Published online Jan 28, 2025. doi: 10.3748/wjg.v31.i4.101933
Revised: November 8, 2024
Accepted: December 6, 2024
Published online: January 28, 2025
Processing time: 89 Days and 18.3 Hours
Helicobacter pylori (H. pylori) infection has a protective effect on gastroesophageal reflux disease (GERD). Both of these diseases have a very high incidence and prevalence. As a result, GERD often recurs after anti-Helicobacter therapy. The problem of effective treatment of H. pylori infection and GERD is that the main groups of drugs [proton pump inhibitors (PPIs) and potassium-competitive acid blockers] have the possibility of side effects with use. Such supposed side effects have no evidence in randomized controlled trials that comply with the principles of evidence-based medicine. Morphological changes in the gastric mucosa after long-term use of antisecretory drugs should be considered as compensatory mechanisms of sanogenesis. The greatest concern for doctors who treat patients with antisecretory drugs is the risk of gastric carcinogenesis. This article presents an analysis of morphological and pathophysiological changes that occur after long-term use of antisecretory drugs (PPIs). Hypertrophy (hyperplasia) of G cells, enterochromaffin-like cells and possible fundic gland polyps (hyperplasia) are compensatory mechanisms of sanogenesis during long-term treatment with PPIs. These mechanisms are of primary importance for rehabilitation and prevention of complications in patients with GERD, non-steroidal anti-inflammatory drugs-gastropathy and other diseases during long-term treatment with PPIs. Under
Core Tip: Helicobacter pylori (H. pylori) infection has a protective effect on gastroesophageal reflux disease. Both of these diseases have a very high incidence and prevalence. The main obstacle to effective treatment H. pylori infection and gastroesophageal reflux disease is the possible side effects after use of proton pump inhibitors (PPIs). The article provides a precise analysis of scientific reviews and meta-analyses, as well as experimental scientific morphological studies on this topic. The author presented his original field of vision. He convincingly substantiated his opinion, proposed to interpret possible morphological and functional changes in the gastric mucosa after long-term use of PPIs as compensatory mechanisms of sanogenesis. This field of view will increase the number of indications for the use of PPIs and other antisecretory drugs.
- Citation: Kotelevets SM. Risks of anti-Helicobacter therapy and long-term therapy with antisecretory drugs. World J Gastroenterol 2025; 31(4): 101933
- URL: https://www.wjgnet.com/1007-9327/full/v31/i4/101933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i4.101933
The global prevalence of Helicobacter pylori (H. pylori) infection is very high. It was 52.6% among adults before 1990 and 43.9% in 2022. This reduction was achieved through expanded indications for anti-Helicobacter treatment[1]. H. pylori infection, affecting about half the world’s population, is the strongest risk factor for stomach cancer[2]. The Maastricht VI/Florence consensus report recommends anti-Helicobacter therapy using proton pump inhibitors (PPIs) for all infected patients, with a double dose recommended for increased eradication effectiveness[3]. Many authors discuss the relationship between H. pylori infection and gastroesophageal reflux disease (GERD), and the side effects of PPIs used for these conditions. Study results are often contradictory, with differing views on H. pylori’s protective effect against GERD[4,5]. Doctors don’t always consider the role of cytochrome P450 2C19 (CYP2C19) genetic polymorphisms when using PPIs to treat H. pylori infection and GERD. This large drug group is metabolized by the CYP2C19 enzyme at different rates, requiring personalized use[6]. Patients are categorized as rapid extensive, extensive, or poor metabolizers of PPIs, and genotyping is necessary before administering PPIs at a given dose. Poor metabolizers often experience side effects[7]. Esomeprazole, the S-isomer of omeprazole, is less affected by CYP2C19 polymorphism[8]. The Caucasian population comprises 70% fast, 25%-30% moderate, and 2%-5% slow metabolizers. This should be considered when using esomeprazole and rabeprazole[9]. Genetic polymorphism may influence the effectiveness and side effects of potassium-competitive acid blockers, as these drugs are metabolized in the liver by CYP enzymes[10].
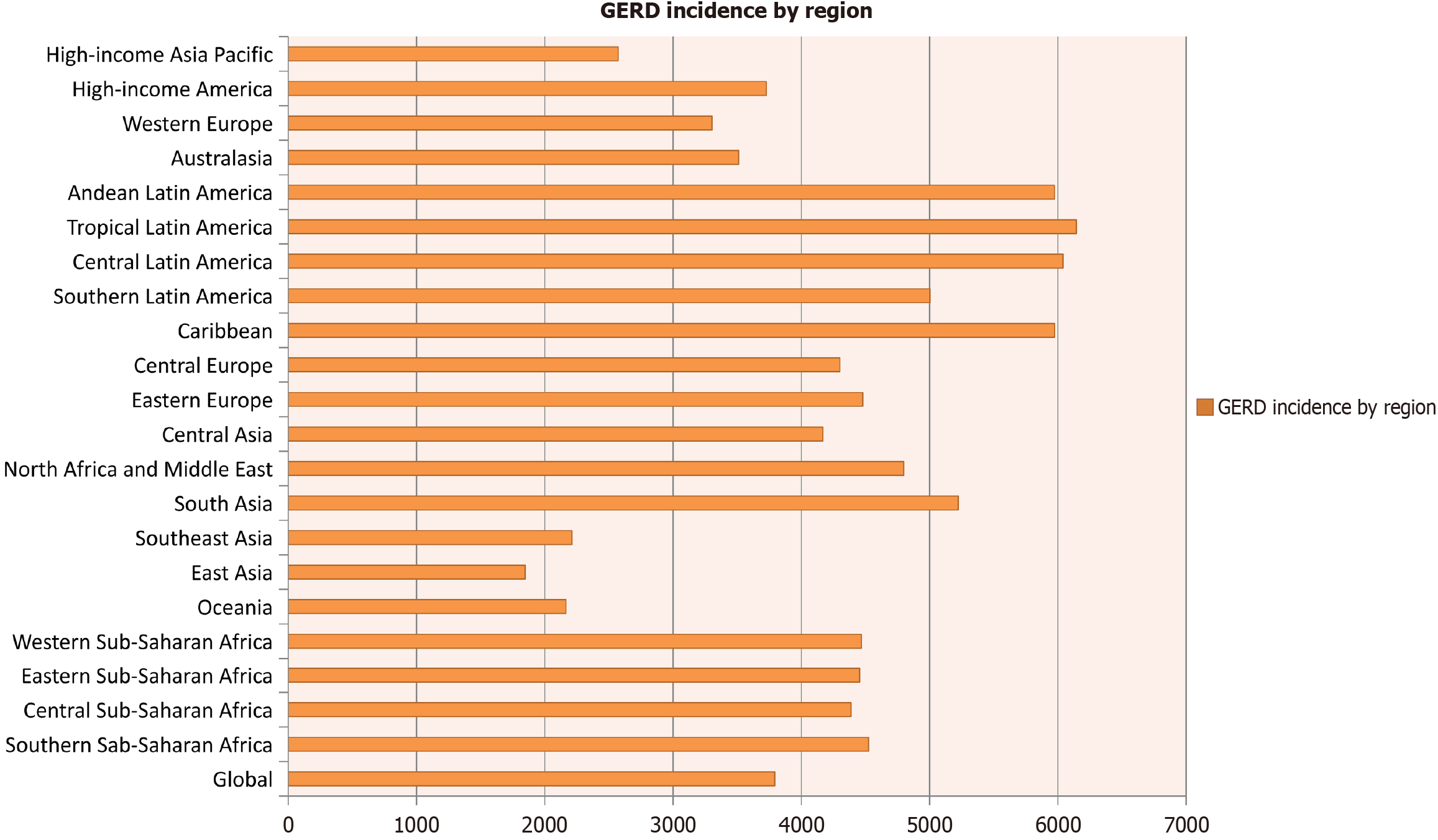
GERD has a very high prevalence and incidence, affecting up to 20% of adults in Western countries. It is a persistent, often recurrent disease[11]. Data on GERD incidence (per 100000) in 2019 show it significantly exceeds the incidence of type 1 diabetes (Figure 1)[12]. GERD’s persistent, recurrent nature necessitates continuous maintenance therapy with PPIs after initial treatment cessation[13]. PPIs are the most effective drugs for GERD pharmacotherapy[14]. Some authors suggest long-term PPI use can promote small intestinal bacterial overgrowth, Clostridium difficile, and other intestinal infections, and affect gut microbiota composition[15]. PPIs are also associated with bone fractures and electrolyte deficiencies[16], and may contribute to diarrhea, pneumonia, and peritonitis potentially caused by various infections[17]. Some recommend short-duration, reduced-dose PPI treatment for GERD to minimize side effects[18]. The lack of evidence on long-term PPI use has led to unfounded concerns about iatrogenic consequences like intestinal infections, bone fractures, malabsorption, gastric polyps, and various cancers. The hypothesized increased risk of gastric and colorectal cancer has not been confirmed[19]. Arab authors note untreated GERD can progress to severe complications, including esophagitis, esophageal erosions, bleeding, ulcers, strictures, Barrett’s esophagus, and adenocarcinoma[20]. Treatment adherence is crucial for drug therapy effectiveness, including PPIs[21]. Negative information about PPIs can obviously affect patient adherence.
Worldwide, 240 million people use non-steroidal anti-inflammatory drugs (NSAIDs) for osteoarthritis[22], and 103 million suffer from lumbar spinal stenosis, also requiring long-term NSAID treatment[23]. Patients using NSAIDs and anticoagulants need long-term gastroprotection with PPIs[24]. Lack of gastroprotection with these ulcerogenic drugs inevitably leads to severe complications like gastrointestinal bleeding and ulcer perforation[25]. The annual incidence of hemorrhagic gastropathy is 84-100 per 100000, with a 3%-10% mortality rate[26]. Gastroprotective PPIs prevent dyspepsia, heartburn, and deaths from upper gastrointestinal hemorrhages[27]. Continuous PPI treatment is necessary for patients on antiplatelet and anticoagulant drugs to prevent hemorrhagic complications[28]. Elwood et al[29] con
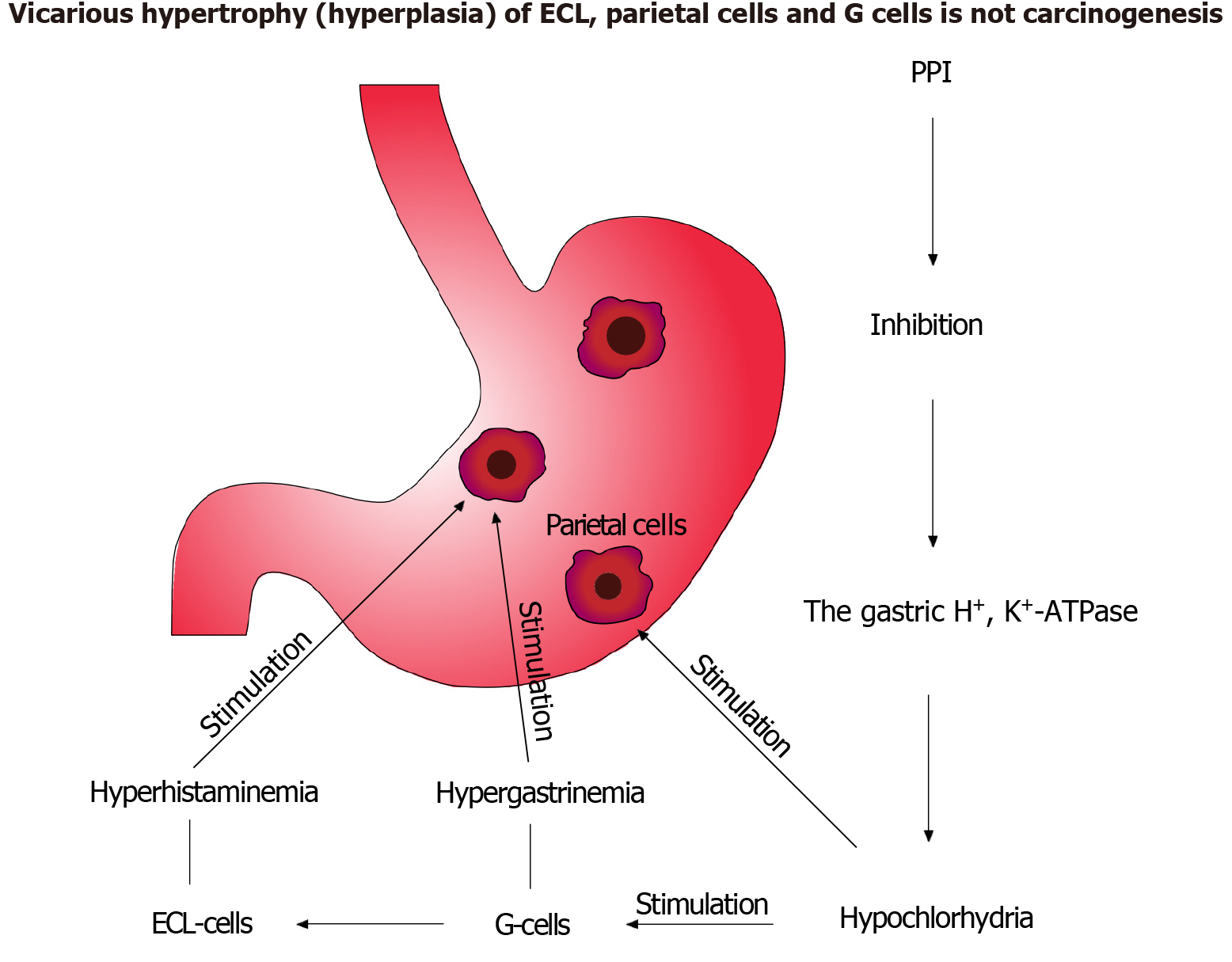
The potential for gastric polyps, carcinoids, and cancer is the most controversial consequence of long-term PPI use (Table 1)[30]. The hypothesis that PPI-induced hypergastrinemia plays a carcinogenic role has not been supported by randomized controlled trials. Bacterial overgrowth’s involvement in gastric carcinogenesis is also unproven. Lack of evidence only allows for assumptions about a relationship between long-term PPI use and gastric cancer[31]. Reviews of existing studies argue these studies lack the rigor of evidence-based medicine. No randomized trials convincingly confirm long-term PPI use’s negative effects[32,33]. The purported association between fundic gland polyps, enterochromaffin-like cell hyperplasia, and long-term PPI use lacks consistent morphological or functional interpretation[33,34]. Early experimental trials confirmed hypochlorhydria, hypergastrinemia, and hyperhistaminemia after long-term PPI use, correctly interpreting the morphological changes. Hypergastrinemia may enhance ulcer healing by increasing cell proliferation. Omeprazole’s suppression of gastric acid did not alter parietal cell turnover[35-37]. An analysis of these contradictions led to an original scheme on long-term PPI safety (Figure 2). The American Gastroenterological Association’s updated Best Clinical Practice Advice regulates PPI use safety in various GERD scenarios[38]. Some suggest potassium-competitive acid blockers are more effective than PPIs for suppressing gastric acid[39]. However, their use is limited by potential side effects, tolerability, and drug interactions. Potassium-competitive acid blockers (e.g., Vonoprazan) require further study[40]. Currently, their efficacy is established only in short-term anti-Helicobacter therapy and other acid-related conditions[41-44]. Observational studies on their long-term use are lacking, so side effects are unknown[45-47]. Some studies noted the risk of vonoprazan side effects in H. pylori infection treatment and experimental observations[48,49]. A meta-analysis of randomized controlled trials found no adverse effects after 8 weeks of treatment for erosive esophagitis and functional dyspepsia[50,51]. Intermittent and on-demand therapy are proposed as alternatives to continuous antisecretory drug administration[52]. Modern drug development utilizes artificial intelligence-driven modeling of pharmacokinetics, pharmacodynamics, and physiological processes, including predicting therapeutic and side effects[53,54]. Primary prevention of common diseases, including H. pylori infection, is the most effective method of control. It should also be taken into account that H. pylori infection is a family epidemiological disease[55]. The strategy of implementing preventive control of common diseases will help avoid frequent use of drugs and their side effects.
| No. | Possible consequences of long-term use of proton pump inhibitors |
| 1 | Significant deficiency of vitamins (B12 and C) and minerals (iron, calcium and magnesium) |
| 2 | Potential risk of congenital malformations in pregnant women |
| 3 | Infections of the intestine including Clostridium difficile, respiratory and urinary tract |
| 4 | Hypergastrinemia, which ultimately mediates the development of gastric polyps, gastric carcinoids and gastric cancer |
| 5 | Concomitant use of proton pump inhibitors with antiplatelet drugs such as clopidogrel may cause serious adverse cardiac events in patients |
| 6 | Osteoporosis-related fractures |
| 7 | Dementia |
| 8 | Rebound effect, hypersecretion after proton pump inhibitor withdrawal |
| 9 | Atrophy of the gastric mucosa (glands) |
G-cell and enterochromaffin-like cell hypertrophy (hyperplasia) and potential fundic gland polyps are compensatory sanogenetic mechanisms during long-term PPI treatment. These are crucial for rehabilitation and preventing complications in patients with GERD, NSAID-gastropathy, and other conditions requiring long-term PPIs. Understanding these compensatory, adaptive, sanogenetic, and carcinogenic mechanisms will expand indications for long-term PPI use safely and effectively. Furthermore, this understanding will allow for predicting the side effects of long-term potassium-competitive acid blocker use.
| 1. | Chen YC, Malfertheiner P, Yu HT, Kuo CL, Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, Wu CY, Lin JT, O'Morain C, Megraud F, Lee WC, El-Omar EM, Wu MS, Liou JM. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology. 2024;166:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 166] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 2. | Engelsberger V, Gerhard M, Mejías-Luque R. Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk. Front Cell Infect Microbiol. 2024;14:1339750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 3. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71:1724-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 630] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 4. | Rubenstein JH, Inadomi JM, Scheiman J, Schoenfeld P, Appelman H, Zhang M, Metko V, Kao JY. Association between Helicobacter pylori and Barrett's esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2014;12:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Qian B, Ma S, Shang L, Qian J, Zhang G. Effects of Helicobacter pylori eradication on gastroesophageal reflux disease. Helicobacter. 2011;16:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Lima JJ, Thomas CD, Barbarino J, Desta Z, Van Driest SL, El Rouby N, Johnson JA, Cavallari LH, Shakhnovich V, Thacker DL, Scott SA, Schwab M, Uppugunduri CRS, Formea CM, Franciosi JP, Sangkuhl K, Gaedigk A, Klein TE, Gammal RS, Furuta T. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin Pharmacol Ther. 2021;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Dickson EJ, Stuart RC. Genetics of response to proton pump inhibitor therapy: clinical implications. Am J Pharmacogenomics. 2003;3:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Kale-Pradhan PB, Landry HK, Sypula WT. Esomeprazole for acid peptic disorders. Ann Pharmacother. 2002;36:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hunfeld NG, Touw DJ, Mathot RA, van Schaik RH, Kuipers EJ. A comparison of the acid-inhibitory effects of esomeprazole and rabeprazole in relation to pharmacokinetics and CYP2C19 polymorphism. Aliment Pharmacol Ther. 2012;35:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Sugimoto M, Hira D, Murata M, Kawai T, Terada T. Effect of Antibiotic Susceptibility and CYP3A4/5 and CYP2C19 Genotype on the Outcome of Vonoprazan-Containing Helicobacter pylori Eradication Therapy. Antibiotics (Basel). 2020;9:645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Li N, Yang WL, Cai MH, Chen X, Zhao R, Li MT, Yan XL, Xue LW, Hong L, Tang MY. Burden of gastroesophageal reflux disease in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of disease study 2019. BMC Public Health. 2023;23:582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Liu S, Li Z, Wang R. Global, regional and national burden of gastroesophageal reflux disease, 1990-2019: update from the GBD 2019 study. Ann Med. 2022;54:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 13. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-28; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1120] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 14. | Clarrett DM, Hachem C. Gastroesophageal Reflux Disease (GERD). Mo Med. 2018;115:214-218. [PubMed] |
| 15. | Kiecka A, Szczepanik M. Proton pump inhibitor-induced gut dysbiosis and immunomodulation: current knowledge and potential restoration by probiotics. Pharmacol Rep. 2023;75:791-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 16. | Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104 Suppl 2:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009;104 Suppl 2:S10-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Iwakiri K, Fujiwara Y, Manabe N, Ihara E, Kuribayashi S, Akiyama J, Kondo T, Yamashita H, Ishimura N, Kitasako Y, Iijima K, Koike T, Omura N, Nomura T, Kawamura O, Ohara S, Ozawa S, Kinoshita Y, Mochida S, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2021. J Gastroenterol. 2022;57:267-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 19. | Hart AM. Evidence-based recommendations for GERD treatment. Nurse Pract. 2013;38:26-34; quiz 34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Shaqran TM, Ismaeel MM, Alnuaman AA, Al Ahmad FA, Albalawi GA, Almubarak JN, AlHarbi RS, Alaqidi RS, AlAli YA, Alfawaz KS, Daghriri AA. Epidemiology, Causes, and Management of Gastro-esophageal Reflux Disease: A Systematic Review. Cureus. 2023;15:e47420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Guadagnoli L, Simons M, McGarva J, Taft TH, van Tilburg MAL. Improving Patient Adherence to Lifestyle Changes for the Management of Gastroesophageal Reflux. Patient Prefer Adherence. 2022;16:897-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 1236] [Article Influence: 309.0] [Reference Citation Analysis (0)] |
| 23. | Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and Management of Lumbar Spinal Stenosis: A Review. JAMA. 2022;327:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 244] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 24. | Kanno T, Moayyedi P. Who Needs Gastroprotection in 2020? Curr Treat Options Gastroenterol. 2020;18:557-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Tuerk E, Doss S, Polsley K. Peptic Ulcer Disease. Prim Care. 2023;50:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Kate V, Sureshkumar S, Gurushankari B, Kalayarasan R. Acute Upper Non-variceal and Lower Gastrointestinal Bleeding. J Gastrointest Surg. 2022;26:932-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 486] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 28. | Lanas A, Dumonceau JM, Hunt RH, Fujishiro M, Scheiman JM, Gralnek IM, Campbell HE, Rostom A, Villanueva C, Sung JJY. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4:18020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 29. | Elwood P, Morgan G, Watkins J, Protty M, Mason M, Adams R, Dolwani S, Pickering J, Delon C, Longley M. Aspirin and cancer treatment: systematic reviews and meta-analyses of evidence: for and against. Br J Cancer. 2024;130:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 30. | Koyyada A. Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations. Therapie. 2021;76:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Chinzon D, Domingues G, Tosetto N, Perrotti M. Safety of long-term proton pump inhibitors: facts and myths. Arq Gastroenterol. 2022;59:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019;12:1756284819834511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Cheung KS, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori. World J Gastrointest Oncol. 2018;10:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706-1719.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 35. | Prinz C, Scott DR, Hurwitz D, Helander HF, Sachs G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am J Physiol. 1994;267:G663-G675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Li H, Helander HF. Parietal cell kinetics after administration of omeprazole and ranitidine in the rat. Scand J Gastroenterol. 1995;30:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Li H, Helander HF. Hypergastrinemia increases proliferation of gastroduodenal epithelium during gastric ulcer healing in rats. Dig Dis Sci. 1996;41:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Targownik LE, Fisher DA, Saini SD. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology. 2022;162:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 39. | Kim GH. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver. 2021;15:646-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | St Onge E, Phillips B. Vonoprazan: A New Potassium-Competitive Acid Blocker. J Pharm Technol. 2023;39:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 41. | Borody TJ, Ng J, Dolai S. Unlocking the path to efficient H. pylori eradication: Embracing potassium-competitive acid blockers (P-CABs). Saudi J Gastroenterol. 2023;29:323-325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Leowattana W, Leowattana T. Potassium-competitive acid blockers and gastroesophageal reflux disease. World J Gastroenterol. 2022;28:3608-3619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 43. | Domingues G, Chinzon D, Moraes-Filho JPP, Senra JT, Perrotti M, Zaterka S. Potassium-competitive acid blockers, a new therapeutic class, and their role in acid-related diseases: a narrative review. Prz Gastroenterol. 2023;18:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Kanu JE, Soldera J. Treatment of Helicobacter pylori with potassium competitive acid blockers: A systematic review and meta-analysis. World J Gastroenterol. 2024;30:1213-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 45. | Scarpignato C, Hunt RH. Potassium-competitive Acid Blockers: Current Clinical Use and Future Developments. Curr Gastroenterol Rep. 2024;26:273-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 46. | Dong Y, Xu H, Zhang Z, Zhou Z, Zhang Q. Comparative efficiency and safety of potassium competitive acid blockers versus Lansoprazole in peptic ulcer: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1304552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Tietto A, Faggin S, Scarpignato C, Savarino EV, Giron MC. Safety of potassium-competitive acid blockers in the treatment of gastroesophageal reflux disease. Expert Opin Drug Metab Toxicol. 2024;1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 48. | Ouyang M, Zou S, Cheng Q, Shi X, Zhao Y, Sun M. Comparative Efficacy and Safety of Potassium-Competitive Acid Blockers vs. Proton Pump Inhibitors for Peptic Ulcer with or without Helicobacter pylori Infection: A Systematic Review and Network Meta-Analysis. Pharmaceuticals (Basel). 2024;17:698. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Edelmuth RCL, Riascos MC, Al Asadi H, Greenberg JA, Miranda IC, Najah H, Crawford CV, Schnoll-Sussman FH, Finnerty BM, Fahey TJ, Zarnegar R. Gastric development of pancreatic acinar cell metaplasia after Vonoprazan therapy in rats. Surg Endosc. 2023;37:9366-9372. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Fang Y, Lou D, Zhou J, Zhang Q, Dai Y, Ren W. Efficacy and Safety of Potassium-competitive Acid Blockers Versus Proton Pump Inhibitors in Treating Erosive Esophagitis: A Meta-analysis Based on Randomized Controlled Trials. J Clin Gastroenterol. 2024;58:841-850. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Huh CW, Youn YH, Jung DH, Cha RR, Kim YJ, Jung K, Song KH, Bang KB, Tae CH, Choi SI, Shin CM; Functional Dyspepsia Research Group Under the Korean Society of Neurogastroenterology and Motility. Efficacy of Tegoprazan in Patients With Functional Dyspepsia: A Prospective, Multicenter, Single-arm Study. J Neurogastroenterol Motil. 2024;30:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | D'Souza S, Udemba S, Fass R. Is It Time for Noncontinuous Therapy for Gastroesophageal Reflux Disease? Gastroenterol Hepatol (N Y). 2024;20:273-280. [PubMed] |
| 53. | Wu K, Li X, Zhou Z, Zhao Y, Su M, Cheng Z, Wu X, Huang Z, Jin X, Li J, Zhang M, Liu J, Liu B. Predicting pharmacodynamic effects through early drug discovery with artificial intelligence-physiologically based pharmacokinetic (AI-PBPK) modelling. Front Pharmacol. 2024;15:1330855. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Uryu K, Imamura Y, Shimoyama R, Mase T, Fujimura Y, Hayashi M, Ohtaki M, Otani K, Hibino M, Horiuchi S, Fukui T, Fukai R, Chihara Y, Iwase A, Yamada N, Tamura Y, Harada H, Shinozaki N, Shimada T, Tsuya A, Fukuoka M, Minami H. Prognostic impact of concomitant pH-regulating drugs in patients with non-small cell lung cancer receiving epidermal growth factor receptor tyrosine kinase inhibitors: the Tokushukai REAl-world Data project 01-S1. Cancer Chemother Pharmacol. 2024;94:197-208. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Zhou JK, Zheng Y, Wang YP, Ji R. Prevalence and associated risk factors of Helicobacter pylori infection in community households in Lanzhou city. World J Gastroenterol. 2024;30:5018-5031. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |