Published online Feb 7, 2025. doi: 10.3748/wjg.v31.i5.98928
Revised: November 5, 2024
Accepted: December 12, 2024
Published online: February 7, 2025
Processing time: 172 Days and 5.5 Hours
Microvascular invasion (MVI) is a critical prognostic factor for postoperative hepatocellular carcinoma recurrence, but the reliability of its current pathological diagnosis remains uncertain.
To evaluate the accuracy of current 7-point sampling methods and propose an optimal pathological protocol using whole-mount slide imaging (WSI) for better MVI detection.
We utilized 40 New Zealand white rabbits to establish VX2 liver tumor models. The entire tumor-containing liver lobe was subsequently obtained, following which five different sampling protocols (A-E) were employed to evaluate the detection rate, accuracy, quantity, and distribution of MVI, with the aim of iden
VX2 liver tumor models were successfully established in 37 rabbits, with an incidence of MVI of 81.1% (30/37). The detection rates [27% (10/37), 43% (16/37), 62% (23/37), 68% (25/37), and 93% (14/15)] and quantity (15, 36, 107, 125, and 395) of MVI increased significantly from protocols A to E. The distribution of MVI showed fewer MVIs farther away from the tumor, but the percentage of MVI detected quantity gradually increased from 6.7% to 48.3% in the distant nonneoplastic liver tissue from protocols A to E. Protocol C was identified as the optimal sampling method by comparing them in sequence. The sampling protocol of three consecutive interval WSIs at the tumor center (WSI3) was further screened to determine the optimal number of WSIs. Protocol A (7-point sampling method) exhibited only 46% accuracy and a high false-negative rate of 67%. Notably, the WSI3 protocol improved the accuracy to 78% and decreased the false-negative rate to 27%.
The current 7-point sampling method has a high false-negative rate in MVI detection. In contrast, the WSI3 protocol provides a practical and effective approach to improve MVI diagnostic accuracy, which is crucial for hepatocellular carcinoma diagnosis and treatment planning.
Core Tip: This study investigated the effectiveness of various pathological sampling protocols for detecting microvascular invasion (MVI) in hepatocellular carcinoma. Conventional 7-point sampling methods have a high false-negative rate for MVI detection. However, the study revealed that whole-mount slide imaging, particularly when three consecutive interval whole-mount slide imaging from the tumor center are used, significantly improves diagnostic accuracy. This innovative approach improves the detection of MVI, especially in distant nonneoplastic liver tissue that have previously been ignored, offering a more reliable method for postoperative prognosis.
- Citation: Li LJ, Wu CQ, Ye FL, Xuan Z, Zhang XL, Li JP, Zhou J, Su ZZ. Histopathological diagnosis of microvascular invasion in hepatocellular carcinoma: Is it reliable? World J Gastroenterol 2025; 31(5): 98928
- URL: https://www.wjgnet.com/1007-9327/full/v31/i5/98928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i5.98928
Hepatocellular carcinoma (HCC) is a highly lethal malignancy, ranking as the second most fatal tumor globally and the fourth leading cause of cancer-related death[1,2]. China alone accounts for approximately half of all HCC cases worldwide and has the highest incidence and mortality rates[3]. A mere 18% 5-year survival rate is reported[1], with over 90% of HCC-related deaths attributed to metastasis and recurrence[4]. The postoperative recurrence rate of HCC is alarmingly high, reaching 70%[5,6]. Microvascular invasion (MVI) significantly increases the risk of postoperative recurrence in HCC patients[7-9], increasing the recurrence rate by 4.4 times[10].
MVI, characterized by the presence of cancer cell nests within an endothelial-lined vascular lumen observed through microscopic examination[11], holds paramount importance as a pathological indicator for assessing recurrence risk and determining therapeutic strategies for HCC[6]. Postoperative histology serves as the established reference standard for MVI diagnosis. However, the detection rate of MVI ranges widely from 7.8% to 74.4%[12], and the sampling protocol used can lead to false-negative results. In 2015, the Chinese guidelines introduced the 7-point sampling method[11], which is widely utilized in clinical pathological examinations of MVI in HCC patients. Notably, previous studies have shown higher MVI-positive rates with the 7-point sampling protocol than with the 3-point sampling protocol (47.1% vs 34.5%), but similar results were obtained with the 13-point sampling method (47.1% vs 51.3%)[13]. Another study revealed that the MVI detection rate reached a plateau when the number of sampling sites exceeded a certain threshold[14]. These findings collectively support the feasibility of using the 7-point sampling approach for MVI detection, emphasizing the limited benefits of surpassing the number of sampling sites threshold.
In a recent study by Yu et al[15], whole-mount slide imaging (WSI) outperformed the 7- and 3-point sampling protocols in detecting MVI (63.7% vs 33.0% or 22.0%), indicating limitations in the current utilization of the 7-point sampling method. Furthermore, the reliability of 7-point sampling for detecting MVI has not been effectively verified, and this protocol has not been widely adopted outside China[12]. Thus, further investigations are warranted to determine the accuracy of the 7-point sampling method in clinical practice. In this study, we developed an animal model using rabbit VX2 in situ liver tumors to investigate the presence of MVI. Serial WSIs of the entire liver lobe were obtained to comprehensively assess peritumoral MVI. Our aim was to evaluate the diagnostic accuracy of various sampling protocols for MVI and develop a practical and viable pathological sampling approach that improves MVI diagnostic precision. Our findings have significant implications for diagnosing MVI and will help optimize future pathological examination strategies.
The ARRIVE guidelines were followed strictly during the conduct of the animal experiments in this study[16]. The protocol was approved by the Institution Animal Care and Use Committee of Zhuhai BesTest Bio-Tech Co., Ltd. in China, protocol number: No. IAC(L)202104001. Forty New Zealand white rabbits weighing 2-3 kg and aged three to four months were used to construct the animal model. The rabbits were housed at Zhuhai BesTest Bio-Tech Co., Ltd. (license number: SYXK 2020-0229), each in an individual cage under trained staff supervision. The facility had a 12-hour light/dark cycle with daylight simulation lamps and good ventilation and air filtration systems. The environmental conditions, such as temperature (20-25 °C) and humidity (60%-70%), were well controlled. Cages and waste trays were cleaned daily to maintain a clean and quiet environment (noise ≤ 60 dB). Food and water were provided freely. Any distress was promptly addressed, and painkillers or anesthetics were administered as needed. The sample size of 40 was established through a statistical power analysis, drawing upon previously published detection rates for various sampling techniques[15], with a two-tailed significance level of 0.05 and a power of 0.8. Factoring in an anticipated 10% sample attrition, this calculation resulted in a target sample size deemed sufficient to ensure statistical robustness.
Anesthesia was induced in the rabbits via intramuscular injections into their hind legs. We used a combination of 0.1 mL/kg xylazine hydrochloride (Dunhua Shengda Animal Medicine Co., Ltd., China), 0.1 mL/kg tiletamine hydrochlo
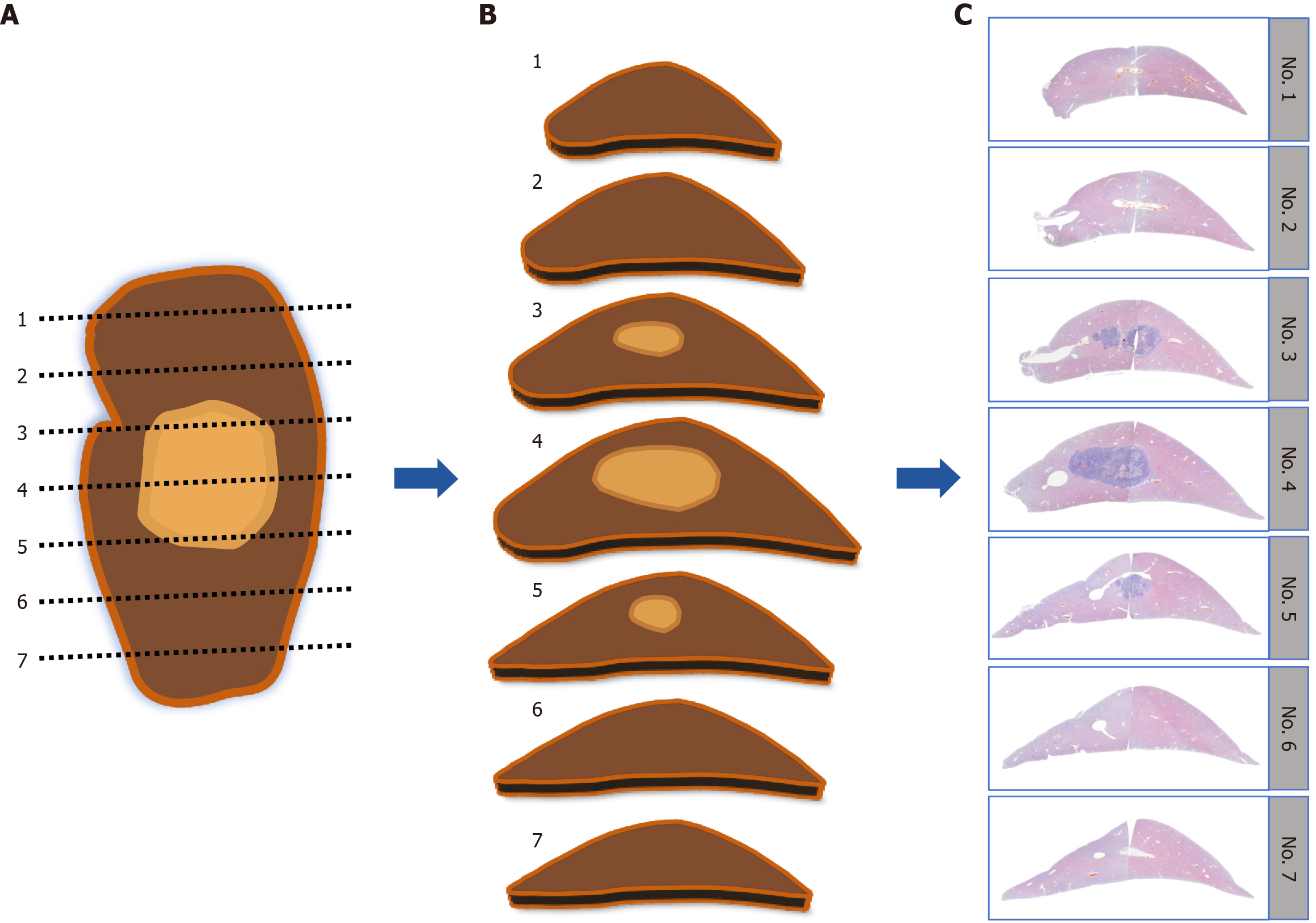
Upon successful establishment of the rabbit VX2 liver tumor model, all the animals were euthanized with air embolism under double-dose anesthesia. The entire liver lobe containing the tumor was then fixed in 4% paraformaldehyde for more than 48 hours. The specimens were subsequently sectioned along the maximal tumor section into consecutive 3-mm-thick tissue blocks parallel to the maximal tumor section, as illustrated in Figure 1A. Each tissue block was placed in the same orientation and then assigned a label with consecutive numbers (as shown in Figure 1B) to maintain accurate specimen identification. If a tissue block was excessively large, it was divided into two parts to ensure appropriate paraffin embedding.
The tissue blocks were dehydrated using an automated tissue dehydrator, embedded in paraffin in the same marked orientation, and then shaped using a paraffin embedding station and mold. Only the first complete tissue section, with a thickness of 5 μm, was prepared from each wax block. These tissue sections were stained with hematoxylin and eosin and digitized into WSIs. For this digitization process, a Pannoramic P250 Flash slide scanner (3D Histech, Budapest, Hungary) was used with the following specific settings: We employed a 20 × objective lens (a resolution of 0.5 μm per pixel), and the output resolution was set to 41 × (0.25 μm per pixel). The scanner was configured for automatic focusing and utilized a bright-field illumination source. With respect to the sample scanning threshold, an automatic setting was employed, and we opted to scan the entire slide (as shown in Figure 1C). The digital images were viewed and analyzed via CaseViewer 2.4 software.
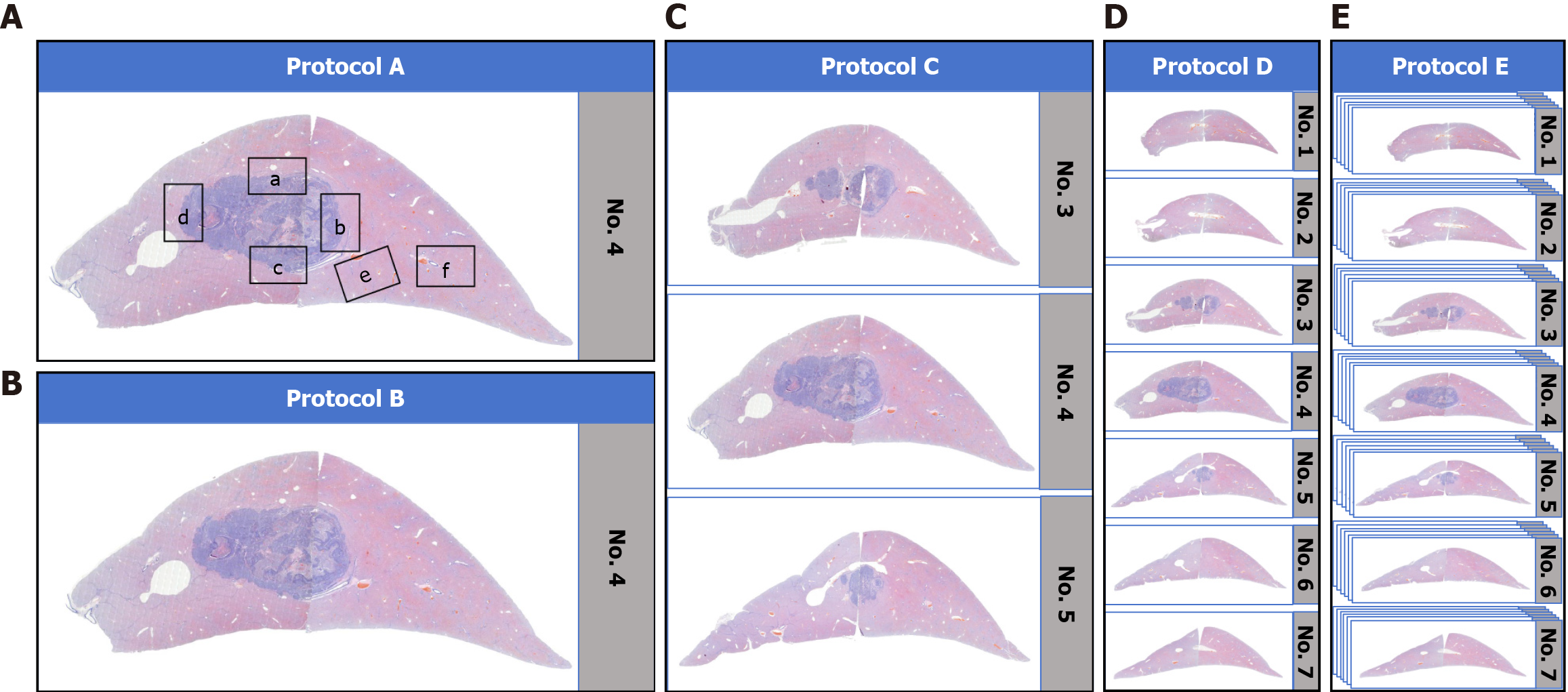
The sampling strategies (protocols A-E) implemented in our study are illustrated in Figure 2.
Protocol A (7-point sampling): Based on the 7-point sampling protocol[11], we preoutlined six specific regions on the WSI of the maximal tumor section (Figure 2A) via CaseViewer 2.4 software while excluding the intratumoral MVI region because it lacks prognostic value[19]. These regions were located at the 12:00, 3:00, 6:00 and 9:00 positions, as were the tumor-adjacent and distant non-neoplastic regions, which are labeled a-f in Figure 2A in sequence. The selected regions were subsequently subjected to examination for the diagnosis of MVI.
Protocol B (WSI sampling): The sampling range of this protocol was the entire WSI of the maximal tumor section (Figure 2B). This approach provided a comprehensive view of the maximal tumor section without focusing on specific subregions, as in protocol A.
Protocol C (tumor 3 mm interval continuous WSI sampling): This protocol’s sampling range was an expansion of that in protocol B. It not only cover the WSI of the maximal tumor section, but also included the adjacent tumor section WSIs. In other words, it included all WSIs of the tumor at 3 mm intervals (Figure 2C) to permit a more extensive examination of the tumor and its adjacent areas.
Protocol D (liver 3 mm intervals continuous WSI sampling): The sampling range was all WSIs of the entire liver lobe, equivalent to obtaining all WSIs of the entire liver lobe at 3 mm intervals (Figure 2D). This provided a broader perspec
Protocol E (liver 0.3 mm interval continuous WSI sampling): In this protocol, fifteen rabbit liver tumor models were randomly selected. All the tissue blocks of the entire liver lobe were sectioned into serial WSIs at 0.3 mm intervals for MVI examination. The sampling range was equivalent to obtaining all WSIs of the entire liver lobe at 0.3 mm intervals (Figure 2E), which enabled a detailed examination of the entire liver lobe with a relatively fine sampling interval.
In accordance with the guidelines for the diagnosis and treatment of primary liver cancer in China, satellite nodules are considered to be in the progressive stage of MVI during the intrahepatic metastasis of HCC. In this study, satellite nodules ≤ 1 mm that were difficult to differentiate from MVI were included in the MVI category[6]. All the sections were examined independently for MVI by two pathologists with over eight years of experience in HCC pathology. Any disa
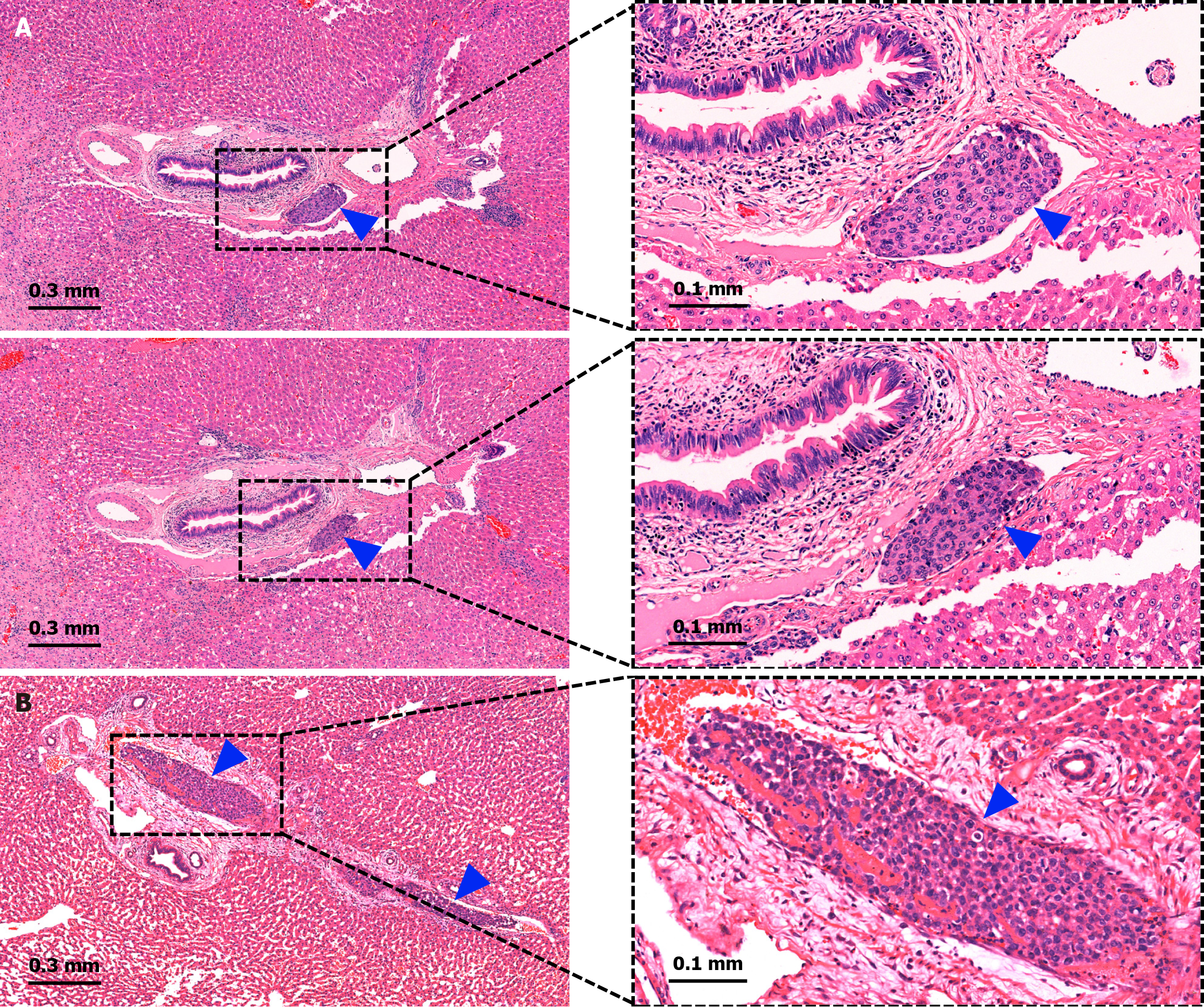
If MVI was detected in any of the sampling protocols, the specimen was deemed positive for MVI. If multiple sections displayed the same MVI lesion (Figure 3A) or involved the same vessel (Figure 3B), it was recorded as a single MVI. The detection rate and quantity of MVI were recorded for each sampling protocol. The metastatic distance between each MVI lesion and the main tumor margin was measured and classified into five groups: ≤ 1.0 mm, 1.1-3.0 mm, 3.1-5.0 mm, 5.1-10.0 mm, and > 10.0 mm. The number of MVI detected by each sampling protocol in various metastatic distance groups was recorded.
The statistical analysis was conducted via SPSS 25.0 (IBM, Armonk, NY, United States). The normality of the quantitative data was assessed via the Shapiro-Wilk test. Descriptive statistics are presented as the means ± SD for continuous variables with a normal distribution and as the medians (ranges) for those with a skewed distribution. Paired t tests or paired Wilcoxon rank-sum tests were applied for paired data, whereas t tests or Mann-Whitney U tests were utilized for unpaired data based on the distribution of variables. Categorical variables are expressed as percentages, and the paired χ2 test was used for paired data, whereas the χ2 test was used for unpaired data with continuity correction based on theoretical frequencies. Statistical significance was defined as P < 0.05.
In this study, the tumor inoculation process was well tolerated by all 40 rabbits without any associated mortality, implant-related infections, or behavioral changes. A total of 37 rabbits (92.5%) were implanted to establish the VX2 in situ liver tumor model. Implantation failed in three cases due to the transplantation of the tumor tissue into subcutaneous or mesentery tissue. Therefore, 37 rabbits were included in the analysis, and their baseline characteristics are shown in Table 1.
| Variables | Results |
| Rabbit liver tumor model | |
| Success | 37 (92.5) |
| Failure | 3 (7.5) |
| Rabbit weight (kg), mean ± SD | |
| Before tumor transplanting | 2.5 ± 0.4 |
| After successfully modeling | 2.8 ± 0.4 |
| Time of tumor growth (day), mean ± SD | 20.4 ± 4.0 |
| Tumor location | |
| Left lateral lobe of liver | 32 (80.0) |
| Left medial lobe of liver | 5 (12.5) |
| Mesentery | 2 (5.0) |
| Subcutaneous | 1 (2.5) |
| Intrahepatic tumor number | |
| Single | 37 (100.0) |
| Multiple | 0 (0.0) |
| Intrahepatic tumor size (cm), mean ± SD | 1.9 ± 0.5 |
| Microvascular invasion | |
| Positive | 30 (81.1) |
| Negative | 7 (18.9) |
| Concomitant > 1 mm satellite nodule | |
| Yes | 9 (30.0) |
| No | 21 (70.0) |
The weights of the rabbits prior to tumor transplantation and after successful modeling were 2.5 ± 0.4 kg and 2.8 ± 0.4 kg, respectively, which were significantly different (P < 0.001). This finding suggested that the weight of the rabbits continued to increase normally after tumor inoculation. The growth of the implanted tumors was monitored for an average of 20.4 ± 4.0 days, during which all rabbits developed a single tumor with a maximum diameter of 1.9 ± 0.5 cm, primarily located in the left lateral lobe (32 cases) and left medial lobe (5 cases). All pathological sections were thoroughly and independently examined by two experienced hepatic histopathologists. During the examination, 56 MVI lesions had diagnostic disagreements, which were resolved through consensus with a third senior pathologist. Ultimately, 441 MVI lesions were determined in total, identifying 30 rabbits as positive for MVI, yielding an overall detection rate of 81% (30/37). Among these rabbits, nine had concurrent satellite nodules larger than 1 mm.
The study included 37 liver lobes to produce WSIs, each of which was sectioned into tissue blocks at 3 mm intervals, resulting in an average of 20.7 ± 2.3 blocks per lobe and a total of 765 blocks. Among these liver lobes, 22 lobes (458 blocks) were sectioned to generate a single WSI per block, resulting in 458 WSIs. The remaining 15 lobes (307 blocks) were sectioned at smaller intervals of 0.3 mm, yielding a median of 8 (range 7-9) WSIs per block and producing a total of 2441 WSIs. For MVI examination, protocol A included six regions per specimen, and a total of 222 regions were examined in 37 specimens, whereas protocols B, C, D, and E included 37, 202, 765, and 2441 WSIs, respectively.
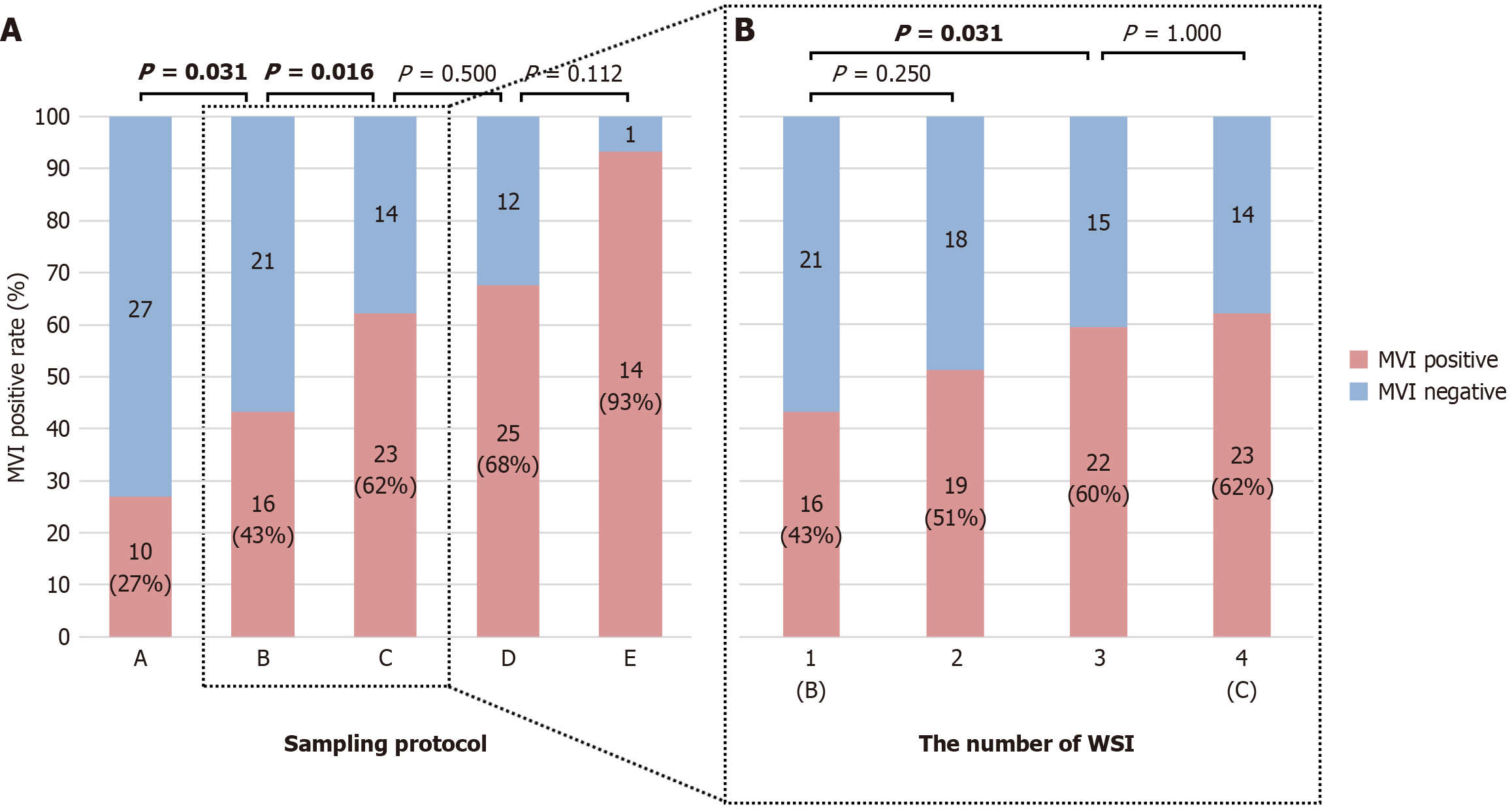
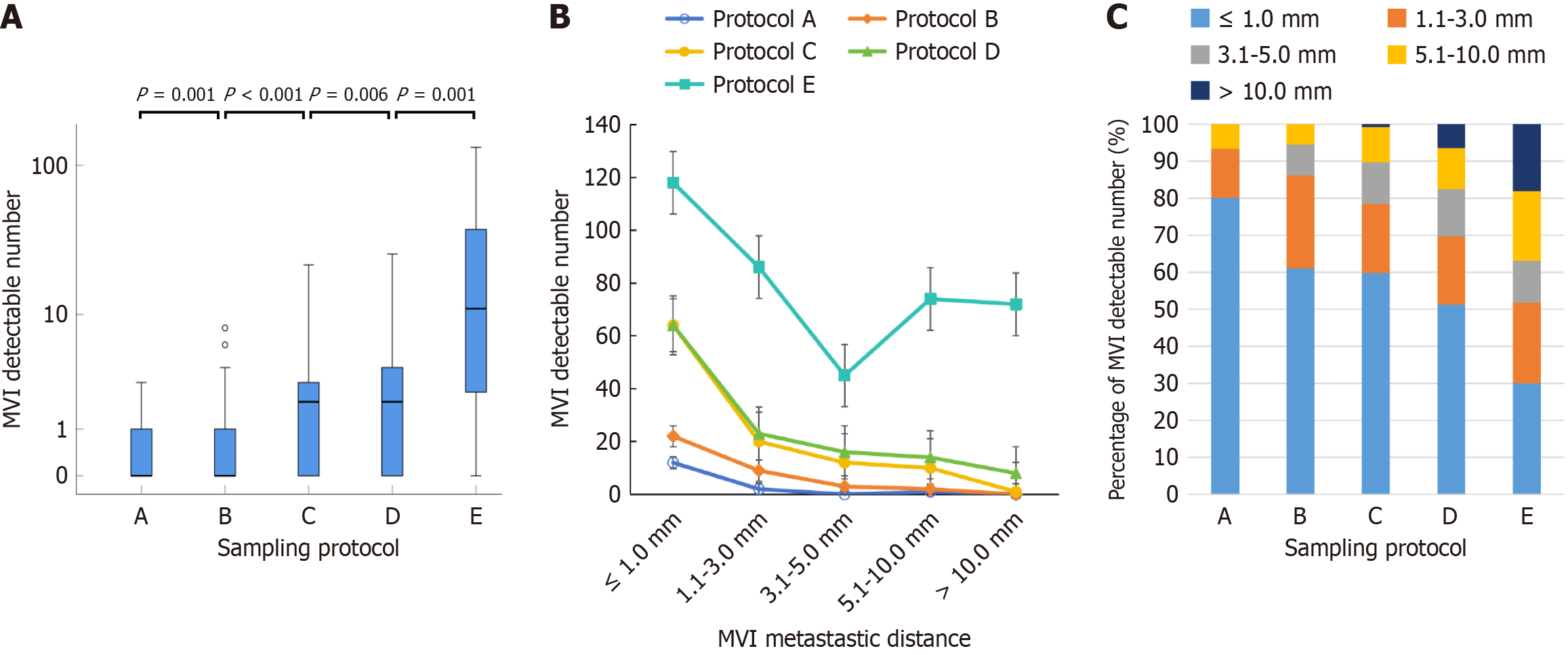
As the sampling range increased from protocols A to E, all MVI-positive cases in a previous protocol were included in the subsequent protocol. The MVI detection rates for protocols A, B, C, D, and E were 27% (10/37), 43% (16/37), 62% (23/37), 68% (25/37), and 93% (14/15), respectively. Figure 4A shows a sequential comparison of the MVI detection rates, which revealed that protocol B was superior to protocol A and that protocol C was superior to protocol B, with statistically significant differences observed (paired χ2 test: P = 0.031, P = 0.016). Although protocol D had a greater detection rate than protocol C and protocol E had a greater detection rate than did protocol D, the differences were not significant (paired χ2 test: P = 0.500; χ2 test: P = 0.112). The results suggested that protocol C, which has a smaller sampling range, resulted in a greater MVI detection rate than the other protocols.
We found that larger tumors produced a greater number of WSIs in protocol C, with a median of 5 WSIs (range 3-9) per tumor in this study. To determine the optimal number of WSIs needed for MVI detection, we incrementally increased the number of adjacent WSIs centered on the maximal tumor section in protocol B. Specifically, WSI1-WSI4 corresponded to WSIs 1-4, respectively. We found that the MVI detection rate was consistent with that of protocol C at WSI4. As shown in Figure 4B, the MVI detection rate did not significantly differ between WSI2 and WSI1 (P = 0.250); however, the MVI detection rate of three consecutive interval WSIs at the tumor center (WSI3) was significantly greater than that of WSI1 (P = 0.031), whereas it did not significantly differ from that of WSI4 (P = 1.000). Based on these findings, we recommend the use of WSI3 for the optimal MVI detection rate, which includes 1 WSI of the maximal tumor section and 2 WSIs of adjacent sections.
In this study, we assessed the performance of five protocols (A, B, C, D, and WSI3) for detecting MVI in 37 specimens, with the overall MVI detection rate as the reference standard (Table 2). Protocol A (7-point sampling) exhibited an accuracy of only 46% and a high false-negative rate of 67%. Conversely, the accuracy of the WSI3 protocol improved to 78%, and the false-negative rate decreased to 27%. Furthermore, protocol E had only 15 specimens, yet its detection rate reached 93% (14/15). The corresponding detection rates of protocols A, B, C, D, and WSI3 were 40% (6/15), 53% (8/15), 60% (9/15), 60% (9/15), and 60% (9/15), respectively. In comparison, the detection rate of protocol E was significantly greater than that of each of the other protocols (paired χ2 test: All P < 0.05). We evaluated the performance of other protocols for detecting MVI in 15 specimens using protocol E as the reference standard (Table 3). Similarly, protocol A was less accurate (47%) and had a greater false-negative rate (57%), whereas the WSI3 protocol as more accurate (67%) and had a lower false-negative rate (36%). Our analysis indicated that WSI3 emerged as the optimal protocol for detecting MVI because of its greater detection rate and narrower sampling range compared with the other protocols.
| Metrics | Protocol A | Protocol B | WSI3 | Protocol C | Protocol D |
| Positive rate | 0.27 (10/37) | 0.43 (16/37) | 0.59 (22/37) | 0.62 (23/37) | 0.68 (25/37) |
| Accuracy | 0.46 (0.42-0.75) | 0.62 (0.45-0.77) | 0.78 (0.61-0.90) | 0.81 (0.64-0.91) | 0.86 (0.70-0.95) |
| Sensitivity | 0.33 (0.18-0.53) | 0.53 (0.35-0.71) | 0.73 (0.54-0.87) | 0.77 (0.57-0.89) | 0.83 (0.65-0.94) |
| Specificity | 1.00 (0.56-1.00) | 1.00 (0.56-1.00) | 1.00 (0.56-1.00) | 1.00 (0.56-1.00) | 1.00 (0.56-1.00) |
| Positive predictive value | 1.00 (0.66-1.00) | 1.00 (0.76-1.00) | 1.00 (0.82-1.00) | 1.00 (0.82-1.00) | 1.00 (0.83-1.00) |
| Negative predictive value | 0.26 (0.12-0.47) | 0.33 (0.15-0.57) | 0.47 (0.22-0.73) | 0.50 (0.24-0.76) | 0.58 (0.29-0.84) |
| False negative rate | 0.67 (0.47-0.82) | 0.47 (0.29-0.65) | 0.27 (0.13-0.46) | 0.23 (0.11-0.43) | 0.17 (0.06-0.35) |
| Metrics | Protocol A | Protocol B | WSI3/protocol C/protocol D |
| Positive rate | 0.40 (6/15) | 0.53 (8/15) | 0.60 (9/15) |
| Accuracy | 0.47 (0.22-0.73) | 0.60 (0.33-0.83) | 0.67 (0.39-0.87) |
| Sensitivity | 0.43 (0.19-0.70) | 0.57 (0.30-0.81) | 0.64 (0.36-0.86) |
| Specificity | 1.00 (0.05-1.00) | 1.00 (0.05-1.00) | 1.00 (0.05-1.00) |
| Positive predictive value | 1.00 (0.52-1.00) | 1.00 (0.60-1.00) | 1.00 (0.63-1.00) |
| Negative predictive value | 0.11 (0.01-0.49) | 0.14 (0.01-0.58) | 0.17 (0.01-0.64) |
| False negative rate | 0.57 (0.30-0.81) | 0.43 (0.19-0.70) | 0.36 (0.14-0.64) |
We evaluated the impact of different sampling protocols on the quantity and distribution of MVI lesions detected. The total numbers of detected MVI lesions via protocols A, B, C, D, and E were 15, 36, 107, 125, and 395, respectively, as illustrated in Figure 5A. These results show that expanding the sampling range significantly increased the quantity of MVI lesions detected (P < 0.05). Table 4 and Figure 5B compare the number of MVI lesions in different distance groups using various sampling protocols, indicating that most MVI lesions were found in adjacent nonneoplastic tissue, whereas fewer were detected in distant nonneoplastic tissue. All protocols showed a decreasing trend in the quantity of MVI lesions with increasing MVI metastatic distance. Moreover, protocol E had a significantly greater detection quantity of MVI than protocol D in each distance group (Mann-Whitney U test: P < 0.05). In addition, Table 4 and Figure 5C show the percentages of MVI lesions detected of in different distance groups for each sampling protocol. These results showed that, from protocols A to E, the percentage of MVI detected in adjacent (≤ 3 mm) nonneoplastic tissue gradually decreased from 93.3% to 51.7%, whereas the percentage in distant (> 3 mm) nonneoplastic tissue gradually increased from 6.7% to 48.3%. This finding suggests that expanding the sampling range can improve the ability to detect MVI in distant nonneoplastic tissue.
| MVI metastatic distance groups | Sampling protocol | ||||||||
| A | P value1 | B | P value2 | C | P value3 | D | P value4 | E | |
| ≤ 1.0 mm | 12 (80.0) | 0.004 | 22 (61.1) | < 0.001 | 64 (59.8) | 1.000 | 64 (51.2) | 0.005 | 118 (29.9) |
| 1.1-3.0 mm | 2 (13.3) | 0.066 | 9 (25.0) | 0.014 | 20 (18.7) | 0.083 | 23 (18.4) | 0.005 | 86 (21.8) |
| 3.1-5.0 mm | 0 (0.0) | 0.083 | 3 (8.3) | 0.041 | 12 (11.2) | 0.102 | 16 (12.8) | 0.006 | 45 (11.4) |
| 5.1-10.0 mm | 1 (6.7) | 0.317 | 2 (5.6) | 0.039 | 10 (9.4) | 0.102 | 14 (11.2) | 0.014 | 74 (18.7) |
| > 10.0 mm | 0 (0.0) | 1.000 | 0 (0.0) | 0.317 | 1 (0.9) | 0.066 | 8 (6.4) | 0.008 | 72 (18.2) |
Accurate and comprehensive histological diagnosis of MVI is crucial for expediting timely intervention and reducing recurrence in patients with HCC following surgery. Furthermore, the establishment of a preoperative MVI prediction model is highly contingent upon the precise histological identification of MVI after surgery. Therefore, improving the accuracy and reliability of postoperative pathological diagnosis is essential for preoperative evaluation and formulation of postoperative treatment strategies for MVI. In this study, an animal experiment was conducted to assess the reliability of the current pathological diagnosis of MVI. Furthermore, we used WSI-based sampling protocols that offer a more comprehensive MVI pathological diagnosis, especially in detecting distant peritumoral MVI lesions, thereby improving the detection rate. This approach improves the management of HCC patients with MVI and may reduce recurrence rates.
Consistent with previous investigations[15,20-22], our results revealed a relatively low detection rate (27%) of MVI using protocol A (7-point sampling). Protocol A exhibited an accuracy of only 46% and a notable false-negative rate of 67%, indicating the inadequacy of the current clinical sampling protocol in accurately detecting MVI in patients with HCC. Conversely, our study effectively demonstrated a significantly greater MVI detection rate when using protocol B (WSI sampling) than when using the 7-point protocol. This finding substantiates prior research that highlights the efficacy of WSI in increasing the detection rate of MVI compared with conventional pathological sampling methods[15].
Furthermore, we observed a noteworthy improvement in the MVI detection rate by increasing the number of WSIs obtained from tumor sections, as implemented in protocol C, compared to protocol B (62% vs 43%, P = 0.016). However, the detection rates of protocol C and protocol D did not significantly differ (62% vs 68%, P = 0.500), with protocol D involving the acquisition of a greater number of WSIs from distant nontumor sections. The lack of a significant difference may be because MVI mostly occurs around the tumor periphery, with a sparse distribution in distant nontumor tissue and a relatively small sample size. These findings suggest that increasing the number of WSIs in tumor sections provides a more effective approach for MVI detection than using nontumor sections. Notably, protocol E, which involved the acquisition of a substantial number of WSIs from 15 specimens, exhibited the highest MVI detection rate of 93% (14/15). Moreover, the detection rate of this protocol was significantly greater than that of each of the other protocols in these 15 specimens. The noteworthy detection rate of MVI achieved by protocol E emphasizes the potential risk of missed diagnoses associated with other sampling protocols. Additionally, we acknowledge the practical limitations of unrestricted sampling of HCC specimens in clinical practice. To optimize the diagnostic accuracy and alleviate the workload on pathologists, we identified WSI3 as the optimal protocol, achieving an MVI detection rate comparable to that of protocol C with fewer samples. Overall, our study provides valuable insights for developing clinical sampling protocols based on WSIs to increase the accuracy of MVI diagnosis, which can notably impact clinical practice. In terms of diagnosis, it enables more precise tumor staging, which is crucial for formulating appropriate treatment plans. With respect to treatment decisions, more aggressive adjuvant therapies, such as targeted therapies or immunotherapies, may be considered. This strategy ultimately contributes to improved patient outcomes and reduced HCC recurrence rates.
In clinical practice, the number and distribution of MVI lesions play crucial roles in predicting postoperative recurrence and long-term survival in HCC patients[23,24]. Our study explored the quantity and distribution of MVI lesions and revealed a pattern in which the number of MVI lesions significantly increased with increasing sampling range and gradually decreased with increasing distance from the primary tumor, which was consistent with the occurrence and progression of tumor metastasis. Previous studies have reported that 93.4%, 93.9%, and 83.3% of peritumoral MVI lesions are distributed within 1 cm of the primary tumor according to the 7-point baseline protocol[14,25,26], but inadequate sampling via this protocol may lead to missed MVI detection in distant nonneoplastic tissue. Using the same protocol in the animal model, this study confirmed that 93.3% of MVI lesions were detected in liver tissue located < 3 mm from the tumor margin (similar to < 1 cm from the tumor in human HCC). However, the WSI pathology sampling protocol enabled an increased sampling range of distant nonneoplastic tissue and revealed an increasing percentage of MVI detection in > 3 mm distant nonneoplastic tissue from protocols B to E, with the highest percentage in protocol E (48.3%). This finding suggests that the previous 7-point sampling protocol may have overlooked MVI in distant nonneoplastic tissue, which could be a potential explanation for the high recurrence rate of HCC.
The present study utilized an animal model of rabbit VX2 in situ liver tumors, which may not be entirely generalizable to human subjects. However, we contend that this model is appropriate for several reasons. First, our study utilized WSIs of the entire liver lobe to examine the distribution of peritumoral MVI. This comprehensive approach allowed us to provide strong evidence to assess the accuracy of pathology sampling protocols and select the optimal pathological protocol to improve MVI detection. However, achieving such extensive evaluation in human liver tissue may be challenging; therefore, we selected the rabbit model as an alternative. Second, the rabbit VX2 in situ liver tumor model is widely used in liver cancer research and has blood supply features similar to those of human liver cancer[27]. Third, VX2 tumors in the rabbit liver have properties comparable to those of human liver cancer and present a high incidence of MVI. In a previous study by Wang et al[28], 14 rabbit VX2 in situ liver tumor animal models were constructed, and the inci
Despite its notable findings, the present investigation is subject to several limitations. First, the small sample size may have contributed to the lack of significant differences between certain sampling protocols. Given the variability in MVI distribution, a larger sample size would likely increase the robustness and reliability of our findings regarding the performance of different sampling protocols in detecting MVI. Furthermore, the potential effect of tumor size on MVI detection rates across different protocols was not assessed by stratification. Finally, the optimal WSI3 pathological protocol was not validated in human HCC. For human HCC, we recommend obtaining three consecutive WSIs at 1 cm intervals, which are sampled from the center of the tumor. In the future, we will validate the WSI3 protocol in human HCC clinical settings to confirm its feasibility and practicality. Moreover, integrating artificial intelligence or machine learning techniques for automated WSI analysis will further optimize the operation process, facilitating the promotion of this valuable protocol in clinical practice.
In summary, our study underscores the inherent limitations of the current 7-point sampling protocol used in the pathological diagnosis of MVI, attributed to its low accuracy and high false-negative rate. However, we propose a practical and viable approach for pathological examination by obtaining three consecutive interval WSIs from the tumor center. This approach has demonstrated a remarkable increase in the detection rate of MVI and has successfully facilitated the ability to identify MVI in distant nonneoplastic tissue. Consequently, our findings provide valuable insights for optimizing pathological examination strategies, with the ultimate aim of enhancing MVI detection in future clinical practice.
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3139] [Article Influence: 523.2] [Reference Citation Analysis (37)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63874] [Article Influence: 15968.5] [Reference Citation Analysis (174)] |
| 3. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2364] [Article Influence: 394.0] [Reference Citation Analysis (1)] |
| 4. | Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 432] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 5. | Vitale A, Peck-Radosavljevic M, Giannini EG, Vibert E, Sieghart W, Van Poucke S, Pawlik TM. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol. 2017;66:412-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 6. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 565] [Article Influence: 113.0] [Reference Citation Analysis (1)] |
| 7. | Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 8. | Zhang XP, Wang K, Wei XB, Li LQ, Sun HC, Wen TF, Chai ZT, Chen ZH, Shi J, Guo WX, Xie D, Cong WM, Wu MC, Lau WY, Cheng SQ. An Eastern Hepatobiliary Surgery Hospital Microvascular Invasion Scoring System in Predicting Prognosis of Patients with Hepatocellular Carcinoma and Microvascular Invasion After R0 Liver Resection: A Large-Scale, Multicenter Study. Oncologist. 2019;24:e1476-e1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 9. | Zhang XP, Zhou TF, Wang ZH, Zhang F, Zhong CQ, Hu YR, Wang K, Chai ZT, Chen ZH, Wu MC, Lau WY, Cheng SQ. Association of Preoperative Hypercoagulability with Poor Prognosis in Hepatocellular Carcinoma Patients with Microvascular Invasion After Liver Resection: A Multicenter Study. Ann Surg Oncol. 2019;26:4117-4125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Miyata R, Tanimoto A, Wakabayashi G, Shimazu M, Nakatsuka S, Mukai M, Kitajima M. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J Gastroenterol. 2006;41:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 11. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J; Guideline Committee. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 185] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 12. | Hu HT, Wang Z, Kuang M, Wang W. Need for normalization: the non-standard reference standard for microvascular invasion diagnosis in hepatocellular carcinoma. World J Surg Oncol. 2018;16:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Sheng X, Ji Y, Ren GP, Lu CL, Yun JP, Chen LH, Meng B, Qu LJ, Duan GJ, Sun Q, Ye XQ, Li SS, Yang J, Liao B, Wang ZB, Zhou JH, Sun Y, Qiu XS, Wang L, Li ZS, Chen J, Xia CY, He S, Li CY, Xu EW, Geng JS, Pan C, Kuang D, Qin R, Guan HW, Wang ZD, Li LX, Zhang X, Wang H, Zhao Q, Wei B, Zhang WJ, Ling SP, Du X, Cong WM; Liver Cancer Pathology Group of China (LCPGC). A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14:1034-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Chen L, Chen S, Zhou Q, Cao Q, Dong Y, Feng S, Xiao H, Wang Y, Liu X, Liao G, Peng Z, Li B, Tan L, Ke Z, Li D, Peng B, Peng S, Zhu L, Liao B, Kuang M. Microvascular Invasion Status and Its Survival Impact in Hepatocellular Carcinoma Depend on Tissue Sampling Protocol. Ann Surg Oncol. 2021;28:6747-6757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Yu HM, Wang K, Feng JK, Lu L, Qin YC, Cheng YQ, Guo WX, Shi J, Cong WM, Lau WY, Dong H, Cheng SQ. Image-matching digital macro-slide-a novel pathological examination method for microvascular invasion detection in hepatocellular carcinoma. Hepatol Int. 2022;16:381-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617-3624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 507] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 17. | Li L, Chen J, Huang Y, Wu C, Ye D, Wu W, Zhou X, Qin P, Jia T, Lin Y, Su Z. Precise localization of microvascular invasion in hepatocellular carcinoma based on three-dimensional histology-MR image fusion: an ex vivo experimental study. Quant Imaging Med Surg. 2023;13:5887-5901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Cheng Z, Zhang S, Wang L, Huang Z, Wang P, Zhu H, Wei Z, Zhou S. Ultrasound-guided percutaneous implantation of rabbit VX2 carcinoma, using a coaxial technique and gelfoam pellet injection combination to establish a rabbit liver tumor model. Diagn Interv Radiol. 2022;28:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Nitta H, Allard MA, Sebagh M, Ciacio O, Pittau G, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Guettier C, Lewin M, Samuel D, Baba H, Adam R. Prognostic Value and Prediction of Extratumoral Microvascular Invasion for Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:2568-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 20. | Chong HH, Yang L, Sheng RF, Yu YL, Wu DJ, Rao SX, Yang C, Zeng MS. Multi-scale and multi-parametric radiomics of gadoxetate disodium-enhanced MRI predicts microvascular invasion and outcome in patients with solitary hepatocellular carcinoma ≤ 5 cm. Eur Radiol. 2021;31:4824-4838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 21. | Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Zhang R, Xu L, Wen X, Zhang J, Yang P, Zhang L, Xue X, Wang X, Huang Q, Guo C, Shi Y, Niu T, Chen F. A nomogram based on bi-regional radiomics features from multimodal magnetic resonance imaging for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Quant Imaging Med Surg. 2019;9:1503-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 537] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 24. | Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, Kakuma T, Torimura T, Sata M. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Feng LH, Dong H, Lau WY, Yu H, Zhu YY, Zhao Y, Lin YX, Chen J, Wu MC, Cong WM. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Zhou KQ, Sun YF, Cheng JW, Du M, Ji Y, Wang PX, Hu B, Guo W, Gao Y, Yin Y, Huang JF, Zhou J, Fan J, Yang XR. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine. 2020;62:103107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Kuszyk BS, Boitnott JK, Choti MA, Bluemke DA, Sheth S, Magee CA, Horton KM, Eng J, Fishman EK. Local tumor recurrence following hepatic cryoablation: radiologic-histopathologic correlation in a rabbit model. Radiology. 2000;217:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wang Z, Wang W, Liu GJ, Yang Z, Chen LD, Huang Y, Li W, Xie XY, Lu MD, Kuang M. The role of quantitation of real-time 3-dimensional contrast-enhanced ultrasound in detecting microvascular invasion: an in vivo study. Abdom Radiol (NY). 2016;41:1973-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |