, Xie MZ, Chen XP, Yan XX, He QH. [Study on the path of improving the cross-cultural communication ability of traditional Chinese Medicine based on SWOT analysis]. Hunan Zhongyiyao Daxue Xuebao. 2021;41:645-648. [DOI] [Full Text] |
| 15. | Wei H, Gao MJ, Wang L. [Application of SWOT analysis in the evaluation of whole sample prescription of antimicrobial agents]. Zhongguo Heliyongyao Tansuo. 2024;21:28-32. [DOI] [Full Text] |
| 16. | Macaluso FS, Orlando A. Anti-TNF combination therapy in inflammatory bowel disease: de novo or selective? Minerva Gastroenterol Dietol. 2019;65:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Li Z, Ge WS. [Benefits and risks of drug combination therapy in inflammatory bowel disease]. Zhonghua Xiaohuabing Yu Yingxiang Zazhi. 2019;9:193-195. [DOI] [Full Text] |
| 18. | Goessens L, Colombel JF, Outtier A, Ferrante M, Sabino J, Judge C, Saeidi R, Rabbitt L, Armuzzi A, Domenech E, Michalopoulos G, Cremer A, García-Alonso FJ, Molnar T, Karmiris K, Gecse K, Van Oostrom J, Löwenberg M, Farkas K, Atreya R, Ribaldone DG, Selinger C, Hoentjen F, Bihin B, Sebastian S; European COMBIO study group, Rahier JF. Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune-mediated inflammatory diseases: A European retrospective observational study. United European Gastroenterol J. 2021;9:1136-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Quiroga LC, Sabourin AA. Review of Dual Biologics in Specialty Pharmacy Practice. Ann Pharmacother. 2023;57:1094-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Shmidt E, Ho EY, Feuerstein JD, Singh S, Terdiman JP. Spotlight: Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease. Gastroenterology. 2021;160:2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 21. | Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158:1450-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 472] [Article Influence: 94.4] [Reference Citation Analysis (3)] |
| 22. | Xu Z, Davis HM, Zhou H. Clinical impact of concomitant immunomodulators on biologic therapy: Pharmacokinetics, immunogenicity, efficacy and safety. J Clin Pharmacol. 2015;55 Suppl 3:S60-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Bretto E, Ribaldone DG, Caviglia GP, Saracco GM, Bugianesi E, Frara S. Inflammatory Bowel Disease: Emerging Therapies and Future Treatment Strategies. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Fu Y, Huang J, Li CY. [Effect of pharmacists’ pharmaceutical care on compliance behavior and quality of life in patients with hypertension]. Zhongguo Yiyao Zhinan. 2024;22:55-58. [DOI] [Full Text] |
| 25. | Liu D, Saikam V, Skrada KA, Merlin D, Iyer SS. Inflammatory bowel disease biomarkers. Med Res Rev. 2022;42:1856-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 26. | Stankovic B, Dragasevic S, Popovic D, Zukic B, Kotur N, Sokic-Milutinovic A, Alempijevic T, Lukic S, Milosavljevic T, Nikcevic G, Pavlovic S. Variations in inflammatory genes as molecular markers for prediction of inflammatory bowel disease occurrence. J Dig Dis. 2015;16:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Bolin K, Hertervig E, Louis E. The Cost-effectiveness of Biological Therapy Cycles in the Management of Crohn's Disease. J Crohns Colitis. 2019;13:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Atreya R, Neurath MF. Biomarkers for Personalizing IBD Therapy: The Quest Continues. Clin Gastroenterol Hepatol. 2024;22:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | The Lancet Gastroenterology Hepatology. The economic burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2023;8:391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 30. | Beard JA, Franco DL, Click BH. The Burden of Cost in Inflammatory Bowel Disease: A Medical Economic Perspective and the Future of Value-Based Care. Curr Gastroenterol Rep. 2020;22:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Xiong JQ, Fu YF, Qiu JH, Liao WD, Luo LY, Chen SH. Global research trends of immunotherapy and biotherapy for inflammatory bowel disease: a bibliometric analysis from 2002 to 2021. Biomed Eng Online. 2022;21:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Gergely M, Prado E, Deepak P. Management of refractory inflammatory bowel disease. Curr Opin Gastroenterol. 2022;38:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, Boland BS, Collins A, Sandborn WJ, Panaccione R, Battat R. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn's disease. Aliment Pharmacol Ther. 2020;51:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 34. | Zhang S, Chen B, Wang B, Chen H, Li Y, Cao Q, Zhong J, Shieh MJ, Ran Z, Tang T, Yang M, Xu B, Wang Q, Liu Y, Ma L, Wang X, Zhang N, Zhang S, Guo W, Huang L, Schreiber S, Chen M. Effect of Induction Therapy With Olamkicept vs Placebo on Clinical Response in Patients With Active Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2023;329:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 35. | Triantafillidis JK, Papalois AE, Parasi A, Anagnostakis E, Burnazos S, Gikas A, Merikas EG, Douzinas E, Karagianni M, Sotiriou H. Favorable response to subcutaneous administration of infliximab in rats with experimental colitis. World J Gastroenterol. 2005;11:6843-6847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Sakurai T, Saruta M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion. 2023;104:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 37. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1455] [Article Influence: 291.0] [Reference Citation Analysis (0)] |