TO THE EDITOR
One of the most challenging complications of Crohn’s disease (CD) is perianal fistula, with an estimated lifetime risk of 14%-38% in population-based estimates[1,2]. This condition significantly impacts the quality of life through its presentation of pain, perianal discharge, and faecal incontinence, impacting the patients’ physical, social, and sexual well-being. Management of perianal fistulizing CD (PFCD) requires a multidisciplinary approach, encompassing medical interventions, surgical procedures, and other newer forms of treatment such as stem cell therapy. The review article by Pacheco et al[1] presents various medical interventions for PFCD, including antibiotics, thiopurines, tacrolimus, anti-tumor necrosis factor (TNF) agents, vedolizumab, ustekinumab, and hyperbaric oxygen therapy. Endoscopic approaches and innovative surgical techniques such as ligation of the inter-sphincteric fistula tract, advancement flaps, fistula plugs, fibrin glue injection, fistula laser closure, and video-assisted anal fistula treatment, are discussed as well. In addition, the authors highlighted the role of stem cells in the treatment of this disease and the importance of screening to prevent complications such as cancer.
A review of the various modalities of therapeutic approaches revealed that antibiotics, mainly ciprofloxacin in combination with infliximab, demonstrated superior healing outcomes when compared to anti-TNF monotherapy[2]. Notably, higher infliximab drug levels (median level at 2 weeks: 20 μg/mL vs 5.6 μg/mL) correlated with better response rates in terms of fistula healing and closure[3]. The healing process can be monitored through magnetic resonance imaging (MRI), with successful healing characterized by the resolution of T2-weighted image hyperintensity and absence of enhancement 12 months post-treatment[4]. Disease recurrence and maintenance of remission remain significant challenges, hence the decision to continue or stop anti-TNF treatment remains unanswered. Current evidence suggests that disease relapse is associated with discontinuation of treatment, supporting the continuation of anti-TNF therapy to achieve clinical remission[5]. Therapeutic drug monitoring is important to improve fistula healing rates, as higher dosing regimens are required for treating PFCD. While anti-TNF therapy using infliximab and adalimumab remains the preferred medical intervention for perianal CD, some patients demonstrate non-response to anti-TNF therapy[6].
Where PFCD is associated with perianal abscess, adequate drainage of the abscess must precede any definite surgery. The standard surgical procedures in the management of perianal fistula include seton placement and fistulectomy[7]. However, surgical interventions for PFCD demonstrate relatively moderate success rates: For example, ligation of the inter-sphincteric fistula tract achieves 53% success, advancement flaps 61%, fistula plugs 50%-60% and fibrin glue injection 38%. Importantly, surgical fistula closure should only be attempted after endoscopic remission of the proctitis[1]. Recent surgical innovations for PFCD include ablative procedures targeting the fistula tract epithelium, such as fistula laser closure and video-assisted anal fistula treatment[7].
Long-standing CD carries a significant risk of cancer development, particularly squamous cell carcinoma and adenocarcinoma. Patients with CD have a 4- to 20-fold increased risk of colorectal carcinoma[8]. The incidence of cancer arising from CD-associated fistulas is 0.2/1000 person-year, and the overall incidence of fistula-associated anal cancer is 0.3% to 0.7%[9]. The latency period between initial diagnosis of CD and detection of carcinoma varied between 18 and 30 years[10]. Detection of carcinoma in perianal CD is difficult, with malignancy often discovered at the advanced stage during surgical intervention, resulting in poor prognosis. Definitive diagnosis typically requires biopsy or curettage performed during examination under anaesthesia[11].
MRI: The primary radiological modality for PFCD investigation
MRI demonstrates utility not only for evaluation of fistulizing disease, but also in the diagnosis of perianal carcinoma. Characteristic MRI findings suggestive of perianal neoplasia in patients include irregular inner wall morphology, delayed enhancement of internal tissue, and mucinous patterns. While the fistula wall is frequently observed, this finding alone is insufficient to differentiate cancer from inflammation. Additionally, a distinct double-layered enhancement pattern characterized by an early bright external wall enhancement contrasting with a darker delayed-enhancing internal wall may be observed[12].
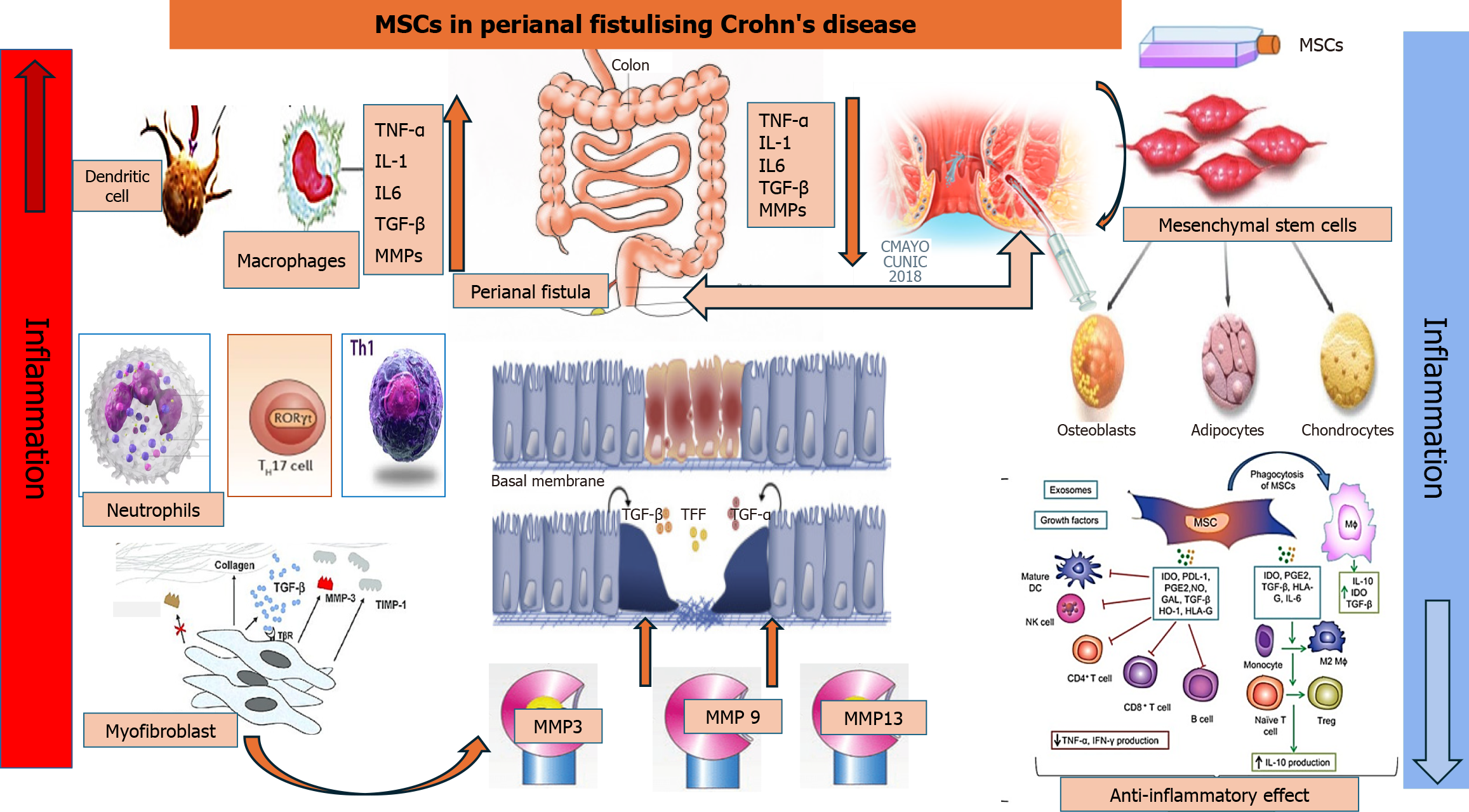
Mechanisms of PFCD: Roles of inflammatory cells, cytokines, and epithelial-to-mesenchymal transition
Perianal fistulas in CD, which can affect the small and large intestines, are characterized by narrow squamous epithelial cells. These cells undergo epithelial-to-mesenchymal transition to become transition cells in response to immunological triggers or bacterial invasion. As a result, the cells lose their phenotypic and functional properties such as polarity and adhesiveness, while acquiring mesenchymal cell traits. This leads to reduced cell-to-cell adhesion and enhanced migratory activity[13]. This anomaly results in a cascade of cellular events, such as infiltration of inflammatory cells, i.e., neutrophils, T helper (Th) cells, M1 macrophages, dendritic cells, and myofibroblasts, as well as the release of inflammatory cytokines and regulatory proteins (Figure 1). Consequently, the peculiar channel of transition cells appears to be the outcome of chronic inflammation spreading through the mucosal wall, which in turn leads to the development of fistulas[14].
Figure 1 Mechanisms of perianal fistulizing Crohn’s disease and therapeutic roles of mesenchymal stem cells.
Enhanced production of inflammatory cytokines by infiltrating macrophages and other inflammatory cells stimulates the epithelial-to-mesenchymal transition, resulting in activation of myofibroblasts and elevation of matrix metalloproteinases, leading to fistula formation. Injection of mesenchymal stem cells into the fistula results in suppression of the inflammatory cells and cytokines and complete resolution of perianal fistulizing Crohn’s disease. MSCs: Mesenchymal stem cells; TNF: Tumor necrosis factor; IL: Interleukin; TGF: Transforming growth factor; MMP: Matrix metalloproteinase; Th: T helper cells; TFF: Trefoil factor family; DC: Dendritic cell; NK: Natural killer; Treg: Regulatory T cell; IDO: Indolamine 2,3-dioxygenase; PDL-1: Programmed death ligand 1; PGE2: Prostaglandin E2; NO: Nitrogen oxide; GAL: Gallic acid; HO-1: Heme oxygenase-1; HLA: Human leukocyte antigen.
Immunohistochemical analyses of CD-related perianal fistulas indicate acute inflammation in 56% of cases[13], characterized by significant infiltration of T cells into the inner wall of the fistula, along with pronounced activation of inflammatory M1 macrophages. Investigations into the phenotypic and functional characteristics of T cells derived from individuals with PFCD revealed significant infiltration of the fistula by T cells with Th17, Th1, and Th17/1 phenotypes[14]. The inflammatory cascade underlying PFCD is predominantly mediated by inflammatory cytokine activation. Severe intestinal inflammation triggers the release of TNF-α, interleukin-6 (IL-6), IL-13, and transforming growth factor-beta (TGF-β). The abundance of these cytokines in the lining of fistula tracts, adjacent tissue, and peripheral blood suggests their involvement in the pathogenesis of PFCD[15].
Matrix metalloproteinases (MMPs) play crucial roles in tissue degradation and remodeling. Excessive MMP activity can cause cancer or inflammatory bowel disease through abnormal degradation of the extracellular matrix[16]. Kiesler et al[17] and Washburn et al[18] identified elevated expression of MMP-3 in CD-associated fistula tissue regardless of the inflammatory state and increased goblet cell presence when compared to controls. Additionally, they found that MMP-3 and MMP-9 were produced by mononuclear cells and fibroblasts, especially in fistulas with active inflammation. Furthermore, MMP-13 protein expression was detected in CD-associated fistulas, but was almost absent in non-fistulizing CD tissue[13]. This provides evidence for the role of MMPs as mediators in the pathogenesis of perianal fistula in CD through abnormal degradation of the extracellular matrix in the bowel wall.
Mesenchymal stem cell therapy in PFCD
Mesenchymal stem cells (MSCs) are multipotent stromal cells with self-renewal and differentiation ability. They are acquired from the umbilical cord, bone marrow, adipose tissue, and various other sources, and can be obtained as autologous or allogeneic, depending on clinical requirements[19,20]. MSCs exhibit well-documented immunomodulatory effects, tissue regeneration properties, and antiaging and anti-inflammatory potential via regulation of function of T cells, B cells, natural killer cells, macrophages, neutrophils and dendritic cells. This regulation occurs via changing of the local inflammatory cell environment, migration to inflammation sites, and reduction of immune response (Figure 1)[21,22]. The precise regulatory mechanism of MSCs in perianal fistula is not well-established and suggested to be via immunosuppression, i.e., controlling the hyperactivity of T-cells, release of inflammatory cytokines such as TGF-β, TNF-α, IL-7, IL-6 and IL-13, and secretion of anti-inflammatory molecules such as IL-10. Through these mechanisms, MSCs maintain an anti-inflammatory environment that is conducive to the repair of eroded tissue in the perianal area, particularly in the context of PFCD[23].
The application of MSCs in phase II and phase III clinical trials has demonstrated significant therapeutic efficacy in PFCD. MSCs, commonly derived from adipose tissue or bone marrow, release anti-inflammatory cytokines through autocrine or paracrine mechanisms to promote blood vessel repair and re-epithelialization[24]. Swaroop et al[25] conducted a trial to examine the therapeutic potential of locally administered human allogeneic bone marrow-derived mesenchymal stromal cells in PFCD patients non-responsive to conventional treatments. In an open-trial, phase I/II, single-arm study, clinical severity and biomarkers were analyzed at baseline through week 104, incorporating MRI scans at weeks 24 and 104. The authors found that across various clinical trials, local administration of MSCs demonstrated favorable safety profile with no significant adverse effects compared to the placebo group[25]. Intraperitoneal injection of human MSCs into SAMP-1/YitFc mice models for CD achieved long-term therapeutic efficacy through sustained anti-inflammatory macrophage programming via efferocytosis[26]. Stem cells have emerged as a promising therapeutic option for Crohn’s fistula, based on its high efficacy and lower incidence of adverse events. Clinical studies have shown that a 3 × 107 cells/mL dosage yields superior outcomes in patients with Crohn’s fistula[27]. However, further clinical and pre-clinical studies are needed to strengthen the evidence base for this approach in the future.
MSC-derived extracellular vesicles therapy for PFCD
Mammalian cells secrete extracellular vesicles (EVs), which serve as vehicles for functional nucleic acids (mRNA, miRNA, and other RNA species) to facilitate cellular communication and uptake both in vitro and in vivo. EVs are categorized based on size into two primary subsets: Exosomes (50–100 nm in diameter), which have an endosomal origin, and microvesicles (100–1000 nm in diameter), derived from cytoplasmic budding[28]. Recently, MSC-derived EVs have emerged as an effective and safe therapeutic modality for the treatment of various autoimmune conditions, including CD[29]. In the dextran sodium sulfate-induced mouse model of colitis, intravenous injection of human umbilical cord-derived exosomes resulted in reduced macrophage infiltration and inflammatory cytokines in colonic tissue, with improvement of colitis[30]. Moreover, a recent phase I clinical trial demonstrated complete tract resolution in 5 of 11 patients with non-CD fistulizing disease following injection of human placental MSCs-derived EVs over a 6-month period[31]. These findings underscore the need for further investigation of both local and systemic administration of EVs in the treatment of PFCD through targeted animal models and expanded clinical trials.
Autophagy modulation in MSCs and EVs for treatment of PFCD
Macroautophagy (hereafter referred to as autophagy) is a prosurvival mechanism for the clearance of damaged cellular components in response to various stressors, such as oxidative stress, inflammation, and hypoxia[32,33]. Therefore, in the context of inflammatory conditions such as CD, autophagy activation in MSCs may serve as a protective mechanism. Recent in vitro investigations reported that autophagy upregulation in MSCs by rapamycin enhanced the immunosuppression of inflammatory CD4+ T cells via secretion of TGF-β1[34]. Moreover, autophagy plays an important role in the differentiation of MSCs at the cellular and molecular levels, thereby influencing their therapeutic potential across a wide range of diseases, including PFCD[35]. Notably, autophagy-induced MSC-derived EVs have demonstrated therapeutic efficiency in attenuating renal fibrosis in vitro via reduction of pro-inflammatory gene expression, including IL-1 and TNF-α[36]. Taken together, these findings suggest that autophagy upregulation in MSCs may enhance their immunosuppressive functions and survival, in addition to the production of effective EVs, potentially advancing the treatment of autoimmune diseases such as PFCD.
Conclusions
PFCD is a common presentation of CD. Various mechanisms are involved in fistula formation, mainly related to the accumulation of inflammatory cells such as M1 macrophages and the production of inflammatory cytokines. This results in epithelial-to-mesenchymal transition, myofibroblast activation, and upregulation of MMPs, culminating in PFCD. While various medical and surgical options target the underlying inflammation, disease recurrence remains a frequent complication. The potential progression to anal carcinoma necessitates screening protocols. MSC therapy has emerged as a relatively novel local therapeutic approach for PFCD, with MSC-derived EVs demonstrating enhanced fistula-healing properties. Further research in vitro and in vivo using specific animal models for PFCD is needed to explore the therapeutic roles of MSCs and EVs in both luminal and fistulizing CD. Moreover, additional studies, including larger randomized controlled trials, long-term follow-up studies, and investigations into patient selection criteria, are required. Additionally, investigation into the potential roles of autophagy modulation in MSCs is needed to enhance their immunosuppressive properties and optimize production of high-quality EVs for treatment of PFCD.