Published online Feb 28, 2025. doi: 10.3748/wjg.v31.i8.98415
Revised: December 11, 2024
Accepted: January 6, 2025
Published online: February 28, 2025
Processing time: 211 Days and 13.3 Hours
Liver transplant (LT) recipients are susceptible to carbapenem-resistant Klebsiella pneumoniae (CRKP) infections. Comprehensive research addressing the incidence, timing, infection sites, resistance patterns, treatment options, and associated risk factors among LT recipients with CRKP is now lacking.
To assess the incidence, resistance, therapy, and risk factors of CRKP infections post-LT, and to evaluate the impact of them on prognosis.
A retrospective study was conducted, including 430 consecutive patients who underwent LT between January 2015 and June 2023. This study aimed to investigate the risk factors for CRKP infections and their influence on outcomes using logistic regression analysis.
Among the 430 patients who underwent LT, 20 (4.7%) experienced at least one documented CRKP infection within 3 months post-transplantation. The median time from LT to the onset of CRKP infections was 6.5 days. The lungs and bloodstream were the most common sites of CRKP infections. CRKP isolates were relatively susceptible to ceftazidime/avibactam (93.7%), polymyxin B (90.6%), and tigecycline (75.0%) treatment. However, all isolates were resistant to piperacillin/tazobactam, ceftazidime, cefepime, aztreonam, meropenem, and levofloxacin treatment. Recipients with CRKP infections had a mortality rate of 35%, the rate was 12.5% for those receiving ceftazidime/avibactam therapy. Multivariate analysis identified female sex [odds ratio (OR) = 3.306; 95% confidence interval (CI): 1.239-8.822; P = 0.017], intraoperative bleeding ≥ 3000 mL (OR = 3.269; 95%CI: 1.018-10.490; P = 0.047), alanine aminotransferase on day 1 post-LT ≥ 1500 U/L (OR = 4.370; 95%CI: 1.686-11.326; P = 0.002), and post-LT mechanical ventilation (OR = 2.772; 95%CI: 1.077-7.135; P = 0.035) as significant variables associated with CRKP. CRKP infections were related to an intensive care unit length (ICU) of stay ≥ 7 days and 6-month all-cause mortality post-LT.
CRKP infections were frequent complications following LT, with poor associated outcomes. Risk factors for post-LT CRKP infections included female sex, significant intraoperative bleeding, elevated alanine aminotransferase levels, and the need for mechanical ventilation. CRKP infections negatively impacted survival and led to prolonged ICU stays.
Core Tip: Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections were frequently seen in liver transplant recipients. CRKP isolates were relatively susceptible to ceftazidime/avibactam and polymyxin B. Risk factors for CRKP infections included female sex, significant intraoperative bleeding, elevated alanine aminotransferase levels, and the need for mechanical ventilation. CRKP infections negatively impacted survival and led to prolonged intensive care unit stay.
- Citation: Liu TH, Chen LH, Wan QQ. Carbapenem-resistant Klebsiella pneumoniae infections after liver transplantation: Drug resistance, risk factors and impact on prognosis. World J Gastroenterol 2025; 31(8): 98415
- URL: https://www.wjgnet.com/1007-9327/full/v31/i8/98415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i8.98415
Liver transplant (LT) recipients are susceptible to infections caused by carbapenem-resistant Klebsiella pneumoniae (CRKP)[1-4]. The reported incidence of CRKP infections following LT varies widely, ranging from 2.5%-35%[1,5-9]. Limited antimicrobial options contribute to poor outcomes associated with CRKP infections, with mortality rates reported between 10%-83% among LT recipients[1,2,4-8,10,11].
While some studies have investigated the risk factors for CRKP infections and their impact on LT recipient prognosis[7,12], comprehensive research addressing the incidence, timing, infection sites, resistance patterns, treatment options, and associated risk factors is still lacking. Furthermore, there is a scant exploration of year-to-year trends in CRKP as a proportion of all Klebsiella pneumoniae (K. pneumoniae) isolates in LT recipients. Integrating these aspects could facilitate the development of effective preventive and therapeutic strategies to enhance outcomes for LT recipients affected by CRKP infections. This study seeks to fill this gap in the current literature.
A total of 439 LT recipients underwent procedures at the Third Xiangya Hospital of Central South University during the study period between January 1, 2015 and June 30, 2023. Finally, 430 cases were included in this study. A total of nine cases were excluded: Four due to donor-derived K. pneumoniae infections, two being minors (< 18 years old), and three due to fatalities within 48 hours from non-functional primary graft or intraoperative hemorrhage. All grafts, except one, originated from brain-dead donors, and a modified piggyback LT technique was uniformly applied. Induction therapy comprised corticosteroids, with over 50% recipients also receiving basiliximab. Maintenance immunosuppression included tacrolimus/cyclosporine, mycophenolate mofetil/enteric-coated mycophenolate sodium, and prednisone, occasionally supplemented with anti-thymocyte globulin in steroid-incompatible cases. Standard perioperative antibiotic prophylaxis consisted of third-generation cephalosporins or carbapenems for 3-5 days. No organs were procured from executed prisoners.
Clinical data spanning a period of 8 years were extracted from electronic medical records, encompassing demographics, infection specifics, and other clinical parameters. Follow-up included microbiological data for 3 months and survival outcomes for 6 months post-LT.
Infections were defined as per the Centers for Disease Control and Prevention/National Healthcare Safety Network criteria, according to which positive cultures and clinical signs or imaging confirming active infection were required[13]. The infection source was validated by culture-positive sites and corresponding clinical manifestations[13]. CRKP was defined as resistance to at least 1 carbapenem, with minimum inhibitory concentrations ≥ 2 μg/mL for ertapenem or ≥ 4 μg/mL for imipenem or meropenem (Clinical and Laboratory Standards Institute, 2017). Donor derived K. pneumoniae infection was defined as K. pneumoniae infection present in the donor that was transmitted to the LT recipients with clear evidence[14]. Reoperations encompassed both re-transplantation and post-LT laparotomy, while acute rejection was confirmed by biopsy[15]. Mortality due to CRKP was defined as death in the setting of persistent infection[7].
Post-LT, routine cultures of blood, urine, sputum, and abdominal drainage fluid were conducted daily for 5-7 days, extending as needed within the initial 3 months. Additional cultures (e.g., bronchoalveolar lavage fluid, bile, and organ preservation solution) were performed based on clinical judgment. Blood cultures utilized the BD9240 automatic system, with microbial identification via the Bruker mass spectrometry. Susceptibility to ceftazidime/avibactam (CZA) and polymyxin B was assessed by disk diffusion or broth microdilution. Other antimicrobial susceptibilities utilized the automated Vitek-2 system (bioMérieux, Marcyl’Etoile, France).
Categorical variables were expressed as frequencies and percentages, continuous variables as mean ± SD or median with interquartile range (IQR). χ2 or Fisher’s exact tests assessed categorical variables. Logistic regression models, utilizing a forward stepwise approach, identified risk factors for post-LT CRKP infections with odds ratios (ORs) and 95% confidence intervals (CIs). Initial models included covariates with P < 0.1, sequentially eliminating nonsignificant predictors. CRKP infection impact on 6-month all-cause mortality was evaluated via the log-rank test and the Kaplan-Meier curves. P < 0.05 was considered significant. Analyses utilized the statistical product and service solutions v26.0 software (IBM Corp., Armonk, NY, United States).
In the cohort of 430 LT recipients, the mean age was 47.2 years, with females comprising 18.1% (78/430) of the group. The median model for end-stage liver disease score at LT was 23.0. Table 1 summarizes the clinical, laboratory, and demographic characteristics of the patients. The leading cause of the primary liver disease was hepatitis virus-related cirrhosis/necrosis/tumor (n = 319), followed by alcoholic liver disease (n = 32) and mixed cirrhosis (n = 24). Infections within 2 months prior to LT accounted for 39.5% (170/430) of the cases, predominantly pulmonary infections (87.6%, 149/170), with a few cases of abdominal/biliary infections (3.5%, 6/170), urinary tract infections (0.6%, 1/170), and multiple-site infections (8.2%, 14/170), all of which initially presented with pulmonary infections.
| Characteristics | Value | Range |
| Recipient age (years) | 47.2 ± 10.6 | 18-73 |
| Recipient gender (female) | 78 (18.1) | |
| Recipient BMI (kg/m2), median (IQR) | 22.8 (20.8-25.1) | 13.8-37.1 |
| Hospital stay prior to LT (days), median (IQR) | 9.0 (1.0-22.0) | 1-161 |
| MELD score at LT, median (IQR) | 23.0 (15.0-30.0) | 6-40 |
| Infection within 2 months prior to LT | 170 (39.5) | |
| Pre-LT use of broad-spectrum antibiotics | 168 (39.1) | |
| Underlying liver diseases | 430 (100) | |
| Viral cirrhosis/necrosis/tumor | 319 (74.2) | |
| Alcoholic cirrhosis | 32 (7.4) | |
| Autoimmune hepatitis | 15 (3.5) | |
| Primary biliary cirrhosis | 13 (3.0) | |
| Mixed cirrhosis | 24 (5.6) | |
| Others1 | 27 (6.0) | |
| Pre-LT type 2 diabetes | 51 (11.9) | |
| Pre-LT creatinine (mg/dL), median (IQR) | 0.8 (0.6-1.0) | 0.1-10.7 |
| Pre-LT WBC count (× 109/L), median (IQR) | 5.2 (3.5-8.0) | 0.5-33.6 |
| Pre-LT lymphocyte count (× 109/L), median (IQR) | 0.8 (0.5-1.2) | 0.1-3.7 |
| Pre-LT platelet count (× 109/L), median (IQR) | 70.0 (43.0-106.3) | 7.0-491.0 |
| Pre-LT albumin level (g/L), median (IQR) | 34.5 (30.7-38.0) | 18.0-55.7 |
| Donor age (years) | 42.2 ± 13.1 | 7-68 |
| Steatosis ≥ 30% | 43 (10.0) | |
| Cold ischemia time (hour) | 6.3 ± 1.4 | 1.5-9.6 |
| Duration of surgery (minutes), median (IQR) | 380.0 (333.0-430.0) | 185-710 |
| Intraoperative bleeding (mL), median (IQR) | 3000.0 (2000.0-5000.0) | 500-27000 |
| Intraoperative RBC transfusion (units), median (IQR) | 12.0 (8.0-18.0) | 0-44.0 |
| Post-LT infections due to Klebsiella pneumoniae | 33 (7.7) | |
| Median interval between the onset of CRKP infections and LT (days), median (IQR) | 6.5 (2.0-17.0) | 1-116 |
| CRKP infections | 20 (4.7) | |
| Post-LT immunosuppressant treatment | 430 (100) | |
| Tacrolimus | 419 (97.4) | |
| Ciclosporin A | 5 (1.2) | |
| Mycophenolate mofetil/enteric-coated mycophenolate sodium | 299 (69.5) | |
| Sirolimus | 5 (1.2) | |
| Glucocorticoid | 430 (100) | |
| Basiliximab | 238 (55.3) | |
| Anti-thymocyte globulin | 18 (4.2) | |
| ALT on day 1 after LT (U/L), median (IQR) | 697.5 (385.0-1276.3) | 54.0-8972.0 |
| Creatinine on day 3 after LT (mg/dL), median (IQR) | 0.9 (0.7-1.4) | 0.3-12.6 |
| Albumin level on day 1 after LT (g/L), median (IQR) | 37.1 (33.6-40.6) | 22.5-51.9 |
| Post-LT mechanical ventilation | 96 (22.3) | |
| Reoperation | 17 (4.0) | |
| Acute rejection | 68 (15.8) | |
| Post-LT renal replacement therapy | 19 (4.4) | |
| ICU stay after LT (days), median (IQR) | 6.0 (5.0-7.0) | 0-32 |
| Hospitalization stay after LT (days), median (IQR) | 26.0 (21.0-30.0) | 3-137 |
| All-cause mortality within 6 months after LT | 37 (8.6) | |
| CRKP infections-related mortality | 7 (1.6) |
Over the 8-year study period, 47 episodes of K. pneumoniae infections occurred among 33 (7.7%) LT recipients. Of these, 32 episodes were identified as CRKP infections, affecting 20 (4.7%) patients within 3 months post-LT. Among the 20 patients with CRKP infections, lung infections were reported in 30.0% (6/20), bacteremia in 15.0% (3/20), intra-abdominal infections in 10.0% (2/20), and multiple-site infections in 45.0% (9/20). The median time from LT to CRKP infections was 6.5 days, with 85% (17/20) occurring within the first month post-LT.
During their hospitalization, 96 patients required mechanical ventilation, 19 underwent renal replacement therapy, and 68 experienced acute rejection post-LT. Additionally, 4.0% (17/430) of cases underwent reoperation. Median lengths of stay in the intensive care unit (ICU) and hospital were 6.0 and 26.0 days, respectively. The 6-month all-cause mortality rate was 8.6% (37/430).
Among the 20 recipients with post-LT CRKP infections, 45.0% (9/20) experienced infections in ≥ two sites, with pneumonia affecting 50.0% (10/20), bloodstream infections affecting 45.0% (9/20), and abdominal/biliary tract infections affecting 45.0% (9/20) (Table 1).
Regarding the CRKP susceptibility to antibiotics, tigecycline demonstrated sensitivity in 75.0% (24/32) of isolates, polymyxin B in 90.6% (29/32), and CZA in 93.8% (30/32). However, CRKP showed resistance to piperacillin/tazobactam, ceftazidime, cefepime, levofloxacin, cefoperazone/sulbactam, aztreonam, and meropenem (Table 2).
| Antimicrobial | Number | Percentage |
| TZP | 32 | 100 |
| CAZ | 32 | 100 |
| CFS | 32 | 100 |
| FEP | 32 | 100 |
| ATM | 32 | 100 |
| MEM | 32 | 100 |
| LVF | 32 | 100 |
| AN | 21 | 65.6 |
| SXT | 18 | 56.3 |
| TIC | 8 | 25.0 |
| POL | 3 | 9.4 |
| CZA | 2 | 6.3 |
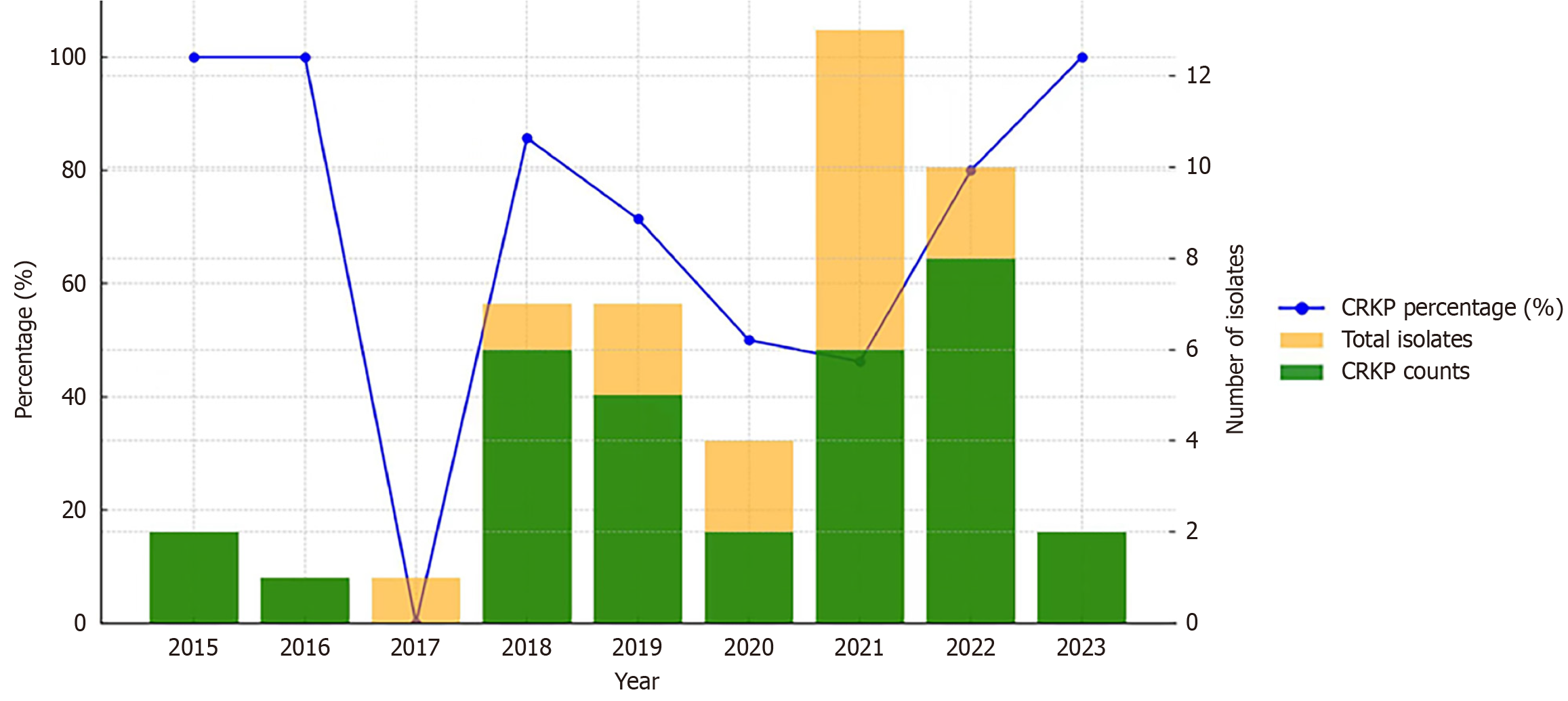
CRKP accounted for 100% (2/2) of all K. pneumoniae isolates in 2015, 100% (1/1) in 2016, 0% (0/1) in 2017, 85.7% (6/7) in 2018, 71.4% (5/7) in 2019, 50% (2/4) in 2020, 46.2% (6/13) in 2021, 80% (8/10) in 2022, and 100% (2/2) in 2023 (Figure 1).
In cases of CRKP infections among LT recipients, CZA was administered to eight patients. Among them, CZA was used as monotherapy in three patients and as a part of the combination therapy in five patients. Combination regimens included CZA with colistin sulfate in four patients and with polymyxin B in one patient. Unfortunately, one patient (12.5%) who received CZA and colistin sulfate combination therapy died due to delayed initiation of both the medications. The median time from CRKP infection onset to CZA initiation was 3.5 days (IQR: 1.3-5.0), and the median duration of CZA treatment was 10.0 days (IQR: 6.3-16.0).
Polymyxin B and meropenem combination therapy was administered to four patients, resulting in death for three (75%). Meropenem alone was administered to three patients, resulting in death in one patient (33.3%). One patient received tigecycline and another received trimethoprim/sulfamethoxazole. Additionally, one patient received gentamicin combined with piperacillin-tazobactam, one patient received doxycycline, and one patient received etimicin, all leading to death.
Univariate analysis indicated that female sex (P = 0.009), surgical durations ≥ 450 minutes (P = 0.015), intraoperative bleeding ≥ 3000 mL (P = 0.004), alanine aminotransferase (ALT) levels on day 1 after LT ≥ 1500 U/L (P < 0.001), post-LT mechanical ventilation (P = 0.003), and post-LT renal replacement therapy (P = 0.052) were potential risk factor for CRKP infections (Table 3).
| Variables | With CRKP infections (n = 20) | Without CRKP infections (n = 410) | P value |
| Female sex | 8 (40.0) | 70 (17.1) | 0.009 |
| Recipient age ≥ 55 years | 7 (35.0) | 98 (23.9) | 0.259 |
| Recipient BMI ≥ 25 | 5 (25.0) | 109 (26.6) | 0.875 |
| MELD score at LT ≥ 25 | 13 (65.0) | 181 (44.1) | 0.067 |
| Hospital stay prior to LT ≥ 7 days | 13 (65.0) | 228 (55.6) | 0.409 |
| Viral cirrhosis/necrosis/tumor | 14 (70.0) | 306 (74.6) | 0.643 |
| Hepatic tumor | 1 (5.0) | 72 (17.6) | 0.248 |
| Alcoholic cirrhosis | 2 (10.0) | 30 (7.3) | 0.992 |
| Pre-LT diabetes | 4 (20.0) | 47 (11.5) | 0.424 |
| Pre-LT use of broad-spectrum antibiotics ≥ 3 days | 11 (55.0) | 157 (38.3) | 0.135 |
| Pre-LT creatinine ≥ 2 mg/dL | 2 (10.0) | 29 (7.1) | 0.959 |
| Infection within 2 months prior to LT | 11 (55.0) | 159 (38.8) | 0.147 |
| Pre-LT WBC count ≥ 10 × 109/L | 2 (10.0) | 59 (14.4) | 0.825 |
| Pre-LT lymphocyte count ≤ 0.5 × 109/L | 3 (15.0) | 103 (25.1) | 0.447 |
| Pre-LT platelet count ≤ 50 × 109/L | 8 (40.0) | 135 (32.9) | 0.512 |
| Pre-LT albumin level < 30 g/L | 4 (20.0) | 85 (20.7) | 1.000 |
| Donor age ≥ 50 years | 8 (40.0) | 134 (32.7) | 0.497 |
| Steatosis ≥ 30% | 2 (10.0) | 41 (10.0) | 1.000 |
| Cold ischemia time ≥ 360 minutes | 11 (55.0) | 193 (47.1) | 0.488 |
| Duration of surgery ≥ 450 minutes | 8 (40.0) | 74 (18.0) | 0.015 |
| Intraoperative bleeding ≥ 3000 mL | 18 (80.0) | 236 (57.6) | 0.004 |
| Intraoperative RBC transfusion ≥ 12 U/L | 13 (65.0) | 223 (54.4) | 0.352 |
| ALT on day 1 after LT ≥ 1500 U/L | 10 (50.0) | 74 (18.0) | < 0.001 |
| Creatinine on day 3 after LT ≥ 2 mg/dL | 3 (15.0) | 60 (14.6) | 1.000 |
| Albumin level on day 1 after LT < 30 g/L | 2 (10.0) | 29 (7.1) | 0.959 |
| Post-LT mechanical ventilation | 10 (50.0) | 87 (21.2) | 0.003 |
| Reoperation | 2 (10.0) | 15 (3.7) | 0.184 |
| Acute rejection | 3 (15.0) | 65 (15.9) | 1.000 |
| Post-LT renal replacement therapy | 3 (15.0) | 16 (3.9) | 0.052 |
| Glucocorticoidse ≥ 1500 mg | 15 (65.0) | 249 (60.7) | 0.702 |
| Basiliximab use ≥ 40 mg | 8 (40.0) | 171 (41.7) | 0.880 |
| Anti-thymocyte globulin use | 2 (10.0) | 16 (3.9) | 0.201 |
Finally, the multivariate analysis identified that the variables significantly associated with CRKP were female sex (OR = 3.306; 95%CI: 1.239-8.822; P = 0.017), intraoperative bleeding ≥ 3000 mL (OR = 3.269; 95%CI: 1.018-10.490; P = 0.047), ALT on day 1 after LT ≥ 1500 U/L (OR = 4.370; 95%CI: 1.686-11.326; P = 0.002) and post-LT mechanical ventilation (OR = 2.772; 95%CI: 1.077-7.135; P = 0.035) (Table 4).
| Variables | B | S.E. | Wald | OR (95%CI) | P value |
| Female sex | 1.196 | 0.501 | 5.700 | 3.306 (1.239-8.822) | 0.017 |
| Intraoperative bleeding ≥ 3000 mL | 1.184 | 0.595 | 3.963 | 3.269 (1.018-10.490) | 0.047 |
| ALT on day 1 after LT ≥ 1500 U/L | 1.475 | 0.486 | 9.215 | 4.370 (1.686-11.326) | 0.002 |
| Post-LT mechanical ventilation | 1.020 | 0.482 | 4.469 | 2.772 (1.077-7.135) | 0.035 |
Within 6 months of LT, seven (1.6%) patients with CRKP infections succumbed to death. A significantly higher proportion of patients with CRKP infections stayed in the ICU for ≥ 7 days post-LT compared to those without CRKP infections (70.0% vs 34.4%; P = 0.001, Pearson’s χ2 test). Moreover, patients with CRKP infections had a significantly higher mortality rate within 6 months post-LT compared to those without CRKP infections (35.0% vs 7.3%; P < 0.001). Conversely, no difference was observed in hospitalization stays ≥ 21 days post-LT between recipients with and without CRKP infections (P = 1.000) (Table 5).
| Variables | With CRKP infections (n = 20) | Without CRKP infections (n = 410) | χ2 | P value |
| ICU stay after LT ≥ 7 days | 14 (70.0) | 141 (34.4) | 10.490 | 0.001 |
| Hospitalization stay after LT ≥ 21 days | 16 (80.0) | 326 (19.5) | 0.003 | 1.000 |
| All-cause mortality within 6 months after LT | 7 (35.0) | 30 (7.3) | 18.583 | < 0.001 |
The Kaplan-Meier curves depicting the 6-month all-cause mortality supported these findings. The survival rate was significantly lower for patients with CRKP infections compared to patients without CRKP infections (log-rank P < 0.001) (Figure 2).
The LT recipients face heightened susceptibility to drug-resistant infections due to various factors such as malnutrition, extensive surgical procedures, prolonged antibiotic exposure, frequent hospitalizations, and immunosuppressive therapies[5]. Gram-negative pathogens, K. pneumoniae is frequently isolated, with a substantial proportion exhibiting carbapenem resistance, consistent with findings in our study where CRKP constituted 68.1% of K. pneumoniae isolates[4,5]. The incidence of CRKP infections in our cohort was 4.7%, slightly lower than other reports in LT recipients, suggesting variability in regional and institutional prevalence rates[1,5].
Infections primarily involved the lung and bloodstream, with bloodstream involvement observed in 45% of cases, aligning with prior studies highlighting the predilection of CRKP for these sites in LT recipients[1,3,7]. Notably, no urinary tract infections were documented in our cohort, consistent with data from Giannella et al[3], reflecting stringent diagnostic criteria employed in this study compared to broader definitions in previous literature.
Antimicrobial susceptibility testing revealed universal resistance of CRKP isolates to multiple agents including piperacillin/tazobactam, ceftazidime, cefepime, aztreonam, levofloxacin, and meropenem, while showing relatively high susceptibility to CZA, polymyxin B, and tigecycline. It is important to note that while polymyxin E, amikacin, tigecycline, and carbapenems have been recommended as optimal drugs for CRKP-infected solid organ transplant recipients, their use is limited due to factors such as inferior efficacy, resistance, suboptimal pharmacokinetics, or high toxicity rates[16-19]. Notably, CZA demonstrated a favorable response with a mortality rate of 12.5% among recipients receiving CZA therapy, alone or in combination with other agents, underscoring its efficacy compared with conventional therapies associated with higher mortality rates[5,10].
CZA is a preferred agent for most K. pneumoniae carbapenemase- and oxacillinase-48-like-producing organisms with an overall success rate of about 70% and reduced toxicity compared to other regimens commonly used to treat K. pneumoniae carbapenemase-producing infections, which are often polymyxin-based[20-24].
In a study by Chen et al[25], CZA was used in 21 LT recipients with severe CRKP infections after previous treatment failures with other antimicrobials. The study reported promising results, with mortality rates of 28.6%, 38.1%, and 42.9% at 14 days, 30 days, and an all-cause mortality, respectively. However, limited data is available specifically on the efficacy of CZA for CRKP infections in patients after LT. Although the present study observed a low mortality rate of 12.5% in LT recipients receiving CZA monotherapy or combination therapy, it is important to note that due to the small sample size, the present study cannot definitively conclude whether CZA combination therapy was associated with a reduced risk of clinical failure. Further studies with larger sample sizes should be conducted to provide more data on the cure and survival rates after CZA therapy in LT recipients with CRKP infections.
Female sex, intraoperative bleeding ≥ 3000 mL, elevated ALT levels post-LT, and post-LT mechanical ventilation emerged as significant risk factors for CRKP infections in multivariate analysis. These findings are consistent with existing literature and highlight the importance of these factors in predisposing LT recipients to CRKP infections[2,3,5,7].
The finding that female sex was associated with an increased risk of CRKP infections was in line with the study from Abbott et al[26], claiming that the female sex was associated with hospitalizations for septicemia among kidney transplant recipients. One possible explanation for this association could be that female patients are more prone to developing urinary tract infections, which could potentially contribute to the risk of CRKP infections. However, it is noteworthy that in the present study, no LT recipients developed urinary tract infections caused by CRKP, suggesting that the exact reason for the association between the female sex and CRKP infections in this context remains unclear and warrants further investigation.
Regarding massive bleeding during the surgery, the findings of the present study were consistent with those of a previous study assessing 100 consecutive living donor LT patients where massive operative blood loss was an independent risk factor for post-transplantation bacteremia[27].
Elevated ALT was also associated with post-LT CRKP infections in the present study, which has not been previously reported. Although the reasons for this association are uncertain, a plausible explanation may be that a higher level of ALT in the early stage of LT indicates a severer attack caused by the operation due to massive bleeding or severe hypotension during the operation or indicates poor quality of the graft. These conditions could potentially weaken the immune system, or promote the translocation of the intestinal flora, making LT recipients more susceptible to infections. However, these findings should be validated in future large-scale studies to confirm their significance.
Pre-LT use of broad-spectrum antibiotics and infection within 2 months prior to LT, were more frequent among patients with CRKP, although the difference was not significant. Infections mean increased hospitalization and the use of broad-spectrum antibiotics. Hu et al[28] and colleagues also identified that the K. pneumoniae from inpatients was more likely to be imipenem-resistant than isolates from outpatients. In agreement with our findings, a meta-analysis conducted by Liu et al[29] involving 16 studies which involved 3627 participants confirmed that prior hospitalization and previous antibiotic use were associated with CRKP infection.
The impact of CRKP infections on outcomes was profound, with patients experiencing prolonged ICU stays and significantly higher 6-month all-cause mortality compared to those without CRKP infections. This underscores the critical need for effective preventive strategies and optimized treatment protocols to mitigate these adverse outcomes[5,7,9].
Limitations of our study include its retrospective nature, single-center design, and relatively small sample size of LT recipients with CRKP infections. Future studies should aim to validate these findings in larger cohorts and explore additional factors such as colonization with CRKP pre-LT, which may further elucidate the risk profile for post-LT infections[2,3]. Nonetheless, our study provides valuable insights into the epidemiology, risk factors, and management outcomes of CRKP infections in LT recipients, highlighting the urgency for tailored interventions to improve patient outcomes in this vulnerable population.
CRKP infections were frequent complications following LT, with poor associated outcomes. Risk factors for post-LT CRKP infections included female sex, significant intraoperative bleeding, elevated ALT levels, and the need for mechanical ventilation. CRKP infections negatively impacted survival and prolonged ICU stay.
We would like to thank Professor He QN’s contribution to the study design and his helpful advice in writing of the paper.
| 1. | Kalpoe JS, Sonnenberg E, Factor SH, del Rio Martin J, Schiano T, Patel G, Huprikar S. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Lübbert C, Becker-Rux D, Rodloff AC, Laudi S, Busch T, Bartels M, Kaisers UX. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis. Infection. 2014;42:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Giannella M, Bartoletti M, Morelli MC, Tedeschi S, Cristini F, Tumietto F, Pasqualini E, Danese I, Campoli C, Lauria ND, Faenza S, Ercolani G, Lewis R, Pinna AD, Viale P. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15:1708-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Varghese J, Gomathy N, Rajashekhar P, Venugopal K, Olithselvan A, Vivekanandan S, Naresh S, Sujatha C, Vijaya S, Jayanthi V, Rela M. Perioperative bacterial infections in deceased donor and living donor liver transplant recipients. J Clin Exp Hepatol. 2012;2:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Liu N, Yang G, Dang Y, Liu X, Chen M, Dai F, Ding X, Li W, Li G, Lou J, Chen D, Yu Y. Epidemic, risk factors of carbapenem-resistant Klebsiella pneumoniae infection and its effect on the early prognosis of liver transplantation. Front Cell Infect Microbiol. 2022;12:976408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Lübbert C, Rodloff AC, Laudi S, Simon P, Busch T, Mössner J, Bartels M, Kaisers UX. Lessons learned from excess mortality associated with Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in liver transplant recipients. Liver Transpl. 2014;20:736-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Pereira MR, Scully BF, Pouch SM, Uhlemann AC, Goudie S, Emond JE, Verna EC. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2015;21:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Mouloudi E, Massa E, Papadopoulos S, Iosifidis E, Roilides I, Theodoridou T, Piperidou M, Orphanou A, Passakiotou M, Imvrios G, Fouzas I, Papanikolaou V, Gritsi-Gerogianni N. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae among intensive care unit patients after orthotopic liver transplantation: risk factors for infection and impact of resistance on outcomes. Transplant Proc. 2014;46:3216-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Barchiesi F, Montalti R, Castelli P, Nicolini D, Staffolani S, Mocchegiani F, Fiorentini A, Manso E, Vivarelli M. Carbapenem-Resistant Klebsiella pneumoniae influences the outcome of early infections in liver transplant recipients. BMC Infect Dis. 2016;16:538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Mouloudi E, Massa E, Piperidou M, Papadopoulos S, Iosifidis E, Roilides I, Theodoridou T, Kydona C, Fouzas I, Imvrios G, Papanikolaou V, Gritsi-Gerogianni N. Tigecycline for treatment of carbapenem-resistant Klebsiella pneumoniae infections after liver transplantation in the intensive care unit: a 3-year study. Transplant Proc. 2014;46:3219-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Hand J, Patel G. Multidrug-resistant organisms in liver transplant: Mitigating risk and managing infections. Liver Transpl. 2016;22:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Wu D, Chen C, Liu T, Wan Q. Risk Factors for Acquisition of Carbapenem-Resistant Klebsiella pneumoniae and Mortality Among Abdominal Solid Organ Transplant Recipients with K. pneumoniae Infections. Med Sci Monit. 2020;26:e922996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4732] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 14. | Garzoni C, Ison MG. Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. 2011;92:1297-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Ma Y, Li C, Peng W, Wan Q. The influence of delirium on mortality and length of ICU stay and analysis of risk factors for delirium after liver transplantation. Front Neurol. 2023;14:1229990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG Jr, Paterson DL, Bonomo RA, Evans S; Antibacterial Resistance Leadership Group. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 17. | Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. In Vitro Antimicrobial Activity of a Siderophore Cephalosporin, S-649266, against Enterobacteriaceae Clinical Isolates, Including Carbapenem-Resistant Strains. Antimicrob Agents Chemother. 2016;60:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 570] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 19. | van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. In Vitro Susceptibility to Ceftazidime-Avibactam of Carbapenem-Nonsusceptible Enterobacteriaceae Isolates Collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob Agents Chemother. 2016;60:3163-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Spiliopoulou I, Kazmierczak K, Stone GG. In vitro activity of ceftazidime/avibactam against isolates of carbapenem-non-susceptible Enterobacteriaceae collected during the INFORM global surveillance programme (2015-17). J Antimicrob Chemother. 2020;75:384-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis. 2022;75:187-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 309] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 23. | Sousa A, Pérez-Rodríguez MT, Soto A, Rodríguez L, Pérez-Landeiro A, Martínez-Lamas L, Nodar A, Crespo M. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73:3170-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 25. | Chen F, Zhong H, Yang T, Shen C, Deng Y, Han L, Chen X, Zhang H, Qian Y. Ceftazidime-Avibactam as Salvage Treatment for Infections Due to Carbapenem-Resistant Klebsiella pneumoniae in Liver Transplantation Recipients. Infect Drug Resist. 2021;14:5603-5612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Abbott KC, Oliver JD 3rd, Hypolite I, Lepler LL, Kirk AD, Ko CW, Hawkes CA, Jones CA, Agodoa LY. Hospitalizations for bacterial septicemia after renal transplantation in the united states. Am J Nephrol. 2001;21:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kaido T, Mori A, Ogura Y, Ogawa K, Hata K, Yoshizawa A, Yagi S, Uemoto S. Pre- and perioperative factors affecting infection after living donor liver transplantation. Nutrition. 2012;28:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Hu Y, Liu C, Shen Z, Zhou H, Cao J, Chen S, Lv H, Zhou M, Wang Q, Sun L, Sun Q, Hu F, Wang Y, Zhang R. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008-2018. Emerg Microbes Infect. 2020;9:1771-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 29. | Liu P, Li X, Luo M, Xu X, Su K, Chen S, Qing Y, Li Y, Qiu J. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb Drug Resist. 2018;24:190-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |