INTRODUCTION
Intestinal mucositis (IM) is characterized by as inflammatory and/or ulcerative lesion within the gastrointestinal tract and is a significant adverse effect in patients undergoing cancer therapy[1]. Approximately 80% of patients receiving chemotherapy experience IM, which is accompanied by a spectrum of debilitating symptoms, including ulceration, diarrhea, impaired food intake, weight loss, abdominal pain, fatigue, anemia, and in severe cases, bacteremia and potential death[2,3]. Currently, standardized treatments for IM are lacking, and clinical management is primarily aimed at symptomatic relief. Loperamide (LO), a potent μ-opioid receptor agonist, is commonly used to relieve diarrhea in IM, yet it also poses risks of constipation and cardiotoxicity[4]. Therefore, the development of new treatment strategies and the identification of safe, convenient, and effective drugs for IM treatment are urgently needed.
Traditional Chinese medicine (TCM) is globally acknowledged for its numerous advantages, including minimal adverse effects, cost-effectiveness, widespread availability, and holistic methodology for ameliorating patient symptoms[5]. Modified Pulsatilla decoction (PD), a classical TCM formulation recorded in Zhang Zhongjing's “Essentials from the Golden Cabinet” from the Han Dynasty, is specifically tailored for treating severe debilitation accompanied by diarrhea. This decoction integrates PD, which comprising Pulsatilla Chinensis, Coptis Chinensis, Cortex Phellodendri, and Cortex Fraxini, with licorice (Glycyrrhiza uralensis) and ejiao (Colla corii asini)[6,7]. The PD is specifically formulated to treat diarrhea, whereas supplementation with licorice and ejiao aims to enhance qi and blood, reduce fatigue, and prevent anemia, thereby providing a formula with the ability to clear heat, stop dysentery, augment qi, and enrich blood[8,9]. In light of TCM principles and given that IM is typified by symptoms of diarrhea, abdominal pain, fatigue, and anemia, anti-diarrheal measures and interventions are necessary to fortify and nourish qi and blood. Aligning with the TCM paradigm of syndrome differentiation and treatment, modified PD is especially appropriate for the effective management of IM.
Modified PD components demonstrate pharmacological properties that protect against gastrointestinal ulcers and inflammation, modulate oxidative stress, reshape the gut microbiome, and are therefore effective for treating IM. PD maintains intestinal microecological stability and significantly alleviates ulcerative colitis[10,11]. Berberine, a major active alkaloid isolated from Rhizoma Coptidis (Huanglian), mitigates the effects of 5-fluorouracil (5-FU)-induced IM[12]. Furthermore, active ingredients in Pulsatilla such as anemoside B4[13] and oleanolic acid[14,15], constituents of Cortex Phellodendri including limonin[16] and obacunone[17]; and compounds in Cortex Fraxini such as esculin, esculetin[18], and fraxetin[19], counteract intestinal inflammation and alleviate gastrointestinal diseases through multiple mechanisms. The key components of processed licorice, glycyrrhizic acid, and isoliquiritigenin, have been shown to suppress inflammatory mediators and oxidative stress, thereby protecting against 5-FU-induced IM[20,21]. Similarly, ejiao significantly inhibits lipopolysaccharide (LPS)-mediated NF-κB activation and mitochondrial reactive oxygen species (ROS) production, reduces intestinal inflammation and oxidative stress, combats fatigue and anemia, and stabilizes the gut microbiota. In clinical settings, modified PD has shown significant therapeutic efficacy in the treatment of IM[22]. Therefore, the exact therapeutic effect and mechanism of action of modified PD warrant further investigation.
The pathogenesis of IM is complex and includes inflammatory dysregulation, oxidative stress, cellular apoptosis, and disturbances in gut microbiota. Chemotherapy rapidly induces DNA strand breaks and increases ROS production, subsequently activating the NF-κB signaling pathway[23]. NF-κB has been suggested to be one of the most significant transcription factors that are activated by chemotherapy and ROS, leading to the induction of side effects and the resistance to tumor therapy[24]. Activation of NF-κB can result in the upregulation of up to 200 genes, including those encoding pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which cause mucosal toxicity by directly targeting intestinal epithelial cells and mucosal tissue[25]. Intensified inflammation further induces the destruction and apoptosis of intestinal epithelial cells, leading to damage to the intestinal mucosal barrier. This process also results in a pronounced reduction in mucoproteins in the intestinal mucosa, disruption of tight junctions between intestinal epithelial cells, increased intercellular permeability, and pathogen colonization in areas of mucosal damage, ultimately leading to severe dysbiosis[26]. LPS, a component of Gram-negative bacterial cell walls, can activate the NF-κB signaling pathway via TLR4, exacerbating intestinal inflammation and microbial dysbiosis[27].
In this study, we assessed the effects of modified PD on the TLR4/MyD88/NF-κB pathway. We evaluated the therapeutic efficacy of modified PD in IM mouse model by analyzing its mechanisms and effects on the gut microbiota. Our findings provide insights into the mechanisms by which modified PD mitigates IM and support its clinical utility.
MATERIALS AND METHODS
Materials
All components of the modified PD formula, including Pulsatilla, Rhizoma Coptidis, Cortex Phellodendri, Cortex Fraxini, roasted licorice, and ejiao, were sourced from the First Affiliated Hospital of Guangzhou University of Chinese Medicine, China. LO hydrochloride was obtained from Xi'an Janssen Pharmaceuticals (China). ROS detection reagents (D7008) and the chemotherapeutic agent 5-FU (F6627-5G) were supplied by Sigma-Aldrich, United States. Primary antibodies including anti-TLR4 (AF7017), anti-MyD88 (AF5195), anti-TRAF6 (AF5376), and anti-beta actin (AF7016) were obtained from Affinity Biosciences Group, Australia. Anti-P65 (AB32536), anti-mucin-2 (AB272692), anti-histone H3 (AB176842) were obtained from Abcam, United Kingdom. Anti-Occludin-1 (GB111401), anti-Claudin-1 (GB15032), anti-ZO-1 (GB111402), and Alexa Fluor 488 goat anti-rabbit IgG (GB25303) were obtained from Wuhan Servicebio, China. Horseradish peroxidase-conjugated goat anti-rabbit IgG (BL003A) was purchased from BioSharp Biotechnology Co., Ltd. (Beijing, China). Mouse ELISA kits for the detection of LPS (YJ002277), MPO (YJ002070), TNF-α (YJ002095), IL-1β (YJ301814), IFN-γ (IJ002277), IL-6 (YJ002293), IL-8 (YJ063162), IL-10 (YJ07873), and IL-17 (YJ037866) in serum were procured from Shanghai Enzyme-linked Biotechnology, China.
Drug preparation
A total of 12 g each of Pulsatilla, ejiao, and roasted licorice and 18 g each of Rhizoma Coptidis, Cortex Phellodendri, and Cortex Fraxini were weighed. All herbs except ejiao were soaked in distilled water for 30 minutes. These were then boiled in a TCM decoction pot for 40 minutes, filtered, and subjected to a second boiling step, followed by another filtration. Decoctions resulting from both boiling steps were combined and concentrated using a rotary evaporator. During the final stages of the concentration process, ejiao was added and the mixture was further concentrated until the volume reached 90 mL (equivalent to 1 g/mL). The preparation was then allowed to cool to room temperature and stored at 4 °C for further use.
Ultra-high-performance liquid chromatography mass spectrometer analysis
An aliquot of 100 μL of modified PD was mixed with 300 μL methanol and centrifuged at 20000 × g for 10 minutes at 4 °C. The supernatant was collected for analysis using a Welch AQ-C18 column (150 mm × 2.1 mm, 1.8 μm) under a flow rate of 0.30 mL/min at a column temperature of 35 °C. An autosampler injected 5 μL of the sample. The mobile phases consisted of 0.1% formic acid (A) and methanol (B), with a gradient elution program adjusted over 30 minutes. Mass spectrometry employed an electrospray ionization source in positive and negative modes, utilizing full scan/dd-MS2 at resolutions of 70000 and 17500, respectively, over a mass range of 100.0–1500.0 m/z. The electrospray was set at 3.2 kV, with a capillary temperature of 300 °C and a total data collection time of 30 minutes.
Molecular docking
Molecular docking simulations were conducted for 25 small molecule compounds of modified PD with the receptors LPS, TLR4, MyD88, TRAF6, and NF-κB. The structures of the proteins and compounds were obtained from the RCSB Protein Data Bank and the PubChem Database, respectively. The compounds were preprocessed using AutoDockTools, which involves the removal of water molecules and the addition of hydrogen atoms. The docking affinities were assessed using the Lamarckian Genetic Algorithm and reported in kcal/mol. Interactions were visualized using PyMOL software (Schrödinger LLC, United States).
Animal experiment
Forty-eight male BALB/c mice (8 weeks old, 24-26 g), were sourced from ZhuHai Bestest Biotechnology Co., Ltd. [License No. SCXK (YUE) 2020-0051] and housed in a specific pathogen-free facility at the School of Traditional Chinese Medicine, Guangzhou University of Chinese Medicine. The conditions were controlled at 23-25°C, 40%-70% humidity, and a 12 h light/12 h dark cycle. The experimental protocols were approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine (approval no. ZYD-2023-070).
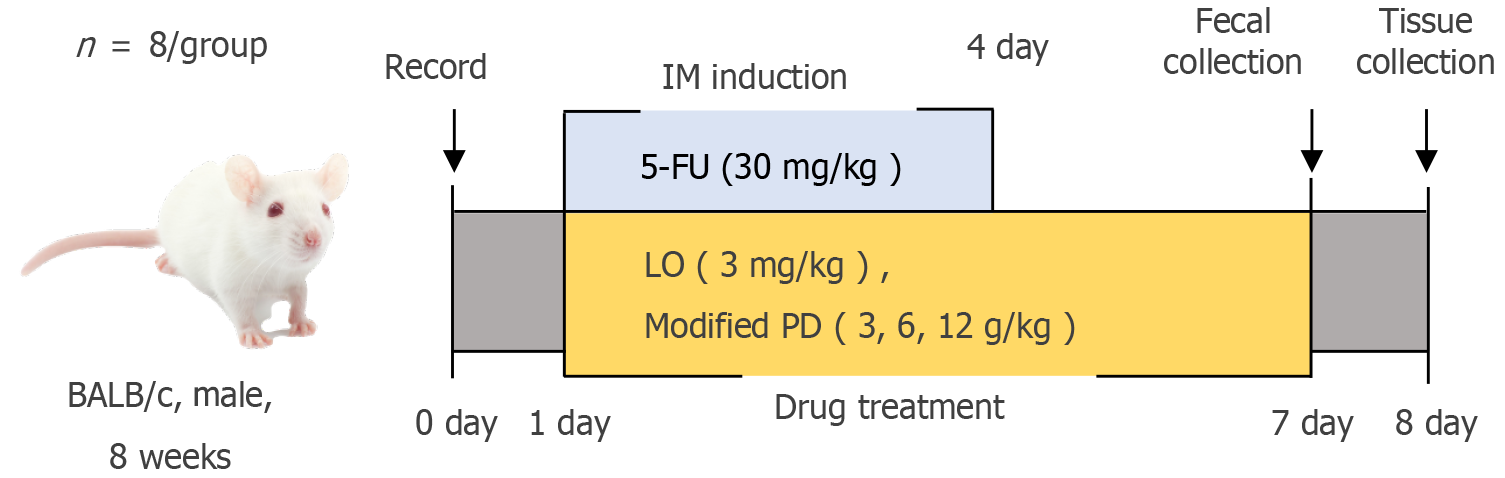
Mice were randomly assigned to six groups (n = 8): Control, model (5-FU), LO (3 mg/kg), and modified PD low-, medium-, and high-dose (3, 6, and 12 g/kg, respectively). IM induction began on day 1, which marked the start of the experiment. IM was induced by administering 5-FU (30 mg/kg); Once daily between 8: 00 AM and 9: 00 AM) on days 1-4. One hour after injection, mice received daily oral treatments with LO or modified PD for seven consecutive days (days 1-7)[28]. Eight hours after the final treatment on day 7, fecal samples were collected using the spot sample collection method[29]. Each mouse was lifted by holding its tail and was positioned on the surface of the cage grid cover. Gentle pressure was applied to the lower abdomen to stimulate defecation. Fresh feces were collected in sterile cryotubes, which were immediately sealed, labeled, and stored on dry ice. The entire fecal collection process took approximately two hours, and the samples were reserved for subsequent 16S rDNA sequencing analysis. After a 12-hour fast, the mice were euthanized by cervical dislocation for tissue collection and analysis. The detailed procedure is illustrated in Figure 1.
Figure 1
Experimental timeline and procedures.
Weight and diarrhea analysis
During the experiment, the overall health status of the mice was closely monitored. Daily data on weight variation and incidence of diarrhea were also collected. Additional observations included fur condition, mental state, and activity level. The severity of diarrhea was objectively quantified using a scoring system[30]: 0 points was assigned for normal feces; 1 point for mild diarrhea, marked by slightly wet and soft feces; 2 points for moderate diarrhea, featuring moderately moist, unformed feces with minor perianal staining; and 3 points for severe diarrhea, presenting as watery stools and often accompanied by persistent seepage.
Histological analysis
Approximately 1 cm of fresh ileal tissue proximal to the cecum was excised, embedded in paraffin, and sectioned. Sections were subsequently stained with hematoxylin and eosin, dehydrated, and mounted for examination. The histopathological score was derived from the sum of four indices: Severity of inflammation severity, crypt damage, villus damage, and epithelial cell damage, each graded on a scale of 0 to 3. Inflammation was scored as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). Crypt damage was scored as 0 (no damage), 1 (loss of 1/3 of the crypts), 2 (loss of 2/3), or 3 (complete loss). Villus damage was graded as 0 (none), 1 (shortening by 1/3), 2 (shortening by 2/3), and 3 (complete degeneration). The epithelial cell damage was scored as follows: 0 (intact), 1 (1/3 loss), 2 (2/3 loss), or 3 (complete loss). Histopathological scores were calculated as described previously[31].
Western blotting
Proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes. The membranes were blocked using blotting buffer and incubated overnight at 4 °C with primary antibodies: Anti-TLR4, anti-MyD88, anti-TRAF6, anti-beta actin (1:500), anti-P65 (1:1000), and anti-histone 3 (1:5000). Following TBST washes, the membranes were incubated with secondary antibody of goat anti-rabbit IgG (BL003A) for 1 h at room temperature and washed three times. Detection was performed using enhanced chemiluminescence. Immunoreactive bands were quantitatively analyzed using ImageJ software.
ROS assays
Frozen tissue sections were thawed, air-dried, and demarcated using an immunohistochemical barrier pen. Autofluorescence was quenched using a specific quenching agent and the sections were rinsed thoroughly. ROS-specific fluorophores were applied to the outlined areas and incubated in the dark. After incubation, the sections were washed several times with PBS. Nuclear structures were delineated using DAPI counterstaining followed by additional washes. Slides were sealed with an anti-fade mounting medium to preserve fluorescence and minimize photobleaching. Images were captured using an ECLIPSE C1 microscope (Nikon, Japan).
Immunofluorescence
Paraffin-embedded sections were dewaxed and subjected to antigen retrieval in citrate buffer (pH 6.0) under high pressure. The sections were boiled until jetting, then removed from the heat and cooled in tap water. After cooling, the sections were blocked with 10% donkey serum to prevent nonspecific binding. The cells were then incubated with primary antibodies against TLR4 (1:50), MyD88 (1:100), TRAF6 (1:50), P65 (1:200), occludin-1 (1:500), claudin-1 (1:200), and ZO-1 (1:200), followed by incubation with secondary anti-rabbit antibodies (1:500). DAPI staining was performed in the dark for 10 min, and the sections were analyzed using a NIKON ECLIPSE C1 microscope (Nikon).
Immunohistochemistry
The paraffin sections were deparaffinized in xylene, rehydrated in a graded alcohol series, and washed. Antigen retrieval was performed in EDTA buffer (pH 9.0) using a microwave: Medium-power for 8 minutes, rest for 8 minutes, and medium-low for 7 minutes. After cooling, sections were washed with PBS (pH 7.4) and blocked with normal rabbit serum. They were then incubated overnight at 4 °C with anti-mucin-2 antibody (1:2000), followed by incubation with goat anti-rabbit secondary antibody (1:200) for 1 h at room temperature. Visualization was performed using DAB, and the sections were counterstained with hematoxylin. Digital images were captured using an Aperio computed tomography 6 digital slide scanner. Mucin-2 staining intensity was evaluated according to the IHC scoring protocol described previously[32]. If the pixel proportion in any specific intensity category (high-positive, positive, low-positive, or negative) exceeded 66.6%, the score for that category was assigned as the final score. Otherwise, a composite score was derived based on the distribution of pixels across the intensity categories and their respective scores, calculated as follows: Total score = ∑ (pixel count in zone × zone score)/total pixel count.
Biochemical analysis
Concentrations of LPS, MPO, TNF-α, IL-1β, IFN-γ, IL-6, IL-8, IL-10, and IL-17 were quantified in mouse small intestine tissues using commercial ELISA kits. All procedures adhered strictly to the manufacturer’s protocol. Tissue samples were homogenized in ice-cold PBS and centrifuged at 3000 × g for 20 minutes at 4 °C to obtain the supernatant for subsequent biomarker analysis.
16S ribosomal DNA gene sequencing
Genomic DNA was extracted from mouse feces using the cetyltrimethylammonium bromide turbidimetric method. The V3-V4 regions of the 16S rRNA gene were amplified using the primers 341F (CCTAYGGGRBGCASCAG) and 806R (GGACTACNNGGGTATCTAAT). PCR products were purified using the MinElute PCR Purification Kit and sequenced libraries were prepared with the TruSeq® DNA PCR-Free Kit, followed by quantification using Qubit and qPCR. Sequencing was performed using the Illumina NovaSeq 6000 system (Metware Biotechnology Co., Ltd., China).
Statistical analysis
All data are presented as mean ± SD. Statistical analyses were performed using SPSS (version 26.0) and GraphPad Prism (version 9.0). Normality was assessed using the Shapiro-Wilk test. For normally distributed data, group differences were analyzed using one-way ANOVA, with the LSD test for homogeneous variances and Dunnett’s T3 test for heterogeneous variances. Non-normally distributed data were analyzed using the Kruskal-Wallis test, with results presented as medians (P25–P75).
RESULTS
Identification of chemical components in modified PD
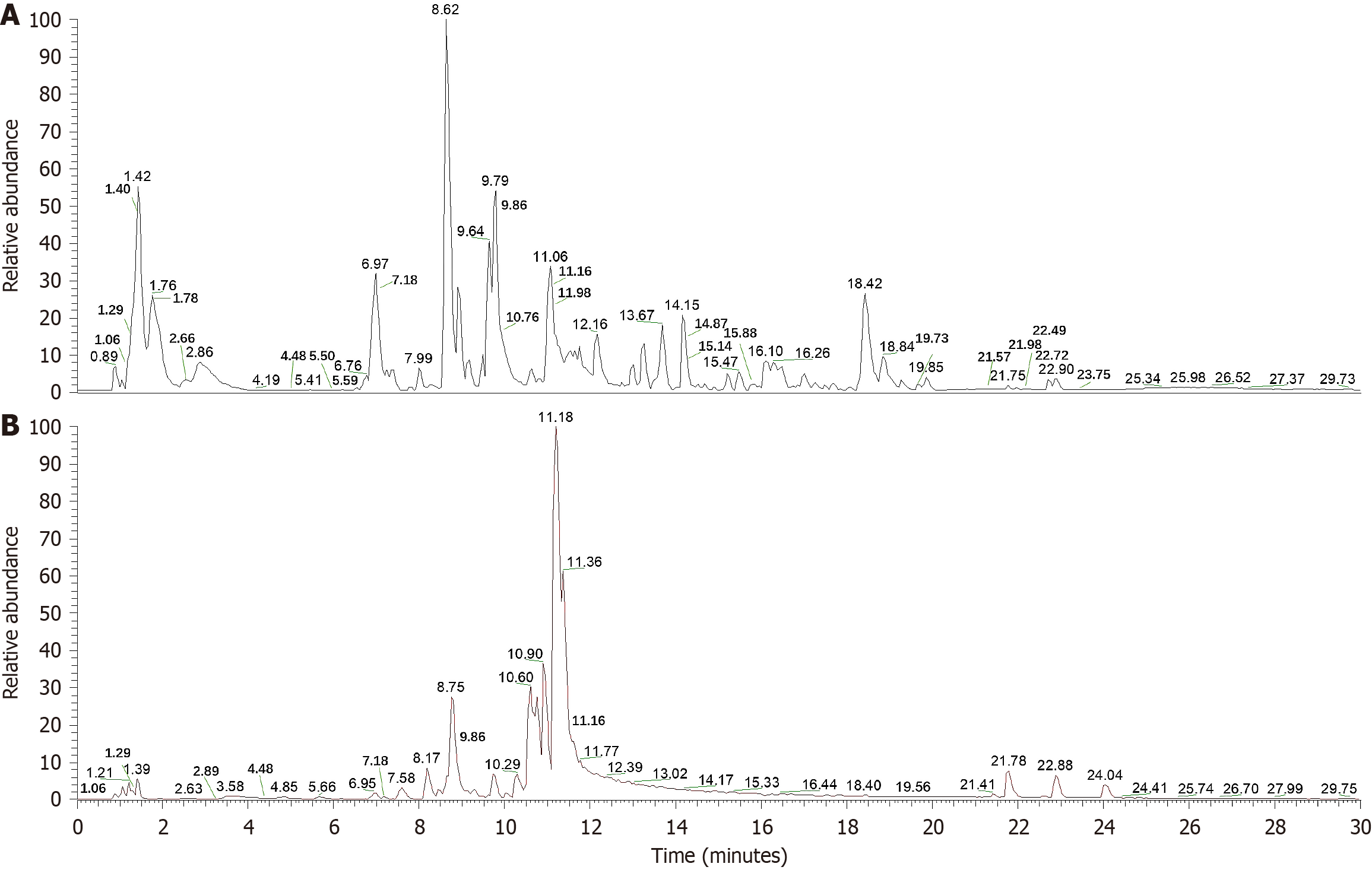
To identify the primary chemical components of the modified PD, liquid chromatography-mass spectrometry was performed. This analysis successfully matched the compounds in the samples with those recorded in the mzCloud database (Supplementary Table 1 and Figure 2). The identified compounds include a variety of bioactive substances, such as anemoside B4, oleanolic acid, berberine, limonin, and obacunone; coumarins, such as esculetin and fraxetin; and the glycoside esculin. The analysis revealed several important amino acids, including L-phenylalanine, prolylleucine, D-proline, L-histidine, L-glutamic acid, L-tyrosine, D-pyroglutamic acid, and D-tryptophan. In addition, fatty acids such as oleic, stearic, palmitic, palmitoleic, and linoleic acids were detected. Furthermore, other compounds such as glycyrrhizic acid and isoliquiritigenin, along with nucleosides such as adenosine and cytarabine, were also identified.
Figure 2 The total negative and positive ion chromatograms of modified Pulsatilla decoction.
A: Total negative ion chromatograms; B: Total positive ion chromatograms.
Modified PD targets the TLR4/MyD88/NF-κB pathway
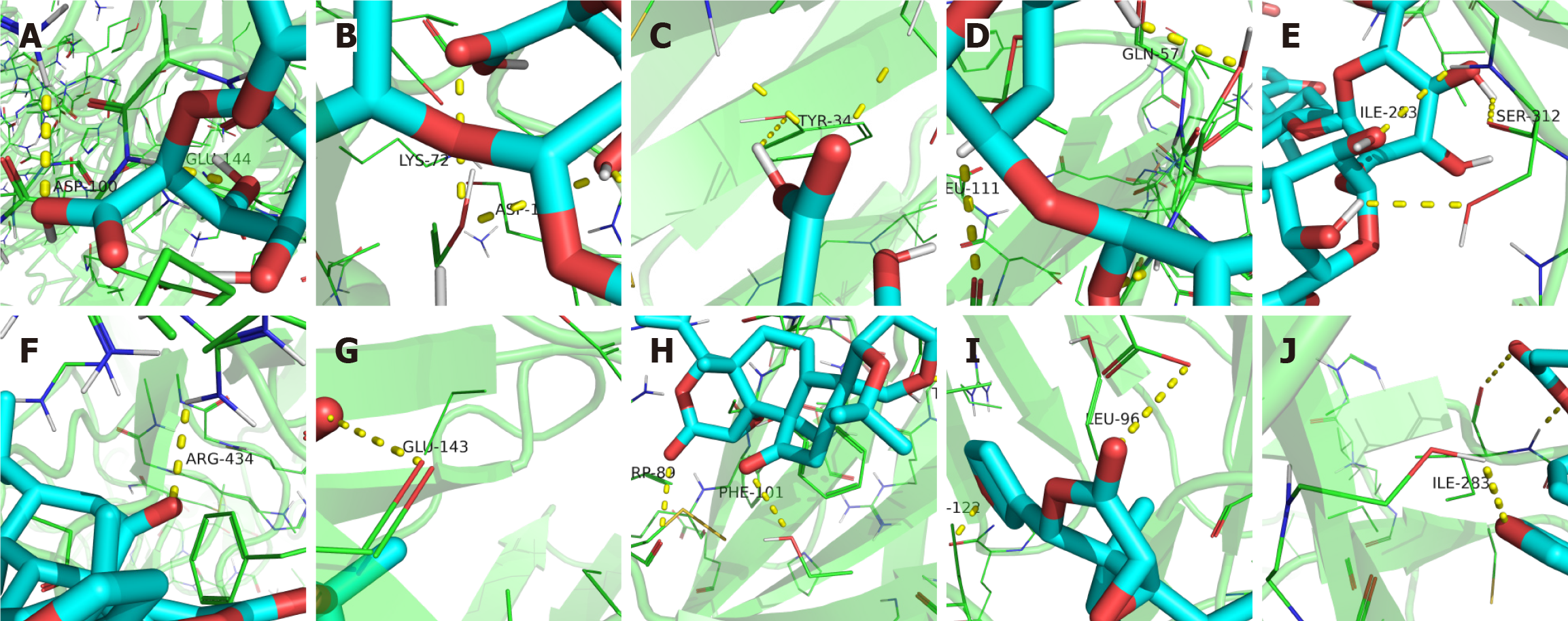
Molecular docking analysis of the 25 compounds in modified PD revealed specific binding sites and substantial binding affinities with key receptors in the TLR4/MyD88/NF-κB signaling pathway, as indicated by negative binding free energy (ΔGbind) values (Supplementary Table 2, Figure 3 and Supplementary Figure 1). Glycyrrhizic acid and limonin exhibited high binding affinities across the five target sites, with clearly defined binding sites. The binding energies of glycyrrhizic acid were as follows: LPS (-11.2 kcal/mol), TLR4 (-10.1 kcal/mol), MyD88 (-8.2 kcal/mol), TRAF6 (-7.4 kcal/mol), and NF-κB (-8.2 kcal/mol), indicating strong and stable interactions. Limonin showed high binding affinity for LPS (-9.8 kcal/mol), TLR4 (-9.4 kcal/mol), MyD88 (-7.6 kcal/mol), TRAF6 (-7.3 kcal/mol), and NF-κB (-8.3 kcal/mol), suggesting robust and stable interactions with the target molecules. Obacunone, oleanolic acid, berberine, anemoside B4, isoliquiritigenin, esculin, esculetin, and fraxetin also exhibited strong binding affinities, contributing to their potential pathway activity. Glycosides, including adenosine and cytarabine, and amino acids, such as d-proline and prolylleucine, display moderate receptor affinities that may provide complementary or synergistic effects. Although fatty acids generally exhibit lower binding affinities, they may enhance pathway stability and auxiliary capacity. Notably, several small-molecule compounds in modified PD showed binding affinities comparable to or exceeding those of LO at distinct sites, suggesting that modified PD may modulate IM through targeted interactions within the TLR4/MyD88/NF-κB pathway.
Figure 3 Modified Pulsatilla decoction compounds ligand dock to the TLR4/MyD88/NF-κB pathway.
A-E: Docking of glycyrrhizic acid with lipopolysaccharide lipopolysaccharide (LPS; A), TLR4 (B), MyD88 (C), TRAF6 (D), and NF-κB (E); F-J: Limonin with LPS (F), TLR4 (G), MyD88 (H), TRAF6 (I), and NF-κB (J).
Modified PD alleviates IM symptoms in mice
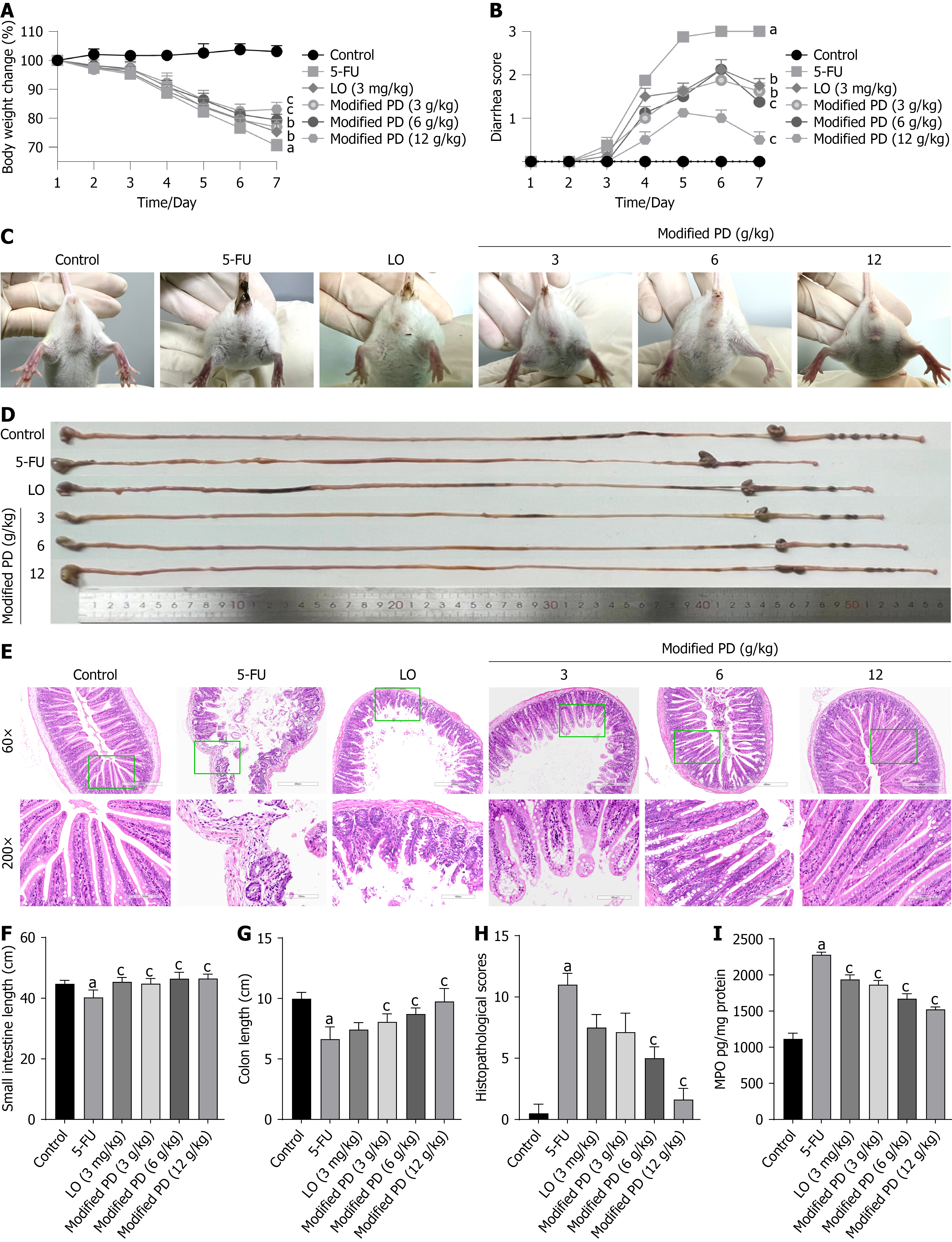
On day 7 of the experiment, the control group mice maintained a body weight of 103.05% ± 2.00% of the initial weight, while the 5-FU group exhibited a significant decline to 70.49% ± 2.13% (P < 0.01). All treatment groups demonstrated an increase in body weight compared to that in the 5-FU group, with the LO group at 75.25% ± 2.96% (P < 0.05), the modified PD group (3 g/kg) at 77.65% ± 1.96% (P < 0.05), the 6 g/kg group at 79.41% ± 1.92% (P < 0.01), and the 12 g/kg group at 83.07% ± 2.39% (P < 0.01; Figure 4A). To assess the severity of diarrhea, we observed the perianal condition of the mice and found that the 5-FU group experienced severe diarrhea (P < 0.01). Each treatment group showed improvement post-treatment, with the modified PD (12 g/kg) demonstrating the most significant effect (P < 0.01), followed by modified PD (6 g/kg; P < 0.01). Additionally, the modified PD (3 g/kg) and LO groups exhibited favorable outcomes (P < 0.05; Figure 4B and C). Furthermore, we measured and analyzed the lengths of the small intestine and colon (Figure 4D), and conducted a microscopic examination of the ileal mucosal condition (Figure 4E). The small intestine length in the Control group was 44.73 ± 1.16 cm, while the 5-FU group was significantly shortened to 40.27 ± 2.45 cm (P < 0.01). All treatment groups effectively reversed the small intestine shortening (P < 0.01), with the LO group measuring 45.42 ± 1.43 cm, Modified PD (3 g/kg) at 44.80 ± 1.67 cm, Modified PD (6 g/kg) at 46.45 ± 2.08 cm, and Modified PD (12 g/kg) at 46.48 ± 1.48 cm (Figure 4F). The colon length in the control group was 9.98 ± 0.52 cm, whereas that in the 5-FU group showed a reduction to 6.65 ± 1.01 cm (P < 0.01). All modified PD groups showed reversal of colon shortening (P < 0.01), with modified PD (3 g/kg) at 8.07 ± 0.66 cm, the 6 g/kg group at 8.72 ± 0.50 cm, and the 12 g/kg group at 9.75 ± 1.08 cm, while the LO group exhibited no significant difference compared to that of the 5-FU group (P > 0.05; Figure 4G). Pathologically, 5-FU-treated mice displayed significant intestinal mucosal damage compared with that in the control group, characterized by shortened villi, shallower crypt depth, increased inflammatory cell infiltration, and structural damage to the intestinal glands (P < 0.01). Treatment with modified PD (6 g/kg and 12 g/kg) significantly reversed these pathological changes (P < 0.01), whereas no significant alterations were observed in the LO and modified PD (3 g/kg) groups (P > 0.05; Figure 4H). MPO is a critical marker of intestinal mucosal inflammation and ulceration. During intestinal inflammation and ulceration, neutrophil infiltration increases, leading to elevated MPO levels in tissue samples[33]. In the 5-FU group, the MPO levels in the intestinal tissue were significantly elevated (P < 0.01). However, treatment with modified PD and LO significantly reduced MPO levels, with modified PD (12 g/kg) demonstrating the greatest effect (P < 0.01; Figure 4I). These results suggest that modified PD effectively mitigates weight loss, diarrhea, and shortening of the intestinal tract induced by 5-FU chemotherapy, while significantly reducing mucosal ulceration and inflammation.
Figure 4 Modified Pulsatilla decoction alleviates symptoms in intestinal mucositis mice.
A: Percentage of initial body weight (%); B: Diarrhea scores; C: Perianal condition; D: Intestinal length; E: H&E staining (60 × and 200 × magnification); F: Length of the small intestine; G: Length of the colon; H: Histopathological scores; I: MPO expression level. Data are shown as mean ± SD (n = 6-8). aP < 0.01 vs control group; bP < 0.05, cP < 0.01 vs 5-fluorouracil group. 5-FU: 5-fluorouracil; LO: Loperamide; PD: Pulsatilla decoction.
Modified PD inhibits ROS production
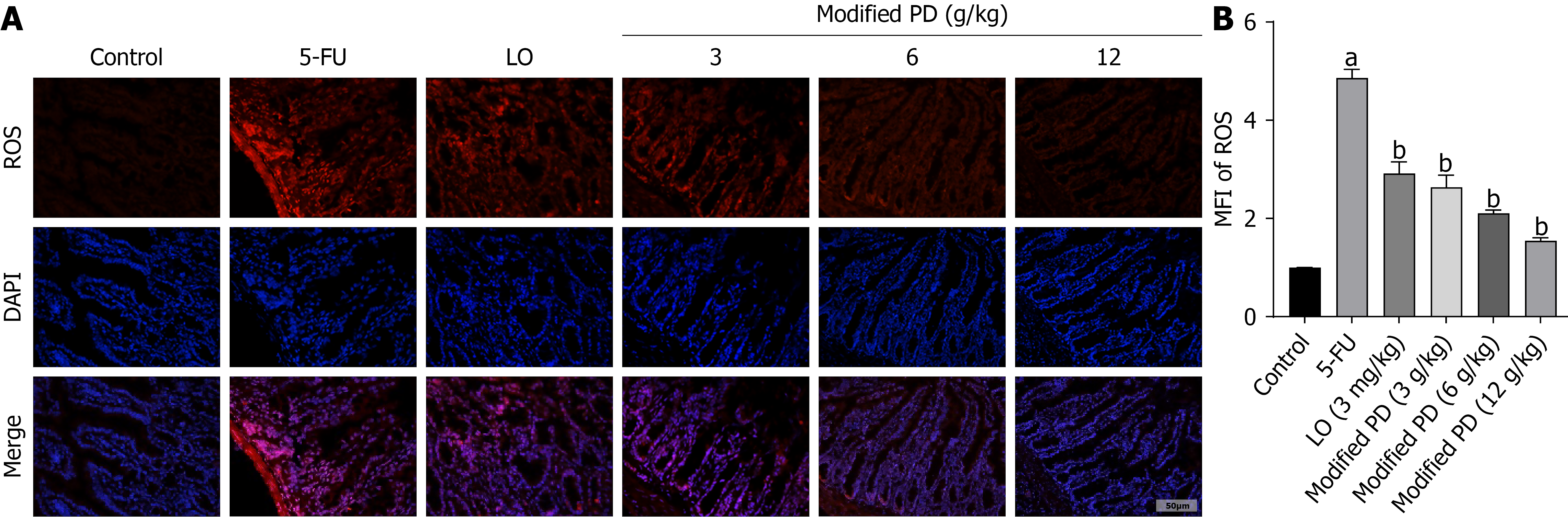
The ROS levels in the small intestines of mice in the 5-FU group were significantly higher than those in the control group (P < 0.01). However, ROS levels significantly decreased following modified PD treatment, with the lowest expression observed in the modified PD (12 g/kg) group (P < 0.01; Figure 5). This indicates that the modified PD effectively suppressed ROS production in the intestinal tract of IM mice to a certain extent.
Figure 5 Modified Pulsatilla decoction inhibits reactive oxygen species production in intestinal mucositis mice.
A: Reactive oxygen species (ROS) staining images of frozen ileum tissue sections; B: Average fluorescence intensity of ROS, normalized to the control group (set as 1). Data are shown as mean ± SD (n = 3). aP < 0.01 vs control group; bP < 0.01 vs 5-fluorouracil group. 5-FU: 5-fluorouracil; LO: Loperamide; PD: Pulsatilla decoction.
Modified PD inhibits the TLR4/MyD88/NF-κB pathway
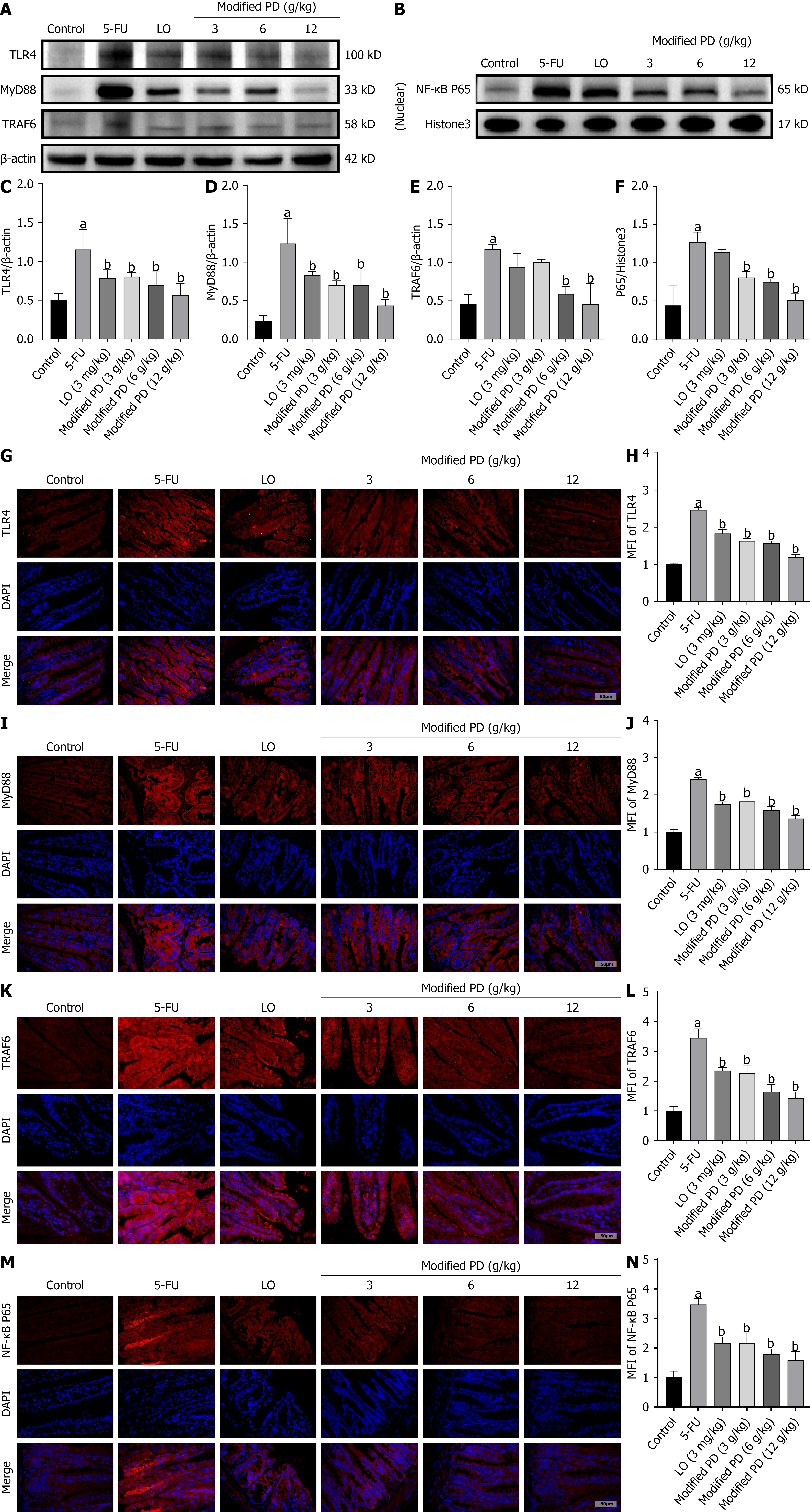
Mice exposed to 5-FU exhibited significantly increased expression levels and fluorescence intensities of TLR4, MyD88, TRAF6, and NF-κB proteins in the small intestine measured using western blotting and immunofluorescence, compared to those in the control group (P < 0.01). Treatment with modified PD at various doses resulted in a reduction in these protein levels (P < 0.01; Figure 6 and Supplementary Figure 2). These findings indicate that modified PD effectively regulates the TLR4/MyD88/NF-κB signaling pathway.
Figure 6 Modified Pulsatilla decoction exerts inhibitory effect on TLR4/MyD88/NF-κB pathway.
A and B: Western blotting images of TLR4, MyD88, TRAF6, β-actin (A), and NF-κB P65, Histone3 (B); C-F: Ratios of TLR4/β-actin (C), MyD88/β-actin (D), TRAF6/β-actin (E), and P65/Histone3 (F); G-N: Immunofluorescent images for TLR4 (G), MyD88 (I), TRAF6 (K), and NF-κB (M) with corresponding fluorescence intensities (H, J, L and N), normalized to the control group (set as 1). Data are shown as mean ± SD (n = 3). aP < 0.01 vs control group; bP < 0.01 vs 5-fluorouracil group. 5-FU: 5-fluorouracil; LO: Loperamide; PD: Pulsatilla decoction.
Modified PD suppresses 5-FU-induced inflammatory response
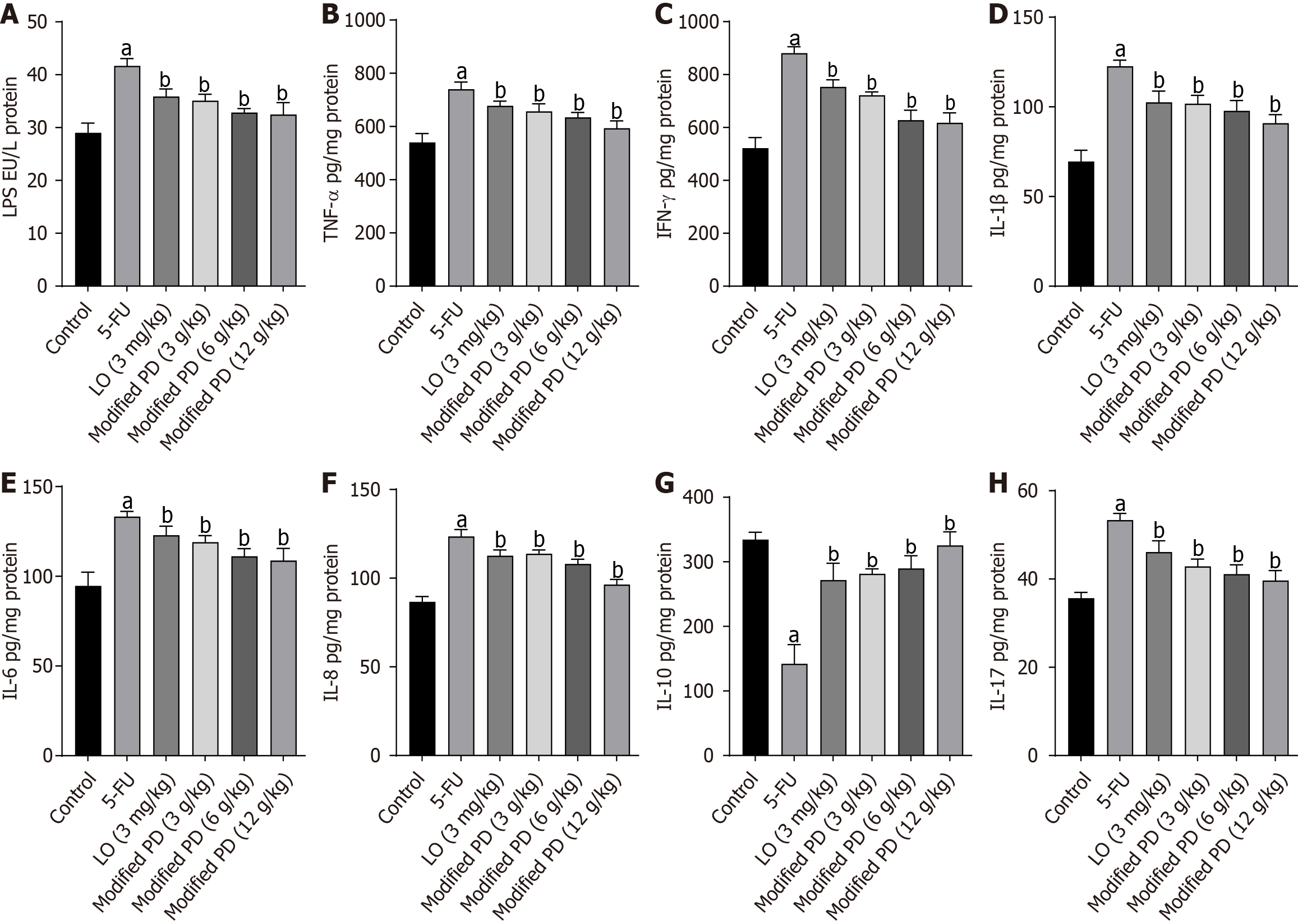
The expression levels of LPS, IL-6, IL-1β, TNF-α, IFN-γ, IL-8, and IL-17 were significantly elevated in the small intestine of the 5-FU group compared to those in the control group, while IL-10 Levels were notably decreased (P < 0.01). However, modified PD significantly decreased the expression levels of LPS, IL-1β, TNF-α, IFN-γ, IL-6, IL-8, and IL-17 that was induced by 5-FU (P < 0.01), as well as increased the IL-10 expression level (P < 0.01; Figure 7). These findings indicated that the modified PD effectively suppressed the inflammatory response.
Figure 7 Modified Pulsatilla decoction promotes inflammatory response balance.
A-H: Levels of inflammatory markers include lipopolysaccharide (A), TNF-α(B), IFN-γ (C), IL-1β(D), IL-6 (E), IL-8 (F), IL-10 (G), IL-17 (H). Data are presented as mean ± SD (n = 6). aP < 0.01 vs control group; bP < 0.01 vs 5-fluorouracil group. 5-FU: 5-fluorouracil; LO: Loperamide; LPS: Lipopolysaccharide.
Modified PD enhances intestinal barrier function
The intestinal mucosal barrier was markedly impaired by 5-FU, as evidenced by the decreased immunofluorescence intensities and immunohistochemical staining of the tight junction proteins occludin-1, claudin-1, ZO-1, and mucin-2 in 5-FU-treated mice compared to those in the control group (P < 0.01). Conversely, pharmacological treatments markedly enhanced the fluorescence intensities of these proteins, with the modified PD treatment effectively increasing protein expression (P < 0.01; Figure 8). These findings indicate that modified PD is effective in reducing intestinal epithelial permeability and reinforcing the intestinal mucosal barrier.
Figure 8 Modified Pulsatilla decoction enhances intestinal barrier function.
A-F: Expression levels and average fluorescence intensity of Occludin-1 (A and B), Claudin-1 (C and D), ZO-1 (E and F); G and H: Immunohistochemical staining and scoring of Mucin-2. Data are presented as mean ± SD (n = 3). aP < 0.01 vs control group; bP < 0.01 vs 5-fluorouracil group. 5-FU: 5-fluorouracil; LO: Loperamide; PD: Pulsatilla decoction.
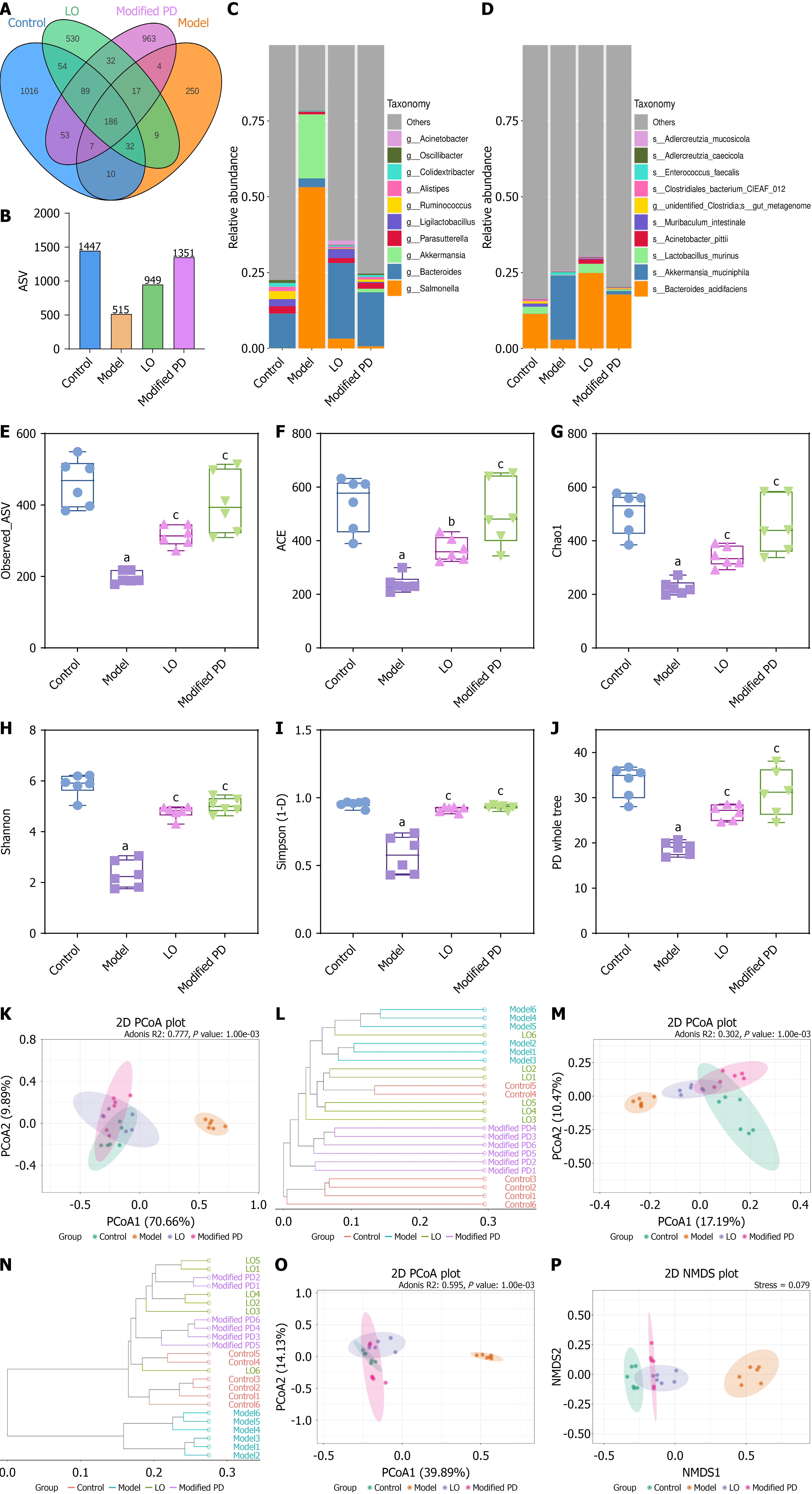
Modified PD ameliorates gut dysbiosis
Gut dysbiosis significantly influences the pathogenesis of IM. We assessed the effects of modified PD (12 g/kg) on gut homeostasis using 16S rDNA sequencing, revealing distinct amplicon sequence variant (ASV) counts of 1447, 515, 949, and 1351 in the control, 5-FU, LO, and modified PD groups, respectively (Figure 9A and B). At the genus level, Bacteroides was the predominant genus in both the control and modified PD groups. The 5-FU group showed elevated levels of Salmonella and Akkermansia, which were reduced by modified PD treatment (Figure 9C). At the species level, Bacteroides acidifaciens was the main species found in the control and modified PD groups, whereas the 5-FU group exhibited elevated concentrations of Akkermansia muciniphila, which was significantly mitigated by modified PD treatment (Figure 9D).
Figure 9 Modified Pulsatilla decoction ameliorates gut microbiota dysbiosis.
A and B: Amplicon sequence variant (ASV) Venn diagram (A) and counts (B); C and D: Stacked bar charts of relative species abundance at genus (C), and species (D) levels; E-J: Alpha diversity indices including observe ASV (E), ACE (F), Chao1 (G), Shannon (H), Simpson (1-D; I), and Pulsatilla decoction whole tree (J); K-P: Beta diversity analysis of Unweighted PCoA (K), Unweighted PCoA with UPGMA (L), Weighted PCoA (M), Weighted PCoA with UPGMA (N), Bray-Curtis PCoA (O) and NMDS (P). aP < 0.01 vs control group; bP < 0.05, cP < 0.01 vs 5-fluorouracil group. ASV: Amplicon sequence variant; LO: Loperamide; PD: Pulsatilla decoction.
Alpha diversity indices, including the observed ASV, ACE, Chao1, Shannon, Simpson, and PD whole trees, reflect the richness and evenness of the microbial communities. The alpha diversity indices revealed that the 5-FU group exhibited significantly lower indices than the control group (P < 0.01). In comparison, the modified PD group demonstrated significant enhancements in these indices relative to those in the 5-FU group (P < 0.01), indicating that modified PD treatment markedly increased microbial richness and diversity (Figure 9E-J). Beta diversity, including PCoA, UPGMA, and NMDS analyses, was used to illustrate the differences in microbial community structures among the groups. The proximity between the modified PD and Control groups suggests a high degree of structural similarity between their microbial communities. In contrast, the considerable distance between the modified PD and 5-FU groups indicated pronounced differences in the community structures (Figure 9K-P).
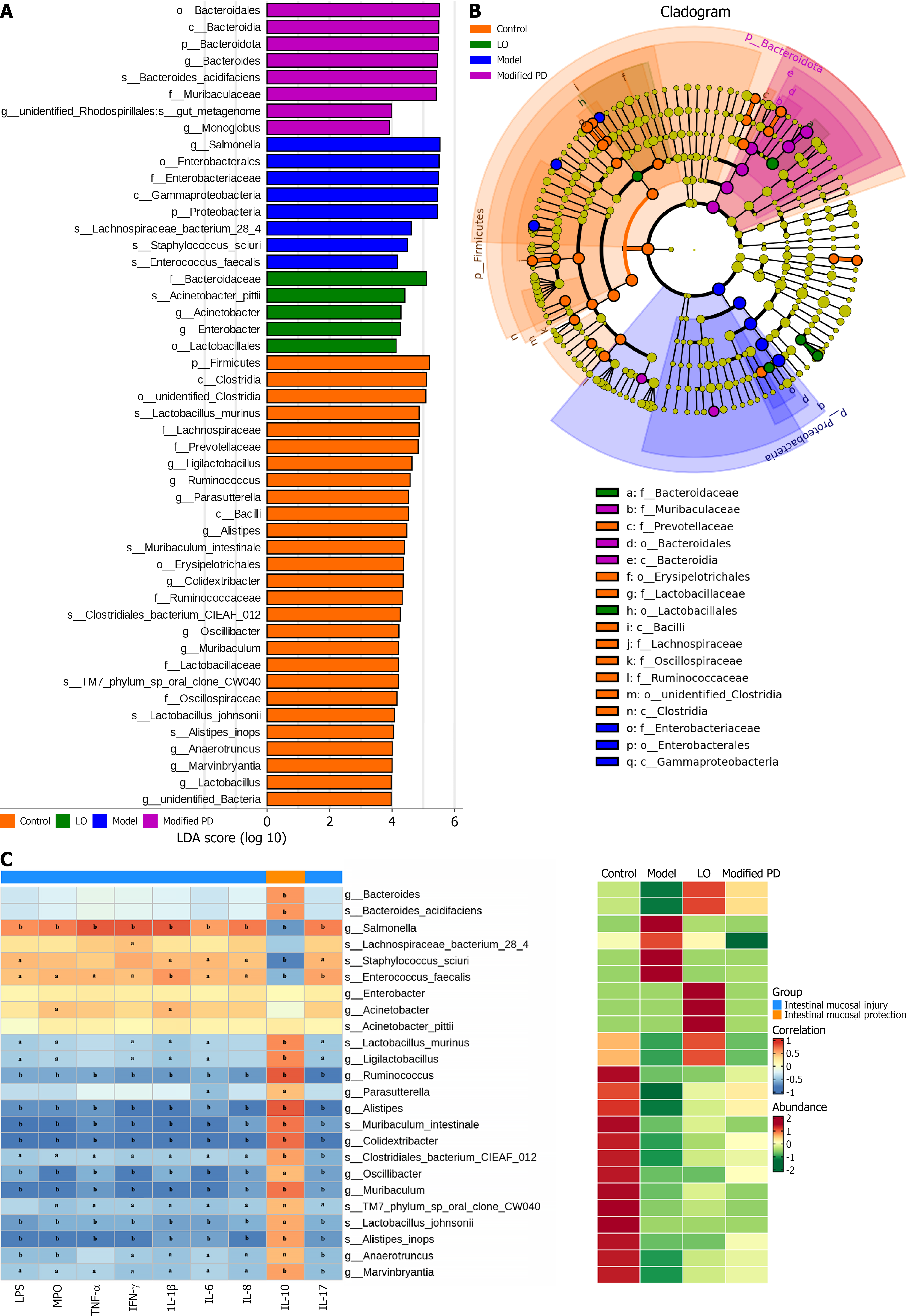
Gut microbiota and biomarker correlations
Using ASV-based LEfSe analysis with an LDA score threshold above four, we identified distinct microbial biomarkers across the groups. The control group displayed a diverse array of beneficial microbes, predominantly within the phylum Firmicutes, including genera such as Lactobacillus, Ruminococcus, and Alistipes. The modified PD group contained key beneficial biomarkers primarily within the phylum Bacteroidota, including Bacteroides and Bacteroides acidifaciens. In contrast, the 5-FU group was characterized by pathogenic microbes from the phylum Proteobacteria, including Salmonella, Staphylococcus sciuri, and Enterococcus faecalis (Figure 10A and B). Pearson correlation analysis of inflammatory mediators revealed that in the 5-FU group, specific microbes, including pathogenic bacteria such as Salmonella, Lachnospiraceae bacterium 28_4, Staphylococcus sciuri, and Enterococcus faecalis, were positively correlated with pro-inflammatory mediators and negatively correlated with anti-inflammatory cytokines. Modified PD treatment significantly reduced the prevalence of these pathogenic microbes, thereby alleviating the damage caused by inflammatory mediators. In the modified PD group, key beneficial microbes, including Bacteroides and Bacteroides acidifaciens, demonstrated positive associations with anti-inflammatory markers and low expression levels of inflammatory mediators (Figure 10C). These findings indicate that modified PD promoted the proliferation of beneficial microbes and reduced the number of pathogenic bacteria in IM mice, enhancing their resistance to inflammation.
Figure 10 The relationships between biological markers of the gut microbiota.
A and B: LDA value distribution bar chart (A) and cladogram (B) from LEfSe analysis; C: The correlation between these biomarkers and the relative abundance of gut microbiota at genus and species levels, with positive correlations shown in red and negative correlations in blue and green, respectively. Significance levels are marked as aP < 0.05, bP < 0.01).
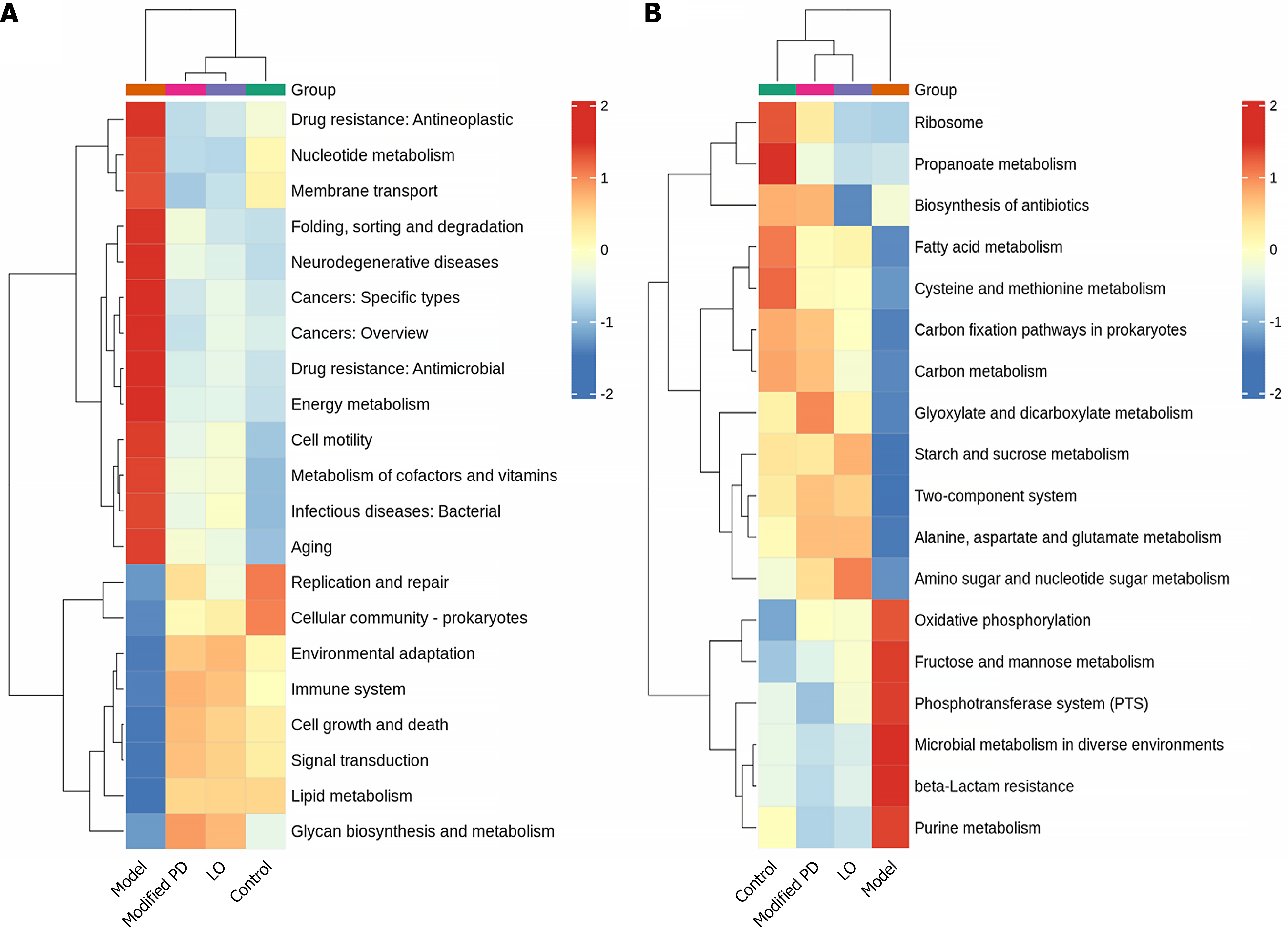
Modified PD may regulate gut microbiome metabolic functions
Metabolic pathway analysis using Tax4Fun2 revealed distinct profiles between groups. At the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway level 2, the control group primarily participated in key metabolic functions, such as ‘replication and repair,’ ‘cellular community - prokaryotes’ and ‘lipid metabolism,’ while the 5-FU group showed increased activity in pathways associated with ‘drug resistance: Antineoplastic’, ‘nucleotide metabolism’, ‘membrane transport’, ‘folding, sorting and degradation’, ‘neurodegenerative diseases’, ‘cancer’, ‘drug resistance: Antimicrobial’, ‘energy metabolism’, ‘cell motility’, ‘metabolism of cofactors and vitamins’, ‘infectious diseases: Bacterial’, and ‘aging’. Modified PD treatment realigned these metabolic profiles to levels closer to those of the control group (Figure 11A). At KEGG pathway level 3, the control group primarily engaged in metabolic pathways related to amino acid metabolism, including ‘amino sugar and nucleotide sugar metabolism’, ‘alanine, aspartate, and glutamate metabolism’, and ‘cysteine and methionine metabolism’. Pathways associated with lipid metabolism included ‘fatty acid metabolism’, ‘glyoxylate and dicarboxylate metabolism’, and parts of ‘carbon metabolism’ which contribute to fatty acid synthesis. Additional pathways with significant activity involve biosynthetic and cellular processes such as ‘two-component systems’, ‘starch and sucrose metabolism’, ‘antibiotic biosynthesis’, ‘carbon fixation in prokaryotes’, and ribosomal functions. In contrast, the model group exhibited elevated activity in pathways related to ‘oxidative phosphorylation’, ‘fructose and mannose metabolism’, ‘the phosphotransferase system’, ‘microbial metabolism in diverse environments’, ‘beta-lactam resistance’, and ‘purine metabolism’. Following modified PD treatment, the metabolic profile aligned more closely with that of the control group and effectively suppressed the active pathways in the model group, suggesting the potential metabolic regulatory effects of modified PD (Figure 11B).
Figure 11 Gut microbiota functional prediction.
A and B: Heatmaps generated by the Tax4Fun2 tool illustrate predicted metabolic pathways across groups at levels 2 (A) and 3 (B). LO: Loperamide; PD: Pulsatilla decoction.
DISCUSSION
5-FU has been the mainstay of chemotherapy for over six decades and is extensively used in the management of head and neck, breast, stomach, bladder, and colorectal cancers[34]. However, 5-FU not only targets cancer cells but also the rapidly dividing intestinal mucosal cells, leading to severe complications including IM[35]. IM is characterized by severe inflammation, ulceration of the intestinal mucosa, and debilitating diarrhea[36]. These symptoms substantially decrease the quality of life, diminish survival rates, and contribute to premature mortality in affected patients. Currently, IM treatments lack standardization of drug administration and primarily focus on symptom relief[37]. Therefore, it is imperative to identify novel pharmacological agents to prevent and treat of IM.
Modified PD is a TCM formula based on PD, enhanced with ejiao and licorice. This combination has been employed clinically for over 1800 years, particularly for treating weak-type patients with diarrhea and post-childbirth weakness, as documented in Essentials from the Golden Cabinet. Unlike conventional treatments that focus mainly on symptom relief, modified PD offers a holistic approach by relieving both the symptoms and basic causes of IM. The synergistic effects of its components, including PD, ejiao, and licorice, contribute to its multitargeted therapeutic actions. PD is primarily used to clear heat and stop dysentery, effectively alleviating severe abdominal pain and diarrhea and has shown efficacy in managing ulcerative colitis-related diarrhea[6,7,10]. Ejiao and licorice serve as both food and medicine, helping to nourish qi and blood and alleviate the fatigue and weakness that are usually exacerbated by chemotherapy-induced bone marrow suppression. Licorice possesses detoxifying, antiulcer, anti-inflammatory, and antioxidant properties[38]. Glycyrrhizic acid, another active component, can reduce inflammation and enhance the activities of antioxidant enzymes such as glutathione S-transferase and catalase[20]. Isoliquiritigenin, another active component, regulates the TNF/NF-κB pathway and suppresses intestinal inflammation, thereby improving symptoms in IM models[21]. Furthermore, ejiao is renowned for its blood-tonifying properties and has proven effective in reversing iron-deficiency anemia, strengthening the intestinal mucosal barrier, reshaping the microbiota ecology, and optimizing microbial structure, thus exerting anti-inflammatory, antioxidant, and anti-apoptotic effects[8,22], highlighting its significant therapeutic potential. This study confirmed that modified PD effectively mitigates key IM symptoms, such as weight loss, severe diarrhea, intestinal shortening, and mucosal pathology, positioning it as a comprehensive treatment option that could overcome the limitations of current mucositis therapies. These findings underscore the need for further exploration of the underlying therapeutic mechanisms.
Gut microbiota dysbiosis plays a key role in exacerbating IM by promoting ROS generation and activating the TLR4/MyD88/NF-κB pathway[39]. During the ulcerative stage, increased intestinal epithelial permeability and disruption of the mucosal barrier provide a conducive environment for colonization by Gram-negative bacteria. LPS from these bacteria can activate TLR4, leading to the recruitment of MyD88 and a TRAF6-associated signaling cascade, culminating in the release and nuclear translocation of NF-κB p65 subunit[26]. Once activated, NF-κB drives the transcription of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, intensifying mucosal inflammation and damage[40]. Our findings indicate that modified PD therapy holds significant promise for modulating the microbiota and inflammatory dysregulation observed in IM. The deterioration of IM is strongly associated with an increased presence of pathogenic species, such as Salmonella, Lachnospiraceae bacterium 28_4, Staphylococcus sciuri, and Enterococcus faecalis. An upsurge in Salmonella disrupts intestinal epithelial integrity and tight junctions, activating the LPS-mediated TLR4/MyD88/NF-κB pathway, which leads to an upregulation of inflammatory cytokines (TNF-α, IL-1β, and IL-6), and a downregulation of the anti-inflammatory cytokine IL-10, culminating in severe gastrointestinal symptoms including nausea, vomiting, abdominal pain, and diarrhea, along with significant dysbiosis[41]. Additionally, exposure to deoxynivalenol promotes the enrichment of Lachnospiraceae bacterium 28_4, exacerbating gastrointestinal disturbances and a spectrum of toxicities such as immunotoxicity, hematotoxicity, and myelotoxicity[42,43]. The presence of Enterococcus faecalis and Staphylococcus sciuri, both equipped with virulence factors that enable biofilm formation and antibiotic resistance, complicates cancer treatment, and produces exotoxins and enzymes that severely damage host tissues, leading to widespread infections and severe sepsis in immunocompromised hosts[44,45]. Our research aligns with these findings, showing that in IM mice, the LPS-mediated TLR4/MyD88/NF-κB pathway is significantly activated, with an associated upregulation of inflammatory mediators such as MPO, IFN-γ, TNF-α, IL-1β, IL-6, and IL-17, and a marked downregulation of the anti-inflammatory cytokine IL-10. The dominant microbiota in these mice correlated with amplified inflammatory responses. However, modified PD treatment resulted in a significant decrease in these microbial populations and effectively suppressed the LPS-mediated signaling pathway, thereby balancing pro- and anti-inflammatory responses and protecting the intestinal mucosal barrier in the IM.
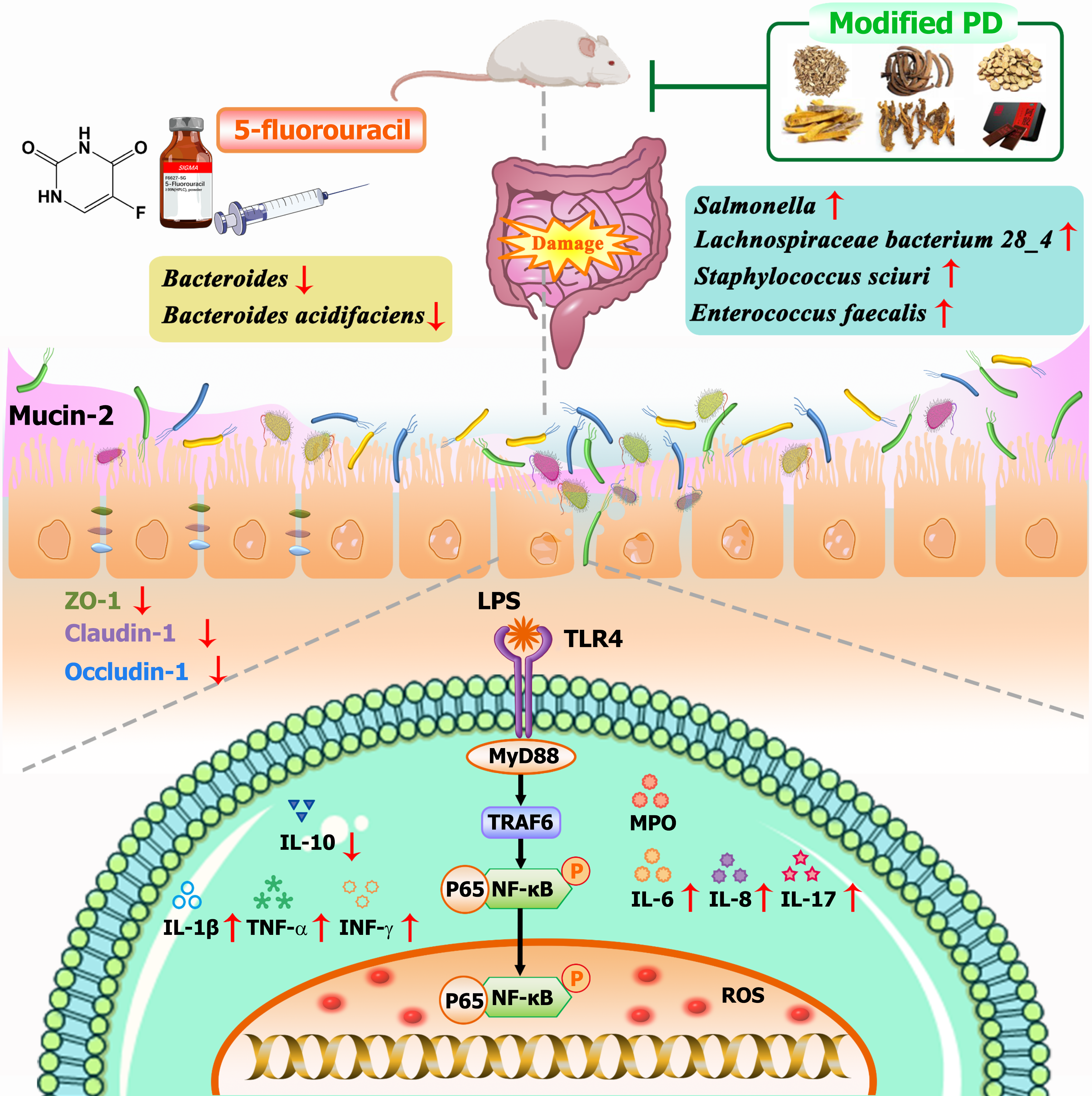
Bacteroides, as one of the predominant microbial genera in the human gut, plays a crucial role in the maintenance of intestinal health. It facilitates immune system regulation by decomposing complex carbohydrates to produce short-chain fatty acids, which help resist intestinal inflammatory disorders, reinforce the gut barrier, and stabilize the gut microbiota[46]. Bacteroides have shown therapeutic potential in ulcerative colitis, Crohn's disease, and diseases induced by Helicobacter hepaticus[47-49]. Specifically, Bacteroides acidifaciens, a novel probiotic, possesses outer vesicles enriched with functional proteins that facilitate mucosal barrier repair, relieve intestinal inflammatory disorders, attenuate microbiota imbalance, and effectively mitigate chemotherapy- and radiotherapy-induced IM[50]. In this study, Bacteroides and Bacteroides acidifaciens, key microbes in the modified PD group, were positively correlated with anti-inflammatory effects but were underexpressed in IM mice. The modified PD treatment significantly increased their relative abundance, effectively combating intestinal inflammation. Tax4fun2 microbial prediction further supports the involvement of these microbes in pathways related to antioxidant, anti-inflammatory, and immune-enhancing activities. Therefore, modified PD may inhibit IM development by blocking the LPS-mediated TLR4/MyD88/NF-κB pathway, enhancing mucosal barrier function, and reprogramming the gut microbiota (Figure 12).
Figure 12 The mechanism of modified Pulsatilla decoction in intestinal mucositis mitigation.
LPS: Lipopolysaccharide; ROS: Reactive oxygen species.
Intestinal mucosal barrier dysfunction is a hallmark of IM, with tight junction proteins and mucins essential for maintaining selective permeability and preventing the translocation of toxins, pathogens, and inflammatory mediators[51,52]. 5-FU chemotherapy compromises intestinal epithelial integrity by downregulating tight junction proteins and mucins, thereby increasing permeability and promoting dysbiosis, which exacerbates IM[53]. In this study, we observed significant downregulation of occludin-1, claudin-1, ZO-1, and mucin-2 in the small intestine of IM mice, confirming barrier disruption. In contrast, treatment with modified PD significantly upregulated these proteins, suggesting that modified PD restores mucosal barrier function and alleviates 5-FU-induced epithelial damage. These findings indicate that modified PD not only modulates inflammatory responses but also preserves intestinal integrity, offering a comprehensive therapeutic strategy for IM.
This study utilized a 5-FU chemotherapy-induced IM model in BALB/c mice to demonstrate that modified PD alleviates IM by modulating the TLR4/MyD88/NF-κB signaling pathway and restoring gut microbiota balance. Previous studies showed that the components of modified PD not only reduce chemotherapy-induced toxicities without compromising therapeutic efficacy but also enhance the oral bioavailability of chemotherapeutic agents and may even potentiate chemotherapy effects. For instance, coptidis rhizoma nanoparticles improve the oral delivery of docetaxel and exhibit potent cytotoxicity against tumor cell lines[54]. Oleanolic acid sensitizes chemotherapy through the PI3K/AKT/mTOR pathway and by upregulating the p53-mediated apoptosis pathway, inducing cancer cell apoptosis while alleviating cisplatin-induced hepatotoxicity[55,56]. Amino acids trigger a rapid ROS burst, enhancing the efficacy of cisplatin[57]. Additionally, other components in modified PD exhibit significant anti-tumor activity. Esculin inhibits the progression of colorectal cancer by regulating the PERK/eIF2α/CHOP and Nrf2/HO-1 pathways[58]. The glycyrrhizin derivative, wighteone inhibits Akt allosterically, resulting in cell cycle arrest and apoptosis in colorectal cancer cells[59]. The major component of coptis, berberine, upregulates miR-17-5p and disrupts the JAK1-STAT3 signaling pathway to suppress bladder cancer cell proliferation[60]. Thus, the diverse components of modified PD exhibit favorable safety profiles, mitigate chemotherapy side effects, potentiate chemotherapy efficacy, and possess intrinsic anti-tumor activity. Given that this study primarily focused on the potential of modified PD to alleviate IM induced by 5-FU chemotherapy, a monotherapy control group was not included. Future research should evaluate its monotherapy anti-tumor effects in tumor-bearing mice and explore its potential for synergistic effects when combined with chemotherapy. Furthermore, genomic analysis should focus on potential regulatory molecules or target molecules (such as Nrf2/Keap1/HO-1 and TGF-β/MAPK) to identify new therapeutic targets[41,61]. Additional studies should investigate the broader therapeutic potential of modified PD, including its antimicrobial, anti-resistance properties, and regulation of lipid and amino acid metabolic pathways, which will enhance its application in IM management and provide theoretical support for related treatments.