Published online Feb 21, 2025. doi: 10.3748/wjg.v31.i7.100051
Revised: December 5, 2024
Accepted: December 23, 2024
Published online: February 21, 2025
Processing time: 167 Days and 2.4 Hours
Colorectal cancer is the third most common malignancy and the fourth leading cause of cancer-related deaths worldwide. Several studies have shown an association between gut microbiota and colorectal cancer. Gut microbiota is unique and can be influenced by geographic factors and habits. This study aimed to determine the diversity and composition of colonic mucosal microbiota in patients with and without colorectal cancer.
To determine the diversity and composition of colonic mucosal microbiota in patients with and without colorectal cancer in Indonesia.
This case-control study included 59 subjects (35 colorectal cancer patients and 24 non-colorectal cancer patients indicated for colonoscopy at Dr. Cipto Mangunkusumo Gastrointestinal Endoscopy Center and Fatmawati Hospital. Microbiota examination was performed using 16S rRNA sequencing. Bioinformatics analysis was performed using the wf-metagenomics pipeline from EPI2Me-Labs (Oxford Nanopore Technologies platform).
Patients with colorectal cancer had a higher median index value on the Shannon index (3.28 vs 2.82, P > 0.05) and a lower value on the Simpson index (0.050 vs 0.060, P > 0.05). Significant differences in beta diversity were observed at the genus (P = 0.002) and species levels (P = 0.001). Firmicutes, Proteobacteria, Bacteroidetes, and Fusobacteria were the dominant phyla. The genera Bacteroides, Campylobacter, Peptostreptococcus, and Parvimonas were found more frequently in colorectal cancer, while Faecalibacterium, Haemophilus, and Phocaeicola were more frequently found in non-colorectal cancer. The relative abundance of Fusobacterium nucleatum, Bacteroides fragilis, Enterococcus faecalis, Campylobacter hominis, and Enterococcus faecalis species was significantly elevated in patients with colorectal cancer. Meanwhile, Faecalibacterium prausnitzii, Faecalibacterium duncaniae, and Prevotella copri were more commonly found in non-colorectal cancer.
Patients with colorectal cancer exhibit distinct differences in the composition and diversity of their colonic mucosal microbiota compared to those with non-colorectal cancer. This study was reviewed and approved by the Ethics Committee of Faculty of Medicine, Universitas Indonesia (No. KET-1517/UN2.F1/ETIK/PPM.00.02/2023).
Core Tip: Previous studies have reported several specific microbiotas with colorectal cancer. However, these studies mainly involved fecal samples instead of colonic mucosal samples and thus could be biased. In addition, studies from Indonesian patients are still very limited and the results may differ from other countries. This study showed that there was a significant difference in the microbiota composition and diversity between patients with colorectal cancer and non-colorectal cancer in Indonesia. These results could potentially be used as a diagnostic and prognostic biomarker for colorectal cancer.
- Citation: Darnindro N, Abdullah M, Sukartini N, Rumende CM, Pitarini A, Nursyirwan SA, Fauzi A, Makmun D, Nelwan EJ, Shatri H, Rinaldi I, Tanadi C. Differences in diversity and composition of mucosa-associated colonic microbiota in colorectal cancer and non-colorectal cancer in Indonesia. World J Gastroenterol 2025; 31(7): 100051
- URL: https://www.wjgnet.com/1007-9327/full/v31/i7/100051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i7.100051
Colorectal cancer is a malignancy of the gastrointestinal system that affects the colon and rectum. It is the third most common malignancy and the fourth leading cause of cancer-related deaths worldwide[1]. Globally, the mortality rate for colorectal cancer reached 8.9 per 100000 individuals[2]. The pathogenesis of colorectal cancer is influenced by various factors, including environmental and genetic factors. Genetic factors are hypothesized to account for only 10%-15% of all colorectal cancer cases, suggesting that environmental factors also play a significant role in carcinogenesis[3]. Among these, the gut microbiota has recently been implicated as a contributing factor.
The gut microbiota plays a vital role in gastrointestinal metabolism. Alterations in its composition, known as dysbiosis, involve changes in the abundance, diversity, or function of microbiota, potentially disrupting homeostasis. Dysbiosis could lead to impaired metabolic processes of the gut, compromised immune function, and induction of chronic inflammation, ultimately triggering tumor development[4].
The gastrointestinal system, in particular, the colon is an organ that houses thousands to millions of microbes, including both commensal and pathogenic species. Several studies have reported alterations in gut microbiota composition in colorectal cancer[5,6]. These alterations include increased levels of Bacteroides fragilis, Escherichia coli, Enterococcus faecalis, Streptococcus gallolyticus, Fusobacterium nucleatum, and Peptostreptococcus in patients with colorectal cancer[3]. In addition, Yusuf et al[7] have also reported decreased amounts of Bifidobacterium in colorectal cancer[7].
These findings highlight a potential link between microbiota and colorectal cancer. However, differences in microbiota composition among individuals and across regions of the colon remain a challenge, as do discrepancies in bacterial abundance between luminal/fecal samples and mucosal/tissue samples. In Indonesia, research on microbiota patterns in colorectal cancer is limited, particularly studies focusing on colonic mucosa or tissue samples. Thus, a study that evaluates the microbiota in colonic mucosa is necessary to identify microbiota changes that could be used as prognostic biomarkers, guide treatment strategies, as well as elucidate the pathogenesis of colorectal cancer. This study aimed to determine the diversity and composition of colonic mucosal microbiota in patients with and without colorectal cancer.
This case-control study was conducted from October 2023 to April 2024 at Dr. Cipto Mangunkusumo Gastrointestinal Endoscopy Center and Fatmawati Hospital. It included patients aged > 18 years who were indicated for colonoscopy owing to suspected colorectal cancer characterized by symptoms such as bloody diarrhea, abdominal pain, unexplained decrease in hemoglobin, chronic diarrhea, or constipation.
Exclusion criteria included patients with conditions such as erosion, ulcers, hyperemia, fistulas, abscesses, ade
The sample size was determined using the formula for comparing the means of two independent groups (colorectal cancer vs non-colorectal cancer). Based on the calculations, the minimal sample required was 25 patients per group or 50 patients in total.
Colonic mucosal samples from the ascending and sigmoid colon were obtained from patients with both colorectal and non-colorectal cancer. The samples were kept in fecal collecting tubes at −80 °C for 24 h. Deoxyribonucleic acid (DNA) extraction was performed using kit QIAamp DNA mini kit (cat No. 51304), with 10 ng of DNA extracted for library preparation. Sequencing was conducted using the Oxford Nanopore Technologies platform. Library preparation was conducted using 16S barcoding kit (cat No. SQK-RAB204) following the manufacturer’s protocol, which included the amplification of target 16S regions and ligation with sequencing adapters before placement in the flow cell (cat No. FLO-MIN106D). Sequencing outputs were generated in FASTQ format and analyzed using the wf-metagenomics pipeline from EPI2ME-Labs (Oxford Nanopore Technologies).
Subsequent data processing was performed using R software and statistical product and service solutions. A generalized linear model with gamma distribution and log link was used to adjust P values for potential confounding variables, including age and gender. This study received ethical approval from the Ethics Committee of Faculty of Medicine, Universitas Indonesia (No. KET-1517/UN2.F1/ETIK/PPM.00.02/2023), and informed consent was obtained from all individual participants.
This case-control study included 59 participants (35 with colorectal cancer and 24 with non-colorectal cancer). The median age was higher in the colorectal cancer group compared to the non-colorectal cancer group (61 years [minimum-maximum: 18-79 years] vs 47 years [minimum-maximum: 21-74 years], P = 0.002). The colorectal cancer group had a higher proportion of male participants compared to non-colorectal cancer group (62.9% vs 41.7%, P = 0.181). Hypertension and diabetes were reported in 25.7% and 14.3% of the patients in the colorectal cancer group, respectively. The distribution of patients with colorectal cancer patients tumor node metastasis stages 1, 2, 3, and 4 was 11.1%, 14.3%, 42.9%, and 31.4%, respectively. The left side of the colon was the most common tumor location in the colorectal cancer group (80%). In the non-colorectal cancer group, the most frequent diagnoses were hemorrhoids (50%), followed by polyp (20.8%), microscopic colitis (16.7%), and irritable bowel syndrome (12.5%). As many as 81.4% of the participants consumed at least one serving of vegetables and fruits daily. While most participants (78.2%) consumed meat in their diet, only 8.5% consumed 100-500 g/day of red meat. Additionally, 44.1% of the participants consumed instant noodles at least 1-2 times per month. The characteristics of the study participants are shown in Table 1.
| Variable | Colorectal cancer (n = 35) | Non-colorectal cancer (n = 24) | Total (n = 59) | P value |
| Age, median (minimum-maximum,) | 61 (18-79) | 47 (21-74) | 55 (18-79) | 0.002a |
| Age (yr) | ||||
| < 30 | 2 (5.7) | 2 (8.3) | 4 (6.8) | 0.012a |
| 30-39 | 2 (5.7) | 6 (25.0) | 8 (13.6) | |
| 40-49 | 1 (2.9) | 6 (25.0) | 7 (11.9) | |
| 50-59 | 10 (28.6) | 4 (16.7) | 14 (23.7) | |
| 60-69 | 13 (37.1) | 5 (20.8) | 18 (30.5) | |
| ≥ 70 | 7 (20.0) | 1 (4.2) | 8 (13.6) | |
| Gender | ||||
| Male | 22 (62.9) | 10 (41.7) | 32 (54.2) | 0.181 |
| Female | 13 (37.1) | 14 (58.3) | 27 (45.8) | |
| Body mass index (kg/m2) | 21.22 ± 3.26 | 22.4 ± 4.20 | 22.1 ± 3.68 | 0.222 |
| Hypertension | ||||
| Yes | 9 (25.7) | 3 (12.5) | 12 (20.3) | 0.326 |
| No | 26 (74.3) | 21 (87.5) | 47 (79.7) | |
| Diabetes mellitus | ||||
| Yes | 5 (14.3) | 5 (20.8) | 10 (16.9) | 0.725 |
| No | 30 (85.7) | 19 (79.2) | 49 (83.1) | |
| Smoking history | ||||
| Active smoker | 0 (0) | 3 (12.5) | 3 (5.1) | 0.075 |
| Stopped smoking | 17 (48.6) | 8 (33.3) | 25 (42.4) | |
| No smoking history | 18 (51.4) | 13 (54.2) | 31 (52.5) | |
| Alcohol history | ||||
| Yes | 7 (20) | 4 (16.7) | 11 (18.6) | 1.000 |
| No | 28 (80) | 20 (83.3) | 48 (81.4) | |
| Vegetable and fruit consumption | ||||
| Almost never | 5 (14.3) | 6 (25.0) | 11 (18.6) | 0.569 |
| 1-2 servings per day | 26 (74.3) | 16 (66.7) | 42 (71.2) | |
| 3-4 servings per day | 4 (11.4) | 2 (8.3) | 6 (10.2) | |
| Red meat consumption | ||||
| < 100 g/day | 30 (85.7) | 24 (100.0) | 54 (91.5) | 0.073 |
| 100-105 g/day | 5 (14.3) | 0 (0.0) | 5 (8.5) | |
| Instant food consumption | ||||
| Never | 14 (40.0) | 9 (37.5) | 23 (39.0) | 0.579 |
| 1-2/month | 17 (48.6) | 9 (37.5) | 26 (44.1) | |
| 3-4/month | 2 (5.7) | 3 (12.5) | 5 (8.5) | |
| 2-3/week | 2 (5.7) | 3 (12.5) | 5 (8.5) | |
| Grilled meat consumption | ||||
| Rarely/never | 31 (88.6) | 19 (79.2) | 50 (84.7) | 0.464 |
| 1-2 servings per week | 4 (11.4) | 24 (20.8) | 9 (15.3) | |
| Colorectal cancer stage | ||||
| I | 4 (11.4) | |||
| II | 5 (14.3) | |||
| III | 15 (42.9) | |||
| IV | 11 (31.4) | |||
| Tumor location | ||||
| Left (rectum, sigmoid, descending) | 28 (80) | |||
| Right (transversum, ascending, caecum) | 7 (20) | |||
| Non-colorectal cancer | ||||
| Hemorrhoid | 12 (50.0) | |||
| Microscopic colitis | 4 (16.7) | |||
| NICE I polyps | 5 (20.8) | |||
| Irritable bowel syndrome | 3 (12.5) |
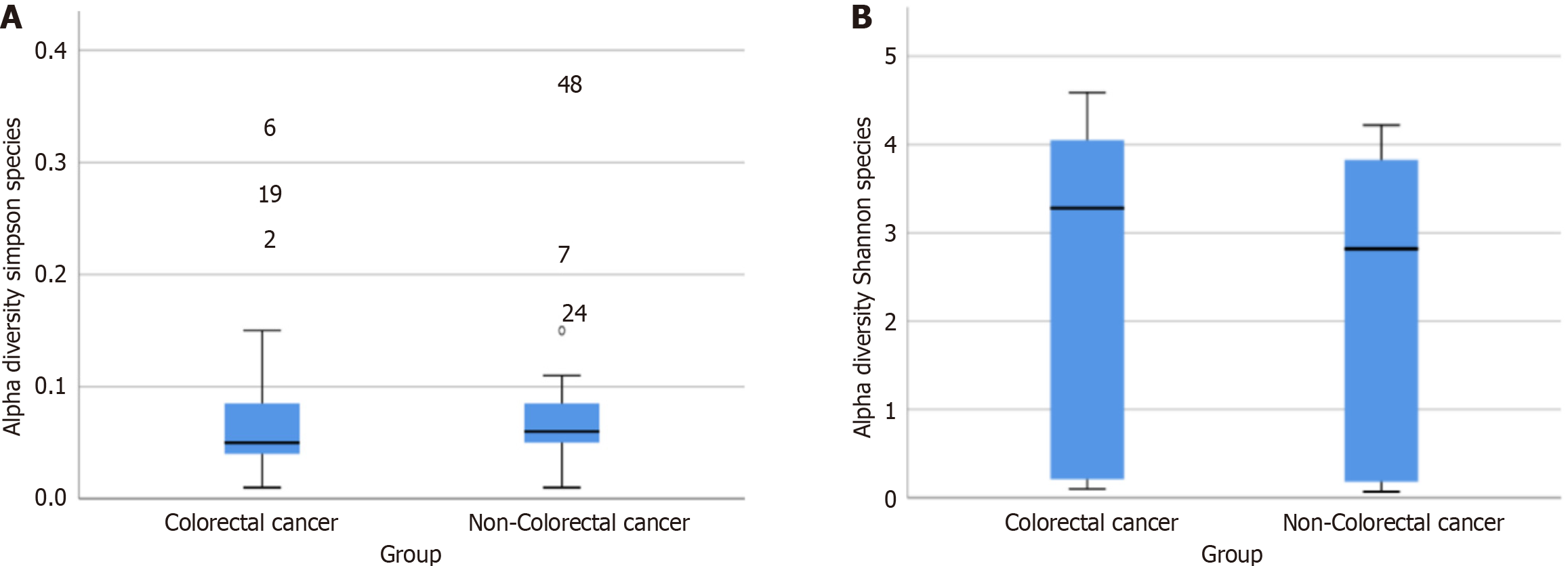
Alpha diversity, which measures the richness and evenness of microbiota within a sample, was evaluated using the Shannon Diversity Index and Simpson Diversity Index. Based on the Shannon index, the colorectal cancer group exhibited a higher median diversity compared to the non-colorectal cancer group (3.28 vs 2.82, P > 0.05, Figure 1A). Conversely, the opposite was observed on the Simpson index (0.050 vs 0.060, P > 0.05, Figure 1B). Although these differences were not statistically significant, there was a trend toward higher alpha diversity in patients with colorectal cancer compared to those with non-colorectal cancer.
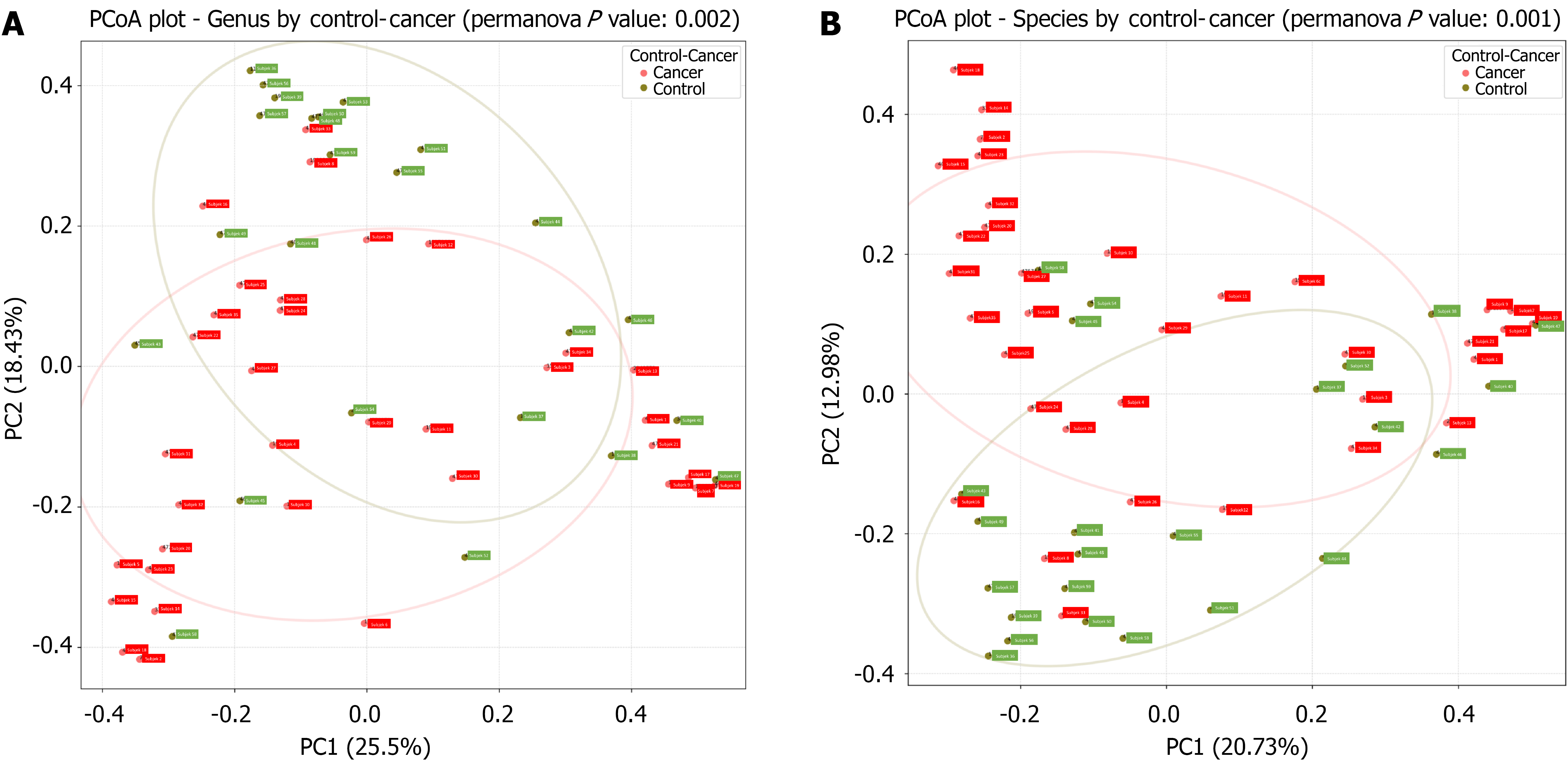
Beta diversity was assessed using principal coordinate analysis (PCoA) with Bray-Curtis dissimilarity (Figure 2). Statistical significance was determined via permutational multivariate analysis of variance conducted in R software. The PCoA analysis was conducted using an R script with the phyloseq (version 1.44.0) package, and the results were visualized accordingly. Beta diversity analysis incorporated taxonomy ranks, phylogenetic distance, and microbiota abundance present in the samples.
The PCoA analysis was conducted based on the taxonomic ranks. In the resulting plot, the red circles represent patients with colorectal cancer, while the green circles represent those with non-colorectal cancer. As shown in Figure 2, distinct clusters were observed between the two groups, with certain clusters unique to either of the groups. This separation was statistically significant at both the genus level (P = 0.002) and species level (P = 0.001).
The gut microbiota composition was evaluated using relative abundance metrics. Taxonomic rank-based abundance analysis was performed using the wf-metagenomics workflow (v2.2.0-g356d8a9), which was executed within a Docker container (v23.0.4) using Nextflow (v23.04.3) and default parameters. Taxonomy classification and abundance results were visualized through tables and stacked bar plots.
The composition analysis was conducted at the phylum, family, genus, and species taxonomic levels. From 59 analyzed samples, a total of 1512980 fragments with an average length of 1532 base pairs were obtained. A total of 38 phyla and 188 genera were identified across the samples.
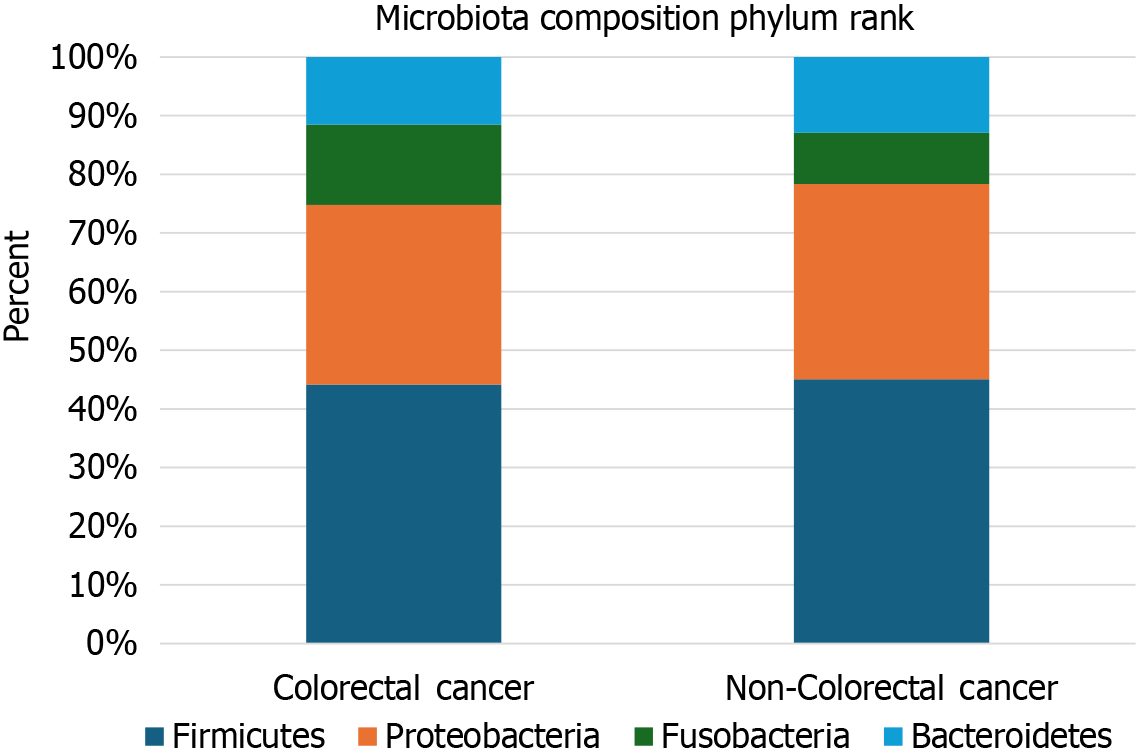
The four most abundant phyla were Firmicutes, Proteobacteria, Bacteroidetes, and Fusobacteria. The mean relative abundance for Phylum Firmicutes across all samples was 40.89%. The mean relative abundance for Phyla Proteobacteria, Bacteroidetes, and Fusobacteria was 29.13%, 11.1%, and 10.7%, respectively (Figure 3).
Elevated levels of Firmicutes and Bacteroidetes were observed in patients with non-colorectal cancer, while Proteobacteria and Fusobacteria were more abundant in patients with colorectal cancer. However, these differences were not statistically significant (Table 2).
| Microbiota | Colorectal cancer, median (minimum-maximum) (n = 35) | Non-colorectal cancer, median (minimum-maximum) (n = 24) | P value | Adjusted P value |
| Firmicutes | 38.4 (4.8-78.9) | 42.1 (0.5-78.3) | 0.84 | 0.83 |
| Proteobacteria | 21.5 (0.9-91.9) | 21.1 (2.67-95.6) | 0.46 | 0.90 |
| Fusobacteria | 4.03 (0-56.9) | 0.06 (0-82.6) | 0.09 | 0.43 |
| Bacteroidetes | 9.83 (0.2-36.9) | 11.0 (0.67-33.1) | 0.45 | 0.68 |
At the family level, variations in microbiota composition were noted. Eight dominant families were identified: Enterobacteriaceae, Lachnospiraceae, Oscillospiraceae, Fusobacteriaceae, Prevotellaceae, Bacteroidaceae, Veillonellaceae, and Clostridiaceae (Table 3). Among these, Oscillospiraceae exhibited a higher mean relative abundance in patients with non-colorectal cancer, a result that remained consistent even after adjusting for age and gender. Conversely, Clostridiaceae showed a higher mean relative abundance in patients with colorectal cancer, although this difference was not statistically significant following adjustment for age and gender.
| Microbiota | Colorectal cancer, median (minimum-maximum) (n = 35) | Non-colorectal cancer, median (minimum-maximum) (n = 24) | P value | Adjusted P value |
| Enterobacteriaceae | 6.53 (0-65.65) | 8.83 (0-63.87) | 0.55 | 0.94 |
| Lachnospiraceae | 11.38 (0.32-61.55) | 9.57 (0.02-44.69) | 0.62 | 0.43 |
| Oscillospiraceae | 4.72 (0.0149.15) | 21.29 (0.01-44.40) | 0.01a | 0.009a |
| Fusobacteriaceae | 4.03 (0-54.94) | 0.06 (0-82.59) | 0.18 | 0.69 |
| Prevotellaceae | 1.94 (0-31.06) | 1.31 (0-28.43) | 0.65 | 0.16 |
| Bacteroidaceae | 2.70 (0.01-16.19) | 2.46 (0-15.27) | 0.68 | 0.30 |
| Veillonellaceae | 1.29 (0-27.40) | 0.56 (0-12.40) | 0.56 | 0.06 |
| Clostridiaceae | 1.20 (0.02-10.15) | 0.65 (0.02-36.03) | 0.02a | 0.11 |
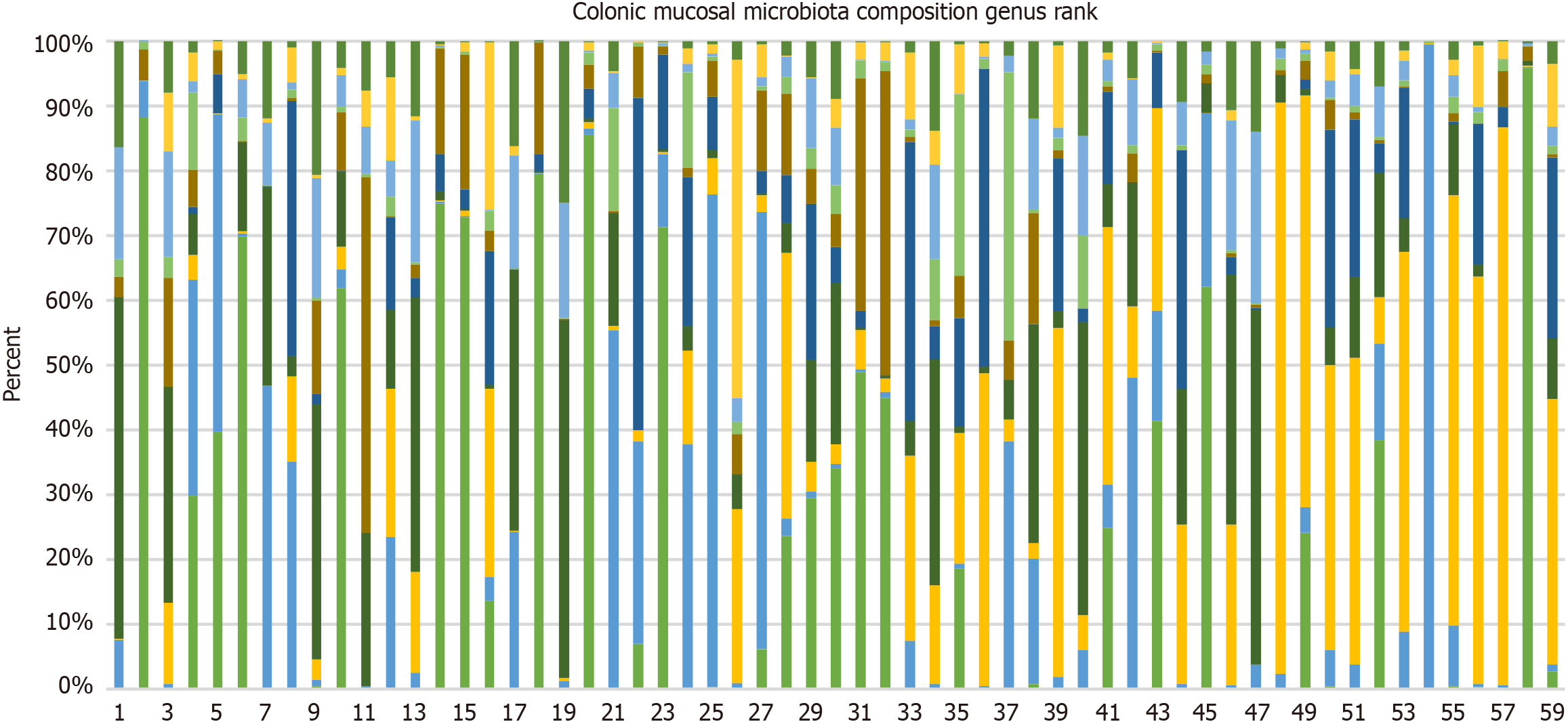
The 10 most common genera identified across all samples were Fusobacterium, Faecalibacterium, Citrobacter, Prevotella, Bacteroides, Clostridium, Kluyvera, Vescimonas, Franconibacter, and Phocaecola. In addition to these, an analysis was conducted on five genera that have been previously associated with colorectal cancer in earlier studies: Campylobacter, Klebsiella, Haemophilus, Peptostreptococcus, and Parvimonas. Figure 4 illustrates the differences in relative abundance at the genus level across samples, with subjects 1-35 being diagnosed with colorectal cancer, and subjects 37-59 being diagnosed with non-colorectal cancer. As shown in Figure 4, Fusobacterium had the highest abundance in the colorectal cancer group. Meanwhile, Faecalibacterium, unknown Bacteria, Fusobacterium, Citrobacter, and Prevotella were the most common genera in patients with non-colorectal cancer.
At the genus level, a statistically significant difference in microbiota composition was observed between the two groups. Bacteroides, Campylobacter, Peptostreptococcus, and Parvimonas were elevated in colorectal cancer, whereas Faecalibacterium, Haemophilus, and Phocaeicola were elevated in non-colorectal cancer (Table 4). These results remained consistent after adjustment for age and gender.
| Microbiota | Colorectal cancer, median (minimum-maximum) (n = 35) | Non-colorectal cancer, median (minimum-maximum) (n = 24) | P value | Adjusted P value |
| Fusobacterium | 4.12 (0-55.0) | 0.06 (0-82.6) | 0.17 | 0.67 |
| Faecalibacterium | 1.15 (0-13.3) | 17.6 (0-41.8) | < 0.001b | < 0.001b |
| Citrobacter | 2.13 (0-23.5) | 3.32 (0-24.1) | 0.43 | 0.73 |
| Prevotella | 1.48 (0-30.7) | 1.34 (0-21.2) | 0.56 | 0.17 |
| Bacteroides | 2.11 (0-16.3) | 0.37 (0-7.70) | 0.02a | 0.03a |
| Clostridium | 0.63 (0-10.2) | 0.60 (0.02-36.3) | 0.90 | 0.48 |
| Kluyvera | 0.82 (0-10.2) | 1.48 (0-11.7) | 0.45 | 0.62 |
| Vescimonas | 0.48 (0-20.6) | 0.36 (0-5.34) | 0.13 | 0.21 |
| Franconibacter | 0.69 (0-10.6) | 0.94 (0-6.22) | 0.61 | 0.85 |
| Phocaeicola | 0.18 (0-6.27) | 1.28 (0-12.4) | 0.03a | < 0.001b |
| Campylobacter | 0.06 (0-23.5) | 0 (0-0.05) | < 0.001b | < 0.001b |
| Klebsiella | 0.51 (0-5.90) | 0.41 (0-2.18) | 0.76 | 0.16 |
| Haemophilus | 0.01 (0-5.02) | 0.34 (0-12.8) | 0.003a | < 0.001b |
| Peptostreptococcus | 0.07 (0-9.41) | 0 (0-0.20) | < 0.001b | < 0.001b |
| Parvimonas | 0.06 (0-6.13) | 0 (0-0.24) | < 0.001b | 0.005a |
Differences in microbiota composition were also observed at the species level (Table 5). Specifically, Fusobacterium nucleatum, Bacteroides fragilis, Parvimonas micra, Peptostreptococcus stomatis, Enterococcus faecalis, and Campylobacter hominis exhibited higher relative abundances in patients with colorectal cancer. Conversely, Faecalibacterium prausnitzii, Haemophilus parainfluenzae, and Prevotella copri showed higher relative abundances in patients with non-colorectal cancer. These differences were statistically significant for all species, even after adjustment for age and gender, with the exception of Parvimonas micra.
| Microbiota | Colorectal cancer, median (minimum-maximum) (n = 35) | Non-colorectal cancer, median (minimum-maximum) (n = 24) | P value | Adjusted P value |
| Citrobacter koseri | 2.49 (0-26.9) | 3.67 (0-26.7) | 0.51 | 0.83 |
| Faecalibacterium prausnitzii | 0.20 (0-8.90) | 3.92 (0-17.5) | 0.001b | < 0.001b |
| Prevotella copri | 0.02 (0-17.5) | 1.10 (0-19.6) | 0.04a | 0.003a |
| Bacteroides fragilis | 0.68 (0-15.2) | 0.03 (0-7.89) | 0.002a | 0.01a |
| Haemophilus parainfluenzae | 0 (0-5.38) | 0.37 (0-11.5) | 0.004a | 0.47 |
| Fusobacterium nucleatum | 0.07 (0-44.6) | 0 (0-0.16) | 0.003a | < 0.001b |
| Prevotella hominis | 0 (0-1.06) | 0.04 (0-1.31) | 0.05 | 0.14 |
| Parvimonas micra | 0.01 (0-6.94) | 0 (0-0.27) | 0.001a | 0.07 |
| Peptostreptococcus stomatis | 0.01 (0-9.81) | 0 (0-0.20) | < 0.001b | 0.68 |
| Enterococcus faecalis | 0 (0-12.3) | 0 (0-0.67) | 0.004a | < 0.001b |
| Campylobacter hominis | 0 (0-5.45) | 0 (0-0.06) | 0.008a | 0.002a |
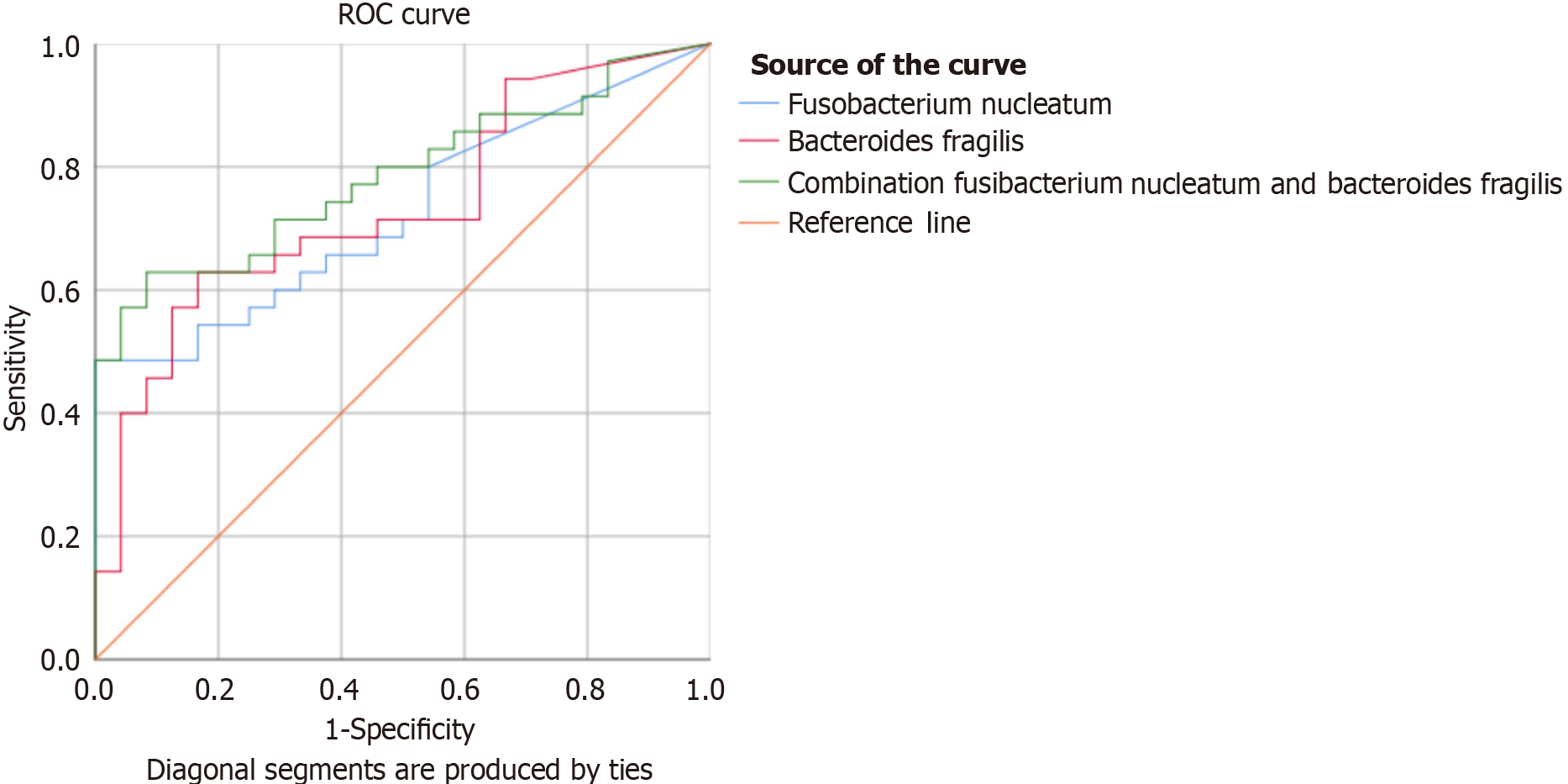
The potential utility of Fusobacterium nucleatum and Bacteroides fragilis, alone and in combination, as diagnostic markers for colorectal cancer in Indonesia was also evaluated. The analysis of 59 samples revealed area under the curve (AUC) values of 0.727 [95% confidence interval (CI: 0.600-0.853)] for Fusobacterium nucleatum, 0.735 (95%CI: 0.607-0.862) for Bacteroides fragilis, and 0.786 (95%CI: 0.671-0.900) for their combination (Table 6 and Figure 5). The cut-off points for the relative abundance of Fusobacterium nucleatum and Bacteroides fragilis were ≥ 0.0117 and ≥ 0.0836, respectively. The combination of these bacteria achieved a sensitivity of 82.8%, specificity of 50%, positive predictive value of 70.7%, and negative predictive value of 66.7%.
| Parameters | Fusobacterium nucleatum | Bacteroides fragilis | Combination of Fusobacterium nucleatum and Bacteroides fragilis |
| AUC (95%CI) | 0.727 (0.600-0.853) | 0.735 (0.607-0.862) | 0.786 (0.671-0.900) |
| P value | 0.003 | 0.002 | < 0.001b |
| Cut-off value | ≥ 0.0117 | ≥ 0.0836 | Fusobacterium nucleatum ≥ 0.0117; Bacteroides fragilis ≥ 0.0836 |
| Sensitivity | 65.7 (47.8-80.9) | 68.6 (50.7-83.2) | 82.8 (66.4-93.4) |
| Specificity | 62.5 (40.6-81.9) | 66.7 (44.7-84.4) | 50 (29.1-70.9) |
| Positive likelihood ratio | 1.75 | 2.06 | 1.66 |
| Negative likelihood ratio | 0.55 | 0.47 | 0.34 |
| Positive predictive value | 71.9 (59.1-81.9) | 75.0 (62.1-84.7) | 70.7 (61.2-78.8) |
| Negative predictive value | 55.6 (41.8-68.5) | 59.3 (45.3-71.9) | 66.7 (46.6-82.1) |
| Accuracy | 64.4 (50.9-76.5) | 67.8 (54.4-79.4) | 69.5 (56.1-80.8) |
The gut microbiota has been implicated in the pathogenesis of colorectal cancer. The study by Ahn et al[8] reported an association between specific colonic microbiota species and the incidence of colorectal cancer. However, a limitation of their study was the use of fecal samples rather than samples taken directly from the colonic mucosa. Some commonly associated bacterial species include Bacteroides fragilis, Escherichia coli, Enterococcus faecalis, and Streptococcus gallolyticus. Other bacterial strains frequently detected in tumor tissues and mucosa include Fusobacterium nucleatum, Parvimonas, Peptostreptococcus, Porphyromonas, and Prevotella[3].
Some researchers hypothesize that gut microbiota not in direct contact with the colonic mucosa may lack the ability to directly stimulate carcinogenesis. Nevertheless, these microbiota can potentially contribute indirectly to carcinogenesis through metabolic alterations, particularly in the context of obesity and a Western diet. Despite these indirect effects, direct contact between gut microbiota and the colonic mucosa appears essential for the initiation and progression of colorectal cancer via mechanisms such as inflammation and DNA damage. Saffarian et al[6] noted differences in the composition of microbiota between intraluminal feces and the colonic mucosa. This finding underscores the limitation of relying on fecal microbiota analysis as a proxy for the microbiota present in the colonic mucosa.
Saffarian et al[6] identified specific oral bacteria in the colonic mucosa and crypts that may contribute to carcinogenesis in colorectal cancer. These bacteria are not only associated with colorectal cancer but also with gastric and pancreatic cancers. It is hypothesized that colonization of the colon by these oral bacteria may lead to epithelial damage and promote carcinogenesis. The primary bacteria implicated are Fusobacterium and Parvimonas micra. Fusobacterium nucleatum can bind to and invade epithelial cells via the Fusobacterium nucleatum adhesin (FadA), triggering the production of reactive oxygen species, transcription factors, Wnt signaling, and inflammatory proteins, all of which could facilitate carcinogenesis. Similarly, Chen et al[5] also reported variations in the abundance and composition of microbiota in tissues obtained from mucosal biopsies. For instance, the Phylum Firmicutes, which derives energy from metabolizing luminal food, is more abundant in intraluminal feces. Meanwhile, Bacteroidetes are predominantly found in the colonic mucosa.
In this study, 59.3% of the participants were patients with colorectal cancer, with a higher prevalence observed in men compared to women (62.9% vs 37.1%). Previous studies by Chen et al[5] and Hibberd et al[9] have also demonstrated that men have a higher risk for colorectal cancer. Although the underlying reasons for this sex-based difference remain unclear, it has been hypothesized that hormones may play a protective role by suppressing bile synthesis and secretion, thereby reducing bile acid levels in the colon[10]. This hypothesis is supported by the finding from Newcomb[11], who observed a 30%-40% reduction in colorectal cancer risk among postmenopausal women who were undergoing hormone replacement therapy[11].
The median age of patients in the colorectal cancer group was higher than that of patients in the non-colorectal cancer group. Hibberd et al[9] observed that individuals with colorectal cancer tend to be older. Similarly, a study in Thailand found that the risk for developing colorectal cancer increases with age. Individuals who were > 50 years old and 60 years old were 3.03 times and 4.29 times more likely to have colorectal cancer, respectively[12]. You et al[13] reported a rising trend for colorectal cancer among younger populations, with the incidence of colon cancer increasing by 1%-2.4% per year and rectal cancer by 2.3%-3.2% per year[13]. In addition to genetics, lifestyle changes may contribute to this increase among younger individuals. For instance, there has been a notable rise in the consumption of fast food, reported to be 3-5 times higher in younger populations, as well as diets high in fat and red meat and low in fiber[13,14]. Furthermore, the prevalence of obesity, diabetes mellitus, and metabolic syndrome has increased in young adults, all of which may play a role in this trend. This rising incidence of colorectal cancer in younger individuals is associated with increased morbidity and mortality, emphasizing the need for colorectal cancer screening starting at 45-50 years of age[15].
In this study, 81.4% of the participants consumed at least one portion of vegetables and fruits per day, and 78.2% consumed red meat, with only 8.5% consuming 100-500 g/day of red meat. Additionally, 44.1% of the participants consumed instant noodles at least once or twice per month. This dietary pattern aligns with the findings of Adelina et al[16], who reported that Indonesian diets are typically moderate to high in vegetable consumption (74%) and include substantial consumption of instant noodles (49.3%). Data from the National Socio-Economic Survey 2016 further support this, showing that individuals living in Java tend to consume more vegetables than meat[17]. Nakayama et al[18] also noted that the Indonesian diet commonly consists of rice, eggs, chicken, and plant-based proteins such as tofu and tempeh. Overall, the dietary habits of the participants in this study align with the typical Indonesian diet.
Although we did not observe a significant difference in alpha diversity at the species level between the colorectal cancer and non-colorectal cancer groups, the Shannon index score was higher in the colorectal cancer group, suggesting a trend toward greater microbial diversity in these patients. This finding was in line with the findings of Hibberd et al[9]. The increased diversity may be attributed to alterations in the microtumor environment, which could be rich in nutrients and potentially promote the replication of pathogenic microbiota.
Additionally, a beta diversity analysis was conducted to assess the differences and similarities between the samples of both groups. A statistically significant difference was found in beta diversity at both the genus (P = 0.002) and species (P = 0.001) levels.
Liu et al[19] reported a significant difference in beta diversity at the species level between healthy patients, the pre-cancerous group, and the colorectal cancer group. Similarly, Sheng et al[20] also observed differences in beta diversity between healthy individuals and those diagnosed with colorectal cancer.
The four most prevalent phyla found in this study were Firmicutes, Proteobacteria, Bacteroidetes, and Fusobacteria. Similar results were reported by Rahayu et al[21], who found that the phyla composition in healthy individuals in Indonesia consisted of Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. A study by Bamola et al[22] in India observed that the dominant phyla in both patients with colorectal cancer and healthy individuals were Firmicutes, followed by Bacteroidetes, Actinobacteria, and Proteobacteria. Variations in the composition and diversity of the colon microbiota at the phylum level may be influenced by regional differences in lifestyle, diseases, and dietary habits. In the study by Bamola et al[22], samples were collected from non-vegetarian subjects consuming a high-calorie diet, rich in dairy products, animal proteins, and plant polysaccharides, which could explain the dominance of Firmicutes.
In this study, an increased abundance of Fusobacteria was observed in patients with colorectal cancer, although this was not statistically significant. This finding aligns with the work of Fang et al[23], who noted the role of Fusobacteria in carcinogenesis, both directly and indirectly through immune system modulation. Given the consistent presence of Fusobacteria in colorectal cancer, it has been proposed as a potential diagnostic and prognostic biomarker for the disease. Disease states such as colorectal cancer can influence the composition of these dominant phyla. The observed increase in Proteobacteria in this study may be related to age, as this phylum is typically detected in individuals aged over 60 years and its abundance increases with age[24].
At the family level, an increase in the Oscillospiraceae family was noted among patients without colorectal cancer. The Oscillospiraceae family encompasses the genus Faecalibacterium, a commensal bacterium commonly found in healthy individuals without colorectal cancer. This finding contrasts with the findings of Chen et al[5], who reported significantly higher levels of bacteria from the Porphyromonadaceae, Fusobacteriaceae, and Peptostreptococcaceae families, while Bifidobacteriaceae and Alcaligenaceae were more prevalent in healthy individuals.
The five most prevalent genera identified in this study, in order of prevalence, were: Fusobacterium, Faecalibacterium, Citrobacter, Prevotella, and Bacteroides. In the study by Liu et al[19], the dominant genera in healthy individuals were Prevotella, Bacteroides, unclassified genera, Faecalibacterium, Escherichia, Roseburia, and Megamonas, accounting for 30%, 30%, 13%, 12%, 7%, 4%, and 4%, respectively[19]. Research on the gut microbiota of healthy individuals in Indonesia is limited. Rahayu et al[21] conducted a study in Yogyakarta and Bali involving 80 healthy participants, identifying Clostridium as the most prevalent genus, followed by Prevotella, Atopobium, Bifidobacterium, and Bacteroides[21]. This study found that Prevotella was more frequently observed compared to Bacteroides among healthy individuals in Indonesia.
The relatively high abundance of the genus Prevotella in both groups reflects the diet of the Indonesian population, which is rich in fiber and vegetables[23]. In this study, 81.4% of subjects reported consuming at least one serving of vegetables and fruits daily. The consumption of vegetables and fiber is associated with an increase in short-chain fatty acid-producing bacteria, which is beneficial for maintaining the balance and diversity of the microbiota[25,26]. These findings align with the research by Nakayama et al[18], which identified Prevotella as a characteristic genus in the Indonesian microbiota[27]. Nakayama et al[18] also noted that this microbiota is linked to the consumption of rice, soybean-based foods (tempeh and tofu), eggs, and chicken meat.
This study also found a significant increase in the relative abundance of Fusobacterium nucleatum in colorectal cancer. This finding was consistent with previous studies that have linked Fusobacterium nucleatum with colorectal cancer[19,20,23,28]. Fusobacterium nucleatum is a gram-negative anaerobic bacterium that typically colonizes the oral cavity[29]. It has also been detected in tumor tissues and feces, where it plays a critical role in tumorigenesis and resistance to chemotherapy[24]. Fusobacterium nucleatum can secrete the adhesin FadA, which binds to E-cadherin, leading to the activation of b-catenin signaling pathways and nuclear factor kappa-B production, thereby triggering tumorigenesis[23,28,30]. With the help of the protein Fap2, this bacterium could also bind to N-acetyl-D-galactosamine found on the surface of tumor cells. Additionally, it can inhibit the activity of cytotoxic T cells and native killer cells against tumors through Fap 2[19].
We also observed an increase in the relative abundance of Bacteroides fragilis in patients with colorectal cancer, consistent with previous studies[5,6,28]. Bacteroides fragilis is an anaerobic commensal microbiota in the colon that could become pathogenic when the gut barrier is disrupted[31]. It belongs to the Bacteroidetes phylum, Bacteroidaceae family, and Bacteroides genus. Furthermore, we found a significant increase in Bacteroides genus in colorectal cancer, aligning with several previous studies[5,19,22]. Bacteroides fragilis can directly and indirectly influence the development of colorectal cancer. The bacterium secretes a zinc-dependent metalloprotease known as Bacteroides fragilis enterotoxin, which induces inflammation, increases intestinal permeability, and facilitates pathogen transmigration. Additionally, Bacteroides fragilis can activate the Wnt signaling pathway via E-cadherin, leading to the release of β-catenin, which is involved in carcinogenesis[14,30].
Although the increase in Parvimonas micra in the colorectal cancer group was not significant after adjusting for age and gender in our study, several studies have consistently linked Parvimonas micra with colorectal cancer[32]. Saffarian et al[6] also reported that Parvimonas micra was associated with left-sided colon tumors. Parvimonas micra is a gram-positive anaerobic coccus bacteria that resides in the oral cavity. It belongs to the Firmicutes phylum, Peptoniphilaceae family, and Parvimonas genus. This bacterium exists in two distinct phylotypes: phylotype A and B. Phylotype A is particularly adept at colonizing the lumen and mucosa of the large intestine, where it may induce DNA methylation changes and disrupt gene expression, contributing to colorectal cancer pathogenesis[33]. Murine studies have shown that Parvimonas micra can increase the levels of interleukins (IL)-17, IL-22, and IL-23, which are associated with Th17 cells. This pathway can activate Wnt signaling, further promoting tumorigenesis. Zhao et al[32] noted that Parvimonas micra is not only linked to the pathogenesis of colorectal cancer but also correlates with lower five-year survival rates in patients with colorectal cancer.
Additionally, a high relative abundance of the genus Peptostreptococcus was observed in patients with colorectal cancer. Several previous studies have also implicated Peptostreptococcus in colorectal cancer[8,23,24,28]. This genus is classified within the phylum Firmicutes and class Clostridia. Peptostretococcus, particularly its species P. anaerobius, has a role in promoting tumorigenesis[24]. P. anaerobius is capable of increasing reactive oxygen species, which interact with Toll-like receptors 2 and 4 on colon cells, thereby enhancing cholesterol synthesis and cell proliferation, as well as promoting dysplasia[4]. Additionally, Peptostreptococcus forms biofilms that protect cancer cells from the immune system[23].
The genus Campylobacter and its species Campylobacter hominis have been found to exhibit significantly higher relative abundance in patients with colorectal cancer. In a sub-analysis based on the cancer stage of patients with colorectal cancer, higher abundances of Campylobacter and Campylobacter hominis were observed in patients with advanced-stage colorectal cancer compared to those with early-stage colorectal cancer. Colonization by bacteria from this genus typically begins in childhood and can trigger inflammation and immune system disruptions. Campylobacter jejuni, a species from this genus, produces a genotoxin that may damage DNA and initiate tumorigenesis[4,34].
Although Enterococcus faecalis exhibited minimal relative abundance, it was still significantly more abundant in the colorectal cancer group compared to the non-colorectal cancer group. This result remained consistent even after adjusting for age and gender. Enterococcus faecalis belongs to the phylum Firmicutes, family Enterococcaceae, and genus Enterococcus. Research suggests that Enterococcus faecalis can produce biliverdin, which may stimulate growth and angiogenesis in colorectal cancer by regulating the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin/vascular endothelial growth factor A pathway[35,36].
In this study, we observed an increase in the relative abundance of Haemophilus genus in individuals without colorectal cancer. This genus is a part of the Proteobacteria phylum, Gammaproteobacteria class, and Pasteurellaceae family, and consists of gram-negative coccobacilli bacteria. A study by Fortoul et al[37] found that Hemophilus influenzae, a member of this genus, exerts a protective effect against inflammation and may reduce the risk of colorectal cancer.
We also observed an increase in Faecalibacterium genus and the bacterium Faecalibacterium prausnitzii in patients with non-colorectal cancer. Zhang et al[24] reported that the Faecalibacterium genus is significantly more prevalent in younger patients without colorectal cancer. Similarly, Liu et al[19] found a higher relative abundance of Faecalibacterium in healthy individuals compared to patients with colorectal cancer (12% vs 4%). Additionally, Chen et al[5] reported that Faecalibacterium was detected in much smaller quantities in tumor cells compared to healthy mucosa. Faecalibacterium prausnitzii is known for its protective and anti-inflammatory effects. It is one of the bacteria capable of producing short-chain fatty acids, which help maintain mucosal integrity, support nutritional status, regulate immune function, and reduce the risk of colorectal cancer. Specifically, Faecalibacterium prausnitzii has been shown to suppress IL-12 and interferon production, as well as increase IL-10 secretion[38,39]. Gopalakrishnan et al[40] also reported that colonization by Faecalibacterium prausnitzii could enhance responses to chemotherapy and improve prognosis.
Furthermore, our study revealed an increased relative abundance of Prevotella copri in non-colorectal cancer. Prevotella copri is a member of the Bacteroidetes phylum and Prevotella genus. Prevotella and Bacteroides are the most common genera in Bacteroidetes phylum. A study in Kenya found that both Prevotella copri and Faecalibacterium prausnitzii were present in higher amounts in healthy individuals, indicating a healthy gut microbiome[41]. Although these bacteria have been associated with inflammation in animal models, studies in humans have shown otherwise. A meta-analysis reported a higher prevalence of Prevotella copri in healthy individuals who have a diet rich in fiber and vegetables[42].
As previously mentioned, numerous studies have established a link between gut microbiota and colorectal cancer. The microbiota plays a significant role in colorectal cancer, either as an initiator or as a stimulator of cancer progression. Therefore, investigating the diversity and composition of gut microbiota in patients with colorectal cancer could potentially aid clinicians in enhancing their screening and diagnostic approaches.
Several studies have highlighted the relationship between microbiota and colorectal cancer. Liang et al[43] conducted a study on 676 subjects to evaluate the role of microbiota in the diagnosis of colorectal cancer. In this study, the abundance of Fusobacterium nucleatum, Lachnoclostridium sp. M3, Bacteroides clarus, and Clostridium hathewayi was calculated and then converted into a score, named 4Bac. Liang et al[43] found that the 4Bac score exhibited higher sensitivity (P < 0.001) but lower specificity (83.8% vs 98.6%) compared to fecal immunochemical test for diagnosing colorectal cancer and advanced adenomas. Notably, the combination of 4Bac and fecal immunochemical tests led to improved sensitivity for detecting colorectal cancer (90.9%) and advanced adenoma (48.5%).
A meta-analysis by Zhang et al[44] also explored the potential of the genera Anaerostipes, Porphyromonas, Fusobacterium, Parvimonas, Peptostreptococcus, and Gamela as diagnostic biomarkers for colorectal cancer. The combination of these six genera resulted in an AUC of 0.76. On further analysis, this study also found that utilization of the three main genera Porphyromonas, Parvimonas, and Peptostreptococcus facilitates the distinction of patients with colorectal cancer from healthy subjects and adenoma with an AUC of 0.87 and 0.67, respectively[44].
In addition to diagnostic applications, gut microbiota may also serve as a screening tool. A systematic review by Zwezerijnen-Jiwa et al[45] reported that gut microbiota has an AUC of 0.28-0.98 for detecting colorectal cancer precursor lesions, such as adenomas. Our study found that gut microbiota has an AUC of 0.65-0.93 for detecting early-stage colorectal cancer. Hence, microbiota has the potential to be utilized as a biomarker for both diagnostic and screening purposes in colorectal cancer.
In this study, we observed a relatively high abundance of Fusobacterium nucleatum and Bacteroides fragilis in patients with colorectal cancer. The increased abundance of these two microorganisms could potentially serve as screening and diagnostic tools for colorectal cancer. In this research, the use of Fusobacterium nucleatum as a diagnostic tool yielded an AUC value of 0.727 (0.600-0.853), while Bacteroides fragilis showed an AUC of 0.735 (0.607-0.862). The combination of these two microorganisms demonstrated an AUC of 0.786 (0.671-0.900). These findings are consistent with those of Huang et al[46], whose meta-analysis reported a diagnostic AUC of 0.8 for the use of Fusobacterium nucleatum in the diagnosis of colorectal cancer. Additionally, our study found that the combination of Fusobacterium nucleatum and Bacteroides fragilis demonstrated a sensitivity of 82.8%, specificity of 50%, positive predictive value of 70.7%, and negative predictive value of 66.7%. A meta-analysis of six journals reported that Fusobacterium nucleatum had a sensitivity of 0.68 (95%CI: 0.64-0.72) and a specificity of 0.78 (95%CI: 0.75-0.81).
These results suggest that microbiota may hold significant potential for development as diagnostic tools for colorectal cancer. Current screening methods recommended by the World Health Organization and the American College of Gastroenterology are based on the early detection of pre-cancerous abnormalities. Unlike traditional screening tools, utilization of microbiota in colorectal cancer screening has the advantage of detecting potential malignancy risks even before the emergence of pre-cancerous lesions.
The strengths of this study include the utilization of cutting-edge technologies, such as third-generation sequencing from Oxford Nanopore Technologies, which enables the generation of longer sequences and increases the likelihood of identifying microbiota at the species level. To the best of our knowledge, this is the first study that investigated mucosal microbiota in colorectal cancer in Indonesia.
One of the limitations of this study is that non-colorectal cancer samples were obtained from patients with gas
Future research with more samples is needed to explore the various factors influencing microbiota composition and its role in colorectal cancer.
This study observed significant differences in the composition and abundance of gut microbiota, particularly at the genus and species levels, between patients with colorectal cancer and those without colorectal cancer. Notably, there was a significant difference in beta diversity at both the genus and species levels between the two groups.
We would like to acknowledge Putri RR, Aprilicia G, the Faculty of Medicine Universitas Indonesia-Cipto Man
| 1. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3306] [Article Influence: 413.3] [Reference Citation Analysis (3)] |
| 2. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1071] [Article Influence: 178.5] [Reference Citation Analysis (1)] |
| 3. | Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021;21:1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 4. | Kim J, Lee HK. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front Immunol. 2021;12:807648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 5. | Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 788] [Cited by in RCA: 736] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 6. | Saffarian A, Mulet C, Regnault B, Amiot A, Tran-Van-Nhieu J, Ravel J, Sobhani I, Sansonetti PJ, Pédron T. Crypt- and Mucosa-Associated Core Microbiotas in Humans and Their Alteration in Colon Cancer Patients. mBio. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Yusuf F, Ilyas S, Damanik HA, Fatchiyah F. Microbiota Composition, HSP70 and Caspase-3 Expression as Marker for Colorectal Cancer Patients in Aceh, Indonesia. Acta Med Indones. 2016;48:289-299. [PubMed] |
| 8. | Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 714] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 9. | Hibberd AA, Lyra A, Ouwehand AC, Rolny P, Lindegren H, Cedgård L, Wettergren Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4:e000145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Abu Hassan MR, Ismail I, Mohd Suan MA, Ahmad F, Wan Khazim WK, Othman Z, Mat Said R, Tan WL, Mohammed SRNS, Soelar SA, Nik Mustapha NR. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol Health. 2016;38:e2016007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Newcomb PA, Zheng Y, Chia VM, Morimoto LM, Doria-Rose VP, Templeton A, Thibodeau SN, Potter JD. Estrogen plus progestin use, microsatellite instability, and the risk of colorectal cancer in women. Cancer Res. 2007;67:7534-7539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Poomphakwaen K, Promthet S, Suwanrungruang K, Kamsa-ard S, Wiangnon S. Risk Factors for Colorectal Cancer in Thailand. Asian Pac J Cancer Prev. 2015;16:6105-6109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | You YN, Lee LD, Deschner BW, Shibata D. Colorectal Cancer in the Adolescent and Young Adult Population. JCO Oncol Pract. 2020;16:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Abdullah M, Sukartini N, Nursyirwan SA, Pribadi RR, Maulahela H, Utari AP, Muzellina VN, Wiraatmadja A, Renaldi K. Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future. Digestion. 2021;102:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 16. | Adelina R, Nurwanti E, Paramastri R, Cerdasari C, Chao JCJ. Evaluating the food consumption among Indonesian young adults lived in a different environment. JGI. 2021;10:36-44. [DOI] [Full Text] |
| 17. | Hafizah D, Hakim DB, Harianto H, Nurmalina R. Analysing food consumption in Indonesia. IJPSAT. 2020;20:340-347. |
| 18. | Nakayama J, Watanabe K, Jiang J, Matsuda K, Chao SH, Haryono P, La-Ongkham O, Sarwoko MA, Sujaya IN, Zhao L, Chen KT, Chen YP, Chiu HH, Hidaka T, Huang NX, Kiyohara C, Kurakawa T, Sakamoto N, Sonomoto K, Tashiro K, Tsuji H, Chen MJ, Leelavatcharamas V, Liao CC, Nitisinprasert S, Rahayu ES, Ren FZ, Tsai YC, Lee YK. Diversity in gut bacterial community of school-age children in Asia. Sci Rep. 2015;5:8397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 19. | Liu W, Zhang R, Shu R, Yu J, Li H, Long H, Jin S, Li S, Hu Q, Yao F, Zhou C, Huang Q, Hu X, Chen M, Hu W, Wang Q, Fang S, Wu Q. Study of the Relationship between Microbiome and Colorectal Cancer Susceptibility Using 16SrRNA Sequencing. Biomed Res Int. 2020;2020:7828392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Sheng Q, Du H, Cheng X, Cheng X, Tang Y, Pan L, Wang Q, Lin J. Characteristics of fecal gut microbiota in patients with colorectal cancer at different stages and different sites. Oncol Lett. 2019;18:4834-4844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Rahayu ES, Utami T, Mariyatun M, Hasan PN, Kamil RZ, Setyawan RH, Pamungkaningtyas FH, Harahap IA, Wiryohanjoyo DV, Pramesi PC, Cahyanto MN, Sujaya IN, Juffrie M. Gut microbiota profile in healthy Indonesians. World J Gastroenterol. 2019;25:1478-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (4)] |
| 22. | Bamola VD, Kapardar R, Lal B, Sharma A, Chaudhry R. A metagenomic assessment of gut microbiota in Indian colon cancer patients. J Cancer Res Ther. 2022;18:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Fang CY, Chen JS, Hsu BM, Hussain B, Rathod J, Lee KH. Colorectal Cancer Stage-Specific Fecal Bacterial Community Fingerprinting of the Taiwanese Population and Underpinning of Potential Taxonomic Biomarkers. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Zhang YK, Zhang Q, Wang YL, Zhang WY, Hu HQ, Wu HY, Sheng XZ, Luo KJ, Zhang H, Wang M, Huang R, Wang GY. A Comparison Study of Age and Colorectal Cancer-Related Gut Bacteria. Front Cell Infect Microbiol. 2021;11:606490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Therdtatha P, Song Y, Tanaka M, Mariyatun M, Almunifah M, Manurung NEP, Indriarsih S, Lu Y, Nagata K, Fukami K, Ikeda T, Lee YK, Rahayu ES, Nakayama J. Gut Microbiome of Indonesian Adults Associated with Obesity and Type 2 Diabetes: A Cross-Sectional Study in an Asian City, Yogyakarta. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Perler BK, Friedman ES, Wu GD. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu Rev Physiol. 2023;85:449-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 27. | Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 1507] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 28. | Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, Sunagawa S, Coelho LP, Schrotz-King P, Vogtmann E, Habermann N, Niméus E, Thomas AM, Manghi P, Gandini S, Serrano D, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Waldron L, Naccarati A, Segata N, Sinha R, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25:679-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1007] [Cited by in RCA: 795] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 29. | Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 30. | Liu Y, Lau HC, Cheng WY, Yu J. Gut Microbiome in Colorectal Cancer: Clinical Diagnosis and Treatment. Genomics Proteomics Bioinformatics. 2023;21:84-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 31. | Elsaghir H, Reddivari AKR. Bacteroides Fragilis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 32. | Zhao L, Zhang X, Zhou Y, Fu K, Lau HC, Chun TW, Cheung AH, Coker OO, Wei H, Wu WK, Wong SH, Sung JJ, To KF, Yu J. Parvimonas micra promotes colorectal tumorigenesis and is associated with prognosis of colorectal cancer patients. Oncogene. 2022;41:4200-4210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 33. | Bergsten E, Mestivier D, Donnadieu F, Pedron T, Barau C, Meda LT, Mettouchi A, Lemichez E, Gorgette O, Chamaillard M, Vaysse A, Volant S, Doukani A, Sansonetti PJ, Sobhani I, Nigro G. Parvimonas micra, an oral pathobiont associated with colorectal cancer, epigenetically reprograms human colonocytes. Gut Microbes. 2023;15:2265138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 34. | Gervaz PA, De Campos Á, Caeiro A. Campylobacter jejuni causes colorectal cancer. World J Colorectal Surg. 2022;11:4-7. [DOI] [Full Text] |
| 35. | de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol. 2018;11:1756284818783606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Zhang L, Liu J, Deng M, Chen X, Jiang L, Zhang J, Tao L, Yu W, Qiu Y. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: biliverdin. J Transl Med. 2023;21:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 37. | Fortoul MC, Kim E, Ardeljan AD, Frankel L, Takabe K, Rashid OM. The Role of Hemophilus influenzae Infection and Its Relationship With Colorectal Cancer. World J Oncol. 2023;14:188-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3203] [Article Influence: 188.4] [Reference Citation Analysis (0)] |
| 39. | Song M, Chan AT, Sun J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology. 2020;158:322-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (2)] |
| 40. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3313] [Article Influence: 473.3] [Reference Citation Analysis (0)] |
| 41. | Obuya S, Elkholy A, Avuthu N, Behring M, Bajpai P, Agarwal S, Kim HG, El-Nikhely N, Akinyi P, Orwa J, Afaq F, Abdalla M, Michael A, Farouk M, Bateman LB, Fouad M, Saleh M, Guda C, Manne U, Arafat W. A signature of Prevotella copri and Faecalibacterium prausnitzii depletion, and a link with bacterial glutamate degradation in the Kenyan colorectal cancer patients. J Gastrointest Oncol. 2022;13:2282-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Tett A, Huang KD, Asnicar F, Fehlner-Peach H, Pasolli E, Karcher N, Armanini F, Manghi P, Bonham K, Zolfo M, De Filippis F, Magnabosco C, Bonneau R, Lusingu J, Amuasi J, Reinhard K, Rattei T, Boulund F, Engstrand L, Zink A, Collado MC, Littman DR, Eibach D, Ercolini D, Rota-Stabelli O, Huttenhower C, Maixner F, Segata N. The Prevotella copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host Microbe. 2019;26:666-679.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 43. | Liang JQ, Wong SH, Szeto CH, Chu ES, Lau HC, Chen Y, Fang J, Yu J, Sung JJ. Fecal microbial DNA markers serve for screening colorectal neoplasm in asymptomatic subjects. J Gastroenterol Hepatol. 2021;36:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Zhang H, Wu J, Ji D, Liu Y, Lu S, Lin Z, Chen T, Ao L. Microbiome analysis reveals universal diagnostic biomarkers for colorectal cancer across populations and technologies. Front Microbiol. 2022;13:1005201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Zwezerijnen-Jiwa FH, Sivov H, Paizs P, Zafeiropoulou K, Kinross J. A systematic review of microbiome-derived biomarkers for early colorectal cancer detection. Neoplasia. 2023;36:100868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 46. | Huang Q, Peng Y, Xie F. Fecal fusobacterium nucleatum for detecting colorectal cancer: a systematic review and meta-analysis. Int J Biol Markers. 2018;1724600818781301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |