Published online Jan 28, 2025. doi: 10.3748/wjg.v31.i4.99312
Revised: November 13, 2024
Accepted: November 20, 2024
Published online: January 28, 2025
Processing time: 163 Days and 21.8 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a highly prevalent liver pathology in need of novel pharmacological treatments to com
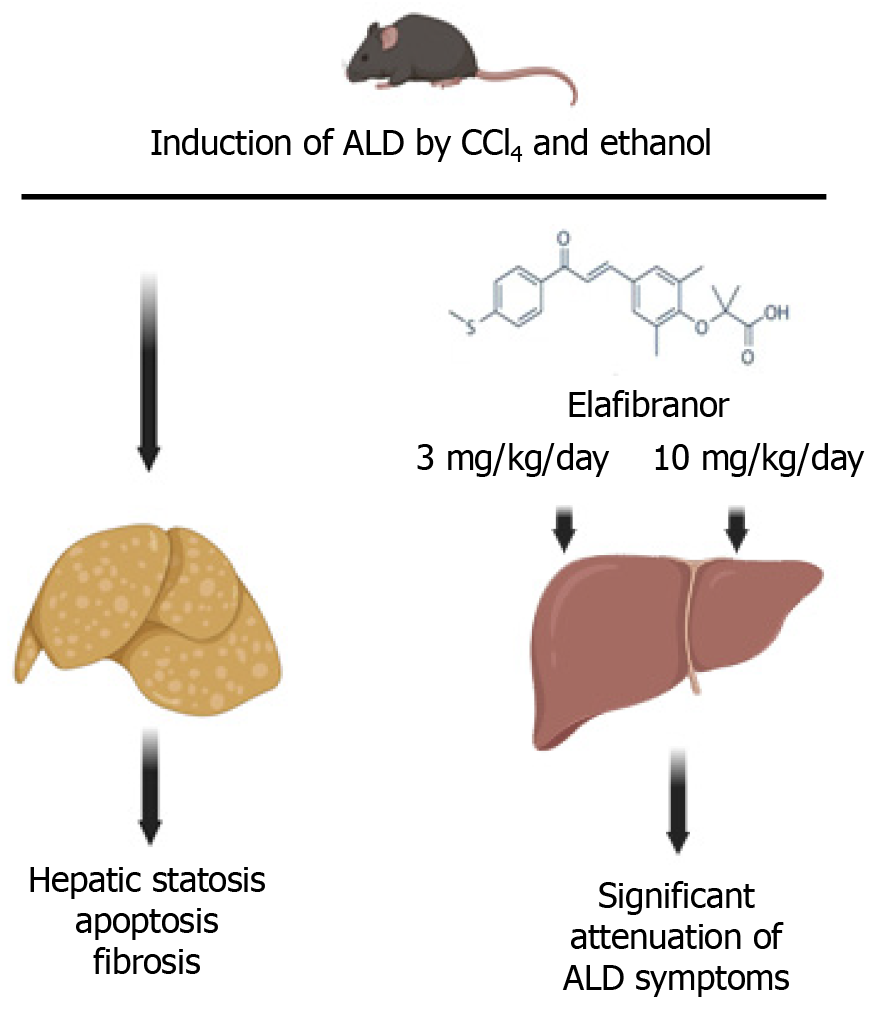
Core Tip: Metabolic dysfunction-associated steatotic liver disease needs novel pharmacological treatments. Elafibranor, a PPAR agonist, remains a promising molecule against steatotic liver disease caused by alcohol consumption. Koizumi et al reported, in World Journal of Gastroenterology, on their use of a mouse model of alcohol-associated liver disease which indicated that hepatic steatosis, liver fibrosis, and hepatocyte apoptosis were alleviated by administration of elafibranor. These data support the potential beneficial action of elafibranor, warranting clinical testing.
- Citation: Pirola L. Elafibranor, a dual PPARα and PPARδ agonist, reduces alcohol-associated liver disease: Lessons from a mouse model. World J Gastroenterol 2025; 31(4): 99312
- URL: https://www.wjgnet.com/1007-9327/full/v31/i4/99312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i4.99312
Metabolic dysfunction-associated steatotic liver disease (MASLD) is defined as a steatotic liver disease (SLD) associated to at last one cardiometabolic risk factor[1]. It is the most prevalent liver pathology worldwide, affecting almost 30% of the adult population[2]. MASLD is projected to further increase its burden in the coming years due to the aging of the population and the ever-increasing incidence of obesity[3].
MASLD pathologies were, until recently, identified as non-alcoholic fatty liver disease [or non-alcoholic steatohepatitis (NASH)]. MASLD is defined by the absence of harmful alcohol intake. Yet, SLD also develops upon excessive alcohol consumption, and pharmacological treatments for alcohol-associated liver disease (ALD) are still being sought.
MASLD progression, leading to metabolic dysfunction-associated steatohepatitis (MASH) characterized by moderate to advanced fibrosis (F2 to F3 stages), has been the object of intensive investigation to identify potential pharmacological agents to be used along with diet and exercise. In the search for therapeutic options for MASLD, nuclear receptors, including THR, and PPARα and δ as molecular targets are under intense scrutiny. As of today, only resmetirom, an orally administered selective agonist of THR-β, has proven its efficacy in the treatment of MASH and has recently obtained conditional approval for marketing[4].
The discovery of a dual PPARα and PPARδ agonist, termed GFT505 and later elafibranor[5], also raised substantial hopes to target MASH, with promising preclincal and clinical early phase results[5]. Following these initial promises, a multicenter double-blind and placebo-controlled phase III study (Resolve-IT) evaluating the safety and efficacy of elafibranor vs placebo in a cohort of participants with NASH did not lead to statistically significant improved primary endpoints at an interim analysis. It consisted of the resolution of NASH without worsening of the stage 2 and 3 fibrotic state as compared to the placebo group. Hence, the study was terminated[6].
Elafibranor works through activation of PPARα and δ targets in multiple cell types and biological processes involved in the pathophysiology of cholestatic liver disease, including cholestasis (impairment of bile flow in the liver), bile toxicity, inflammation and fibrosis, and bile acid output. Elafibranor has also been the subject of clinical investigation as a treatment for primary biliary cholangitis, and the efficacy and safety in this disease was shown[7].
Writing in the World Journal of Gastroenterology, Koizumi et al[8] added a new exploratory aspect to the potential pharmacological action of elafibranor in liver disease by studying its pharmacological effect on experimentally induced ALD in a preclinical mouse model. ALD in mice was induced by continuously feeding mice a liquid Lieber-DeCarli diet containing 2.5% ethanol in conjunction with three weekly intraperitoneal injections of hepatotoxic carbon tetrachloride (CCl4), a well-known inducer of hepatotoxicity and liver fibrosis[9].
Simultaneous addition of elafibranor by daily gavage at a low or high dose (3 and 10 mg/kg of body weight, respectively) improved several pathological parameters observed during the development of SLD. It decreased aspartate aminotransferase and alanine aminotransferase activity, lowered serum triglycerides, lowered liver fat accumulation, and increased lipolysis and fatty acid oxidation.
Elafibranor also: (1) Prevented ALD-induced apoptosis, as measured by the TUNEL assay showing decreased apoptosis; (2) Allowed recovery of hepatocyte proliferation as measured by Ki67 immunostaining; and (3) Improved autophagy, which is strongly impaired in ALD animals. These biochemical improvements resulted in lowered scores of fibrosis and an overall relief from liver inflammation as demonstrated by decreased infiltration of Kupfer cells in the hepatic parenchyma, lowered activation of NF-kB signaling, and reduced expression levels of proinflammatory genes Tnfa, Il1b, Il6, and Ccl2 (encoding monocyte chemoattractant protein 1). Importantly, elafibranor also ameliorated the intestinal barrier function by reinstating the expression of tight junction genes and proteins Zo1, occludin, and claudin 2 and alleviating ALD-induced apoptosis, as observed in the liver.
In summary (Figure 1), the investigation of Koizumi et al[8], using a set of well-designed and informative experimental approaches, supported the notion that elafibranor may have a potential to ameliorate ALD. A small drawback of the study was that the experimental model was boosted by multiple CCl4 injections. Hence, it is difficult to discern the specific contribution to liver pathology caused by CCl4 or the administered ethanol. In this respect, follow-up studies inducing ALD in rodents by administration of the Lieber-DeCarli ethanol-supplemented diet (without CCl4 administration) would prove informative[10]. Also, the induction of ALD and therapeutic treatment by elafibranor were simultaneous, while translational use in humans would occur once the symptoms of ALD had occurred. Finally, it must not be discounted that in most patients with SLD caused by alcohol consumption, alcohol might not be the only contributor, with other nutritional of lifestyle habits contributing to the manifestation of the pathology. Further studies in animal models may be warranted to fully define the positive effects of elafibranor on ALD. As the efficacy and safety profiles of elafibranor have been already proven in humans, clinical testing on patients suffering from ALD may be feasible to potentially define elafibranor as a treatment for the disease.
| 1. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 454] [Article Influence: 454.0] [Reference Citation Analysis (1)] |
| 2. | Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36:912-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 3. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1713] [Article Influence: 244.7] [Reference Citation Analysis (0)] |
| 4. | Sookoian S, Pirola CJ. Resmetirom for treatment of MASH. Cell. 2024;187:2897-2897.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 5. | Cariou B, Zaïr Y, Staels B, Bruckert E. Effects of the new dual PPAR α/δ agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care. 2011;34:2008-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients with Nonalcoholic Steatohepatitis (NASH) (RESOLVE-IT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT02704403 ClinicalTrials.gov Identifier: NCT02704403. |
| 7. | Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, Arrese M, Corpechot C, Francque SM, Heneghan MA, Invernizzi P, Jones D, Kruger FC, Lawitz E, Mayo MJ, Shiffman ML, Swain MG, Valera JM, Vargas V, Vierling JM, Villamil A, Addy C, Dietrich J, Germain JM, Mazain S, Rafailovic D, Taddé B, Miller B, Shu J, Zein CO, Schattenberg JM; ELATIVE Study Investigators’ Group; ELATIVE Study Investigators' Group. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med. 2024;390:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 8. | Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol. 2024;30:3428-3446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (2)] |
| 9. | Unsal V, Cicek M, Sabancilar İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environ Health. 2021;36:279-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 10. | Guo F, Zheng K, Benedé-Ubieto R, Cubero FJ, Nevzorova YA. The Lieber-DeCarli Diet-A Flagship Model for Experimental Alcoholic Liver Disease. Alcohol Clin Exp Res. 2018;42:1828-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |