Published online Jan 28, 2025. doi: 10.3748/wjg.v31.i4.100470
Revised: October 8, 2024
Accepted: December 3, 2024
Published online: January 28, 2025
Processing time: 134 Days and 21.6 Hours
As a new type of pollutant, the harm caused by microplastics (MPs) to organisms has been the research focus. Recently, the proportion of MPs ingested through the digestive tract has gradually increased with the popularity of fast-food products, such as takeout. The damage to the digestive system has attracted increasing attention. We reviewed the literature regarding toxicity of MPs and observed that they have different effects on multiple organs of the digestive system. The mechanism may be related to the toxic effects of MPs themselves, interactions with various substances in the biological body, and participation in various signaling pathways to induce adverse reactions as a carrier of toxins to increase the time and amount of body absorption. Based on the toxicity mechanism of MPs, we propose specific suggestions to provide a theoretical reference for the government and relevant departments.
Core Tip: This paper is a review of the damage caused by microplastics (MPs) to human digestive system organs and the related mechanisms. As an emerging pollutant, MPs have caused irreversible damage to the environment and human body. This paper analyzes and summarizes the mechanism of action of MPs on human digestive organs, and puts forward suggestions for relevant departments to reduce MPs pollution.
- Citation: Wang YF, Wang XY, Chen BJ, Yang YP, Li H, Wang F. Impact of microplastics on the human digestive system: From basic to clinical. World J Gastroenterol 2025; 31(4): 100470
- URL: https://www.wjgnet.com/1007-9327/full/v31/i4/100470.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i4.100470
Microplastics (MPs), or pieces of plastic smaller than 5 mm, were a concept first introduced by Thompson et al[1] of the University of Plymouth, United Kingdom, in the journal of Science in 2004. As a result of human activities, such as the use of cleaning products, cosmetics and medical supplies, a large amount of plastic waste is generated[2]. Most of them have not been properly disposed of but are left to become MPs, which are mainly divided into two categories: Primary MPs and secondary MPs; the former mainly refers to the plastic particles industrial products discharged into the water environment through rivers and sewage treatment plants[3], the latter refers to the plastic particles caused by physical, chemical and biological processes of large plastic waste such as ultraviolet radiation and mechanical degradation[4-6]. MPs are ubiquitous in the environment and are often deposited in the ocean[7,8], soil[9] and freshwater systems[10] in their natural state. They damage the natural environment and make it difficult for humans active in ecosystems to escape their harm[11]. Global demand for plastics continues to grow because of their stability, durability and versatility[12]. More than 6.3 billion tons of plastic waste were produced globally in 2017[13], and a few studies have pointed out that 12 billion tons of plastic waste may be produced by 2050[13]. Human beings have to ingest approximately 39000-52000 MPs every year[14], and 20 MPs in every 10 g of feces of a normal adult[15]. Recently, MPs have been reported in the blood of human[16], which indicates that MPs have been inextricably linked with human life.
MPs enter the human body mainly through the digestive[15], respiratory system[17] and skin systems[18]. Among them, the pathways to enter the digestive system are more diverse, including various media, such as food, air, and drinking water[19]. Natural foods containing high levels of MPs are primarily seafood[20]. A survey of European food-grade sea salt showed a median MP content of 466 MPs/kg with a mean mass estimate of 4.51 ± 6.74 μg/kg[21]. Air and drinking water are also important ways for humans to ingest MPs, we ingest 4400-5800 MPs per year from tap water[12,22]. With the popularity of takeout in recent years and the widespread use of plastic packaging boxes, the MP content in the human body has significantly increased[23]. Schwabl et al[15] first discovered the presence of MPs in human feces in 2019, suggesting that MPs may accumulate in the human digestive system and pose a health threat. Nowadays, their impact on the digestive system is a growing concern. Starting at the upper end of the digestive system, MPs alter the microbiota in the mouth[23], which may induce dental caries, periodontitis, bad breath and other oral problems. They then enter the esophagus, destroy the esophageal epithelial barrier and are associated with eosinophilic esophagitis[24]. MPs affect the distribution of gastrointestinal flora and the absorption of nutrients[25] while affecting the function of the liver and pancreas, including lipid[26] and blood sugar metabolism[27].
As an important system to ensure the normal progress of human metabolism, the digestive system is one of the most important ways to ingest MPs[28], which is greatly affected by them[29]. However, current research on the impact of MPs on the digestive system mostly focuses on discussing individual organs, and lacks a systematic and comprehensive argumentation of the digestive system. This review summarizes the role of MPs in the entire digestive system from a macro perspective.
Although there is no clear definition of the upper and lower limits of the MP size in actual research, the most commonly used is the 2008 definition by the National Oceanic and Atmospheric Administration, which states that plastic particles do not exceed 5 mm[30]. Surveys have shown serious MP pollution in soil[31]. The concentration of MPs in agricultural soil in China is 42960 MPs/kg, while the concentration of MPs in coastal sediments in India is as high as 73100 MPs/kg[32]. Researchers have confirmed that MPs can change soil properties and affect microorganisms and plant growth[33]. For example, MPs are genotoxic and cytotoxic to crops such as broad beans and onions[34], which may be related to their tendency to attach to plant roots and damage the corresponding cell structures[35]. The health of animals that consuming foods containing MPs is also at risk. Animals involved in soil decomposition such as earthworms and nematodes directly ingest MPs through their activities, causing metabolic disorders[36], genetic damage[37], neurotoxicity[38] and other adverse effects. In addition, chickens consuming plants containing MPs are associated with increased cardiotoxicity[39]. For rodents such as mice, studies have reported that MPs can cause great harm to their digestive systems, causing intestinal dysfunction, intestinal flora disorder, and even affecting the liver’s clearance function[40,41]. Moreover, MPs flow into the marine ecosystem[42,43]. Therefore, it is not surprising that MPs have been detected in corals and phytoplankton in the ocean[44]. Smaller shrimps may be more susceptible to their effects because they ingest food that is similar in size to MPs[45]. Commercial fish and crustaceans eat these organisms containing MPs, leading to further accumulation. Undoubtedly, it is difficult for humans at the top of the food chain to escape adverse effects[46,47]. Various studies have shown that they can accumulate and cause damage to the human gastrointestinal tract[28], liver[48], pancreas[49] and other organs.
Various studies have shown that MPs are unfavorable to organisms; they can affect the function of thrombin and the aggregation of platelets through the combination of coagulation factors and promote thrombosis formation[50]. They can affect digestive function by damaging gastrointestinal epithelial cells, adsorbing toxic substances and destroying the ecological balance of digestive tract microorganisms[51]. They can promote the inflammatory response in hepatocytes and the formation of liver fibrosis through the activation of signaling pathways such as the reactive oxygen species (ROS)/transforming growth factor-β (TGF-β)/Smad2/3 signaling axis[52], cGAS/STING pathway[48] and Wnt/β-catenin pathway[53].
As one of the eight major systems of the human body, the digestive system performs physiological functions, such as intake, transport, digestion of food, absorption of nutrients and excretion of waste. At the same time, digestive system diseases have a high incidence, high recurrence rate and high mortality status, which cause huge economic burdens to society and individuals[54]. With the development of society, more new pollutants have gradually entered human life and been ingested by the digestive system, causing various diseases, such as gastritis, peptic ulcers, inflammatory bowel disease, non-alcoholic fatty liver disease (NAFLD), pancreatitis and metabolic disorders. In the digestive system, there are various causes and pathogenesis of diseases. For example, gastrointestinal diseases are mostly caused by pollutants that damage mucosal cells and destroy the defensive barriers. The flora is disordered, the intestinal microecology is destroyed[55], the absorption of nutrients and immune defense is reduced[56] and the regulation of the brain-gut axis[57] is broken. Activation of the inflammatory pathway increases the secretion of inflammatory factors such as interleukin-1 (IL-1), IL-6, and IL-8, which can induce an imbalance of inflammatory factors/anti-inflammatory factors, persistent intestinal inflammation and barrier function impairment[58]. Another important disease, NAFLD, is caused by insulin resistance[59], inflammation and necrosis of hepatocytes, endoplasmic reticulum (ER) stress[60], liver fibrosis, intestinal flora disorders and other factors[61]. To a certain extent, MPs can induce these factors. They are mainly transported and absorbed in the gastrointestinal tract through the lymphatic follicles of the ileum into the digestive system, and harm to the human body is mainly divided into three parts: Inducing apoptosis of normal human cells or regulating gene expression through various signaling pathways[49,62], as a carrier of toxic chemicals such as di(2-ethylhexyl) phthalate (DEHP), polychlorinated biphenyls and polycyclic aromatic hydrocarbons[63], and as a foreign body to stimulate the body and induce oxidative stress reactions to destroy normal cells[64]. Even at non-cytotoxic concentrations, it can cause elevated levels of IL-6, tumor necrosis factor, and malondialdehyde, leading to adverse reactions[65], which requires further exploration.
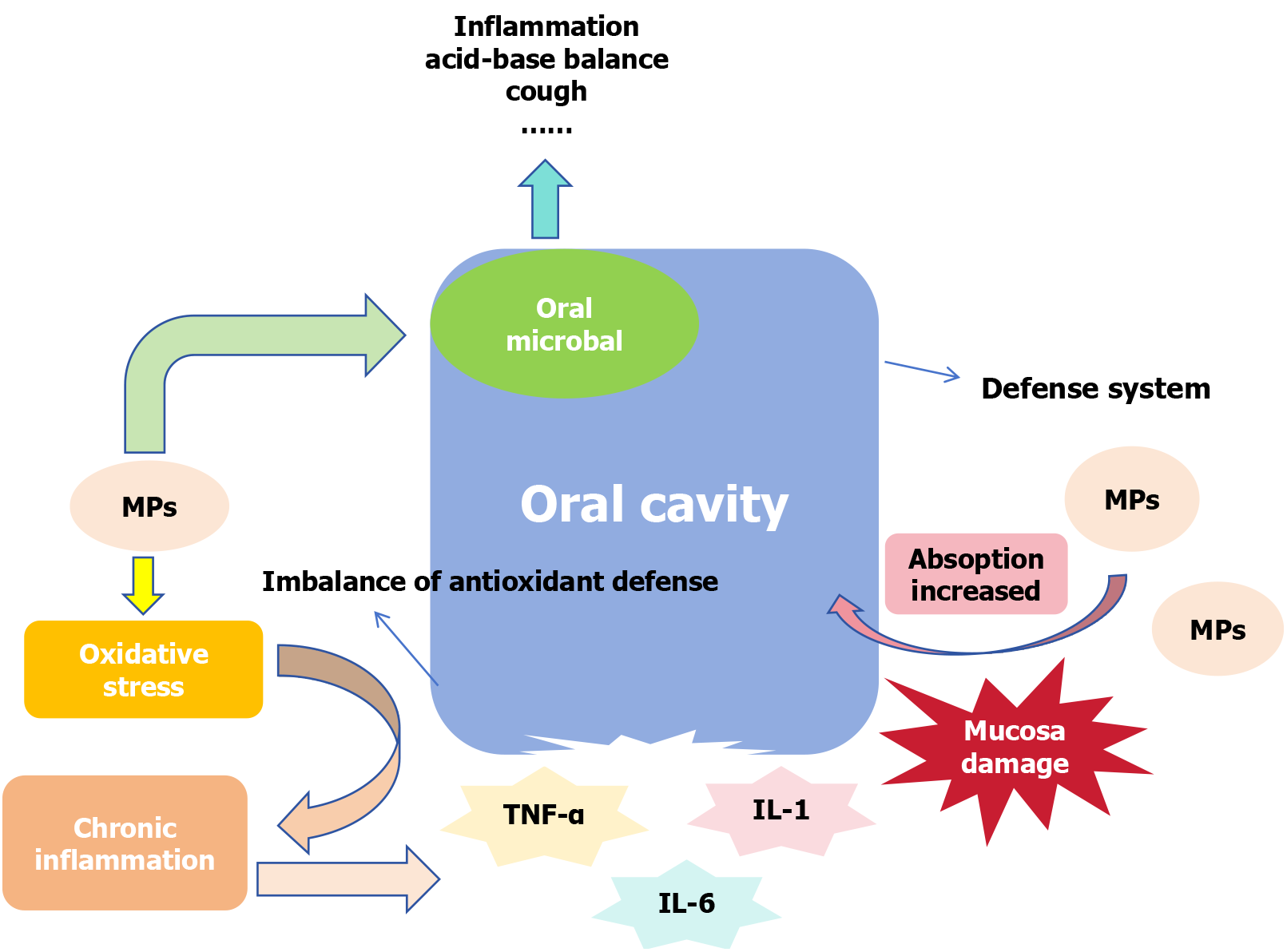
The mouth is usually the first stop point for MPs to enter the digestive system. The daily intake of MPs for average Chinese residents can reach 0.14 items/kg bw/day[66]. Therefore, the effect of MPs on the oral cavity cannot be ignored. MPs have been observed to damage the oral cavity by altering the composition of oral microorganisms[23] and destroying the oral mucosa (Figure 1). Microorganisms in the oral cavity colonize rapidly a few hours after birth[67]. As the second largest microbiota after the intestinal flora, they are mainly composed of bacteria, such as Salivary Streptococ
The concentration of MPs in the esophagus may be lower than that in the gastrointestinal tract, possibly because of the peristaltic movement of the esophagus and its smaller surface area[72]. However, the effects of MPs on the esophagus are undeniable, mainly by destroying the epithelial structure of the esophagus[24], altering the species and concentration of original bacteria in the esophagus, carrying toxic substances, and affecting the function of the brain-gut axis[73]. They can even cause eosinophilic esophagitis[67], esophageal cancer[74] and other diseases.
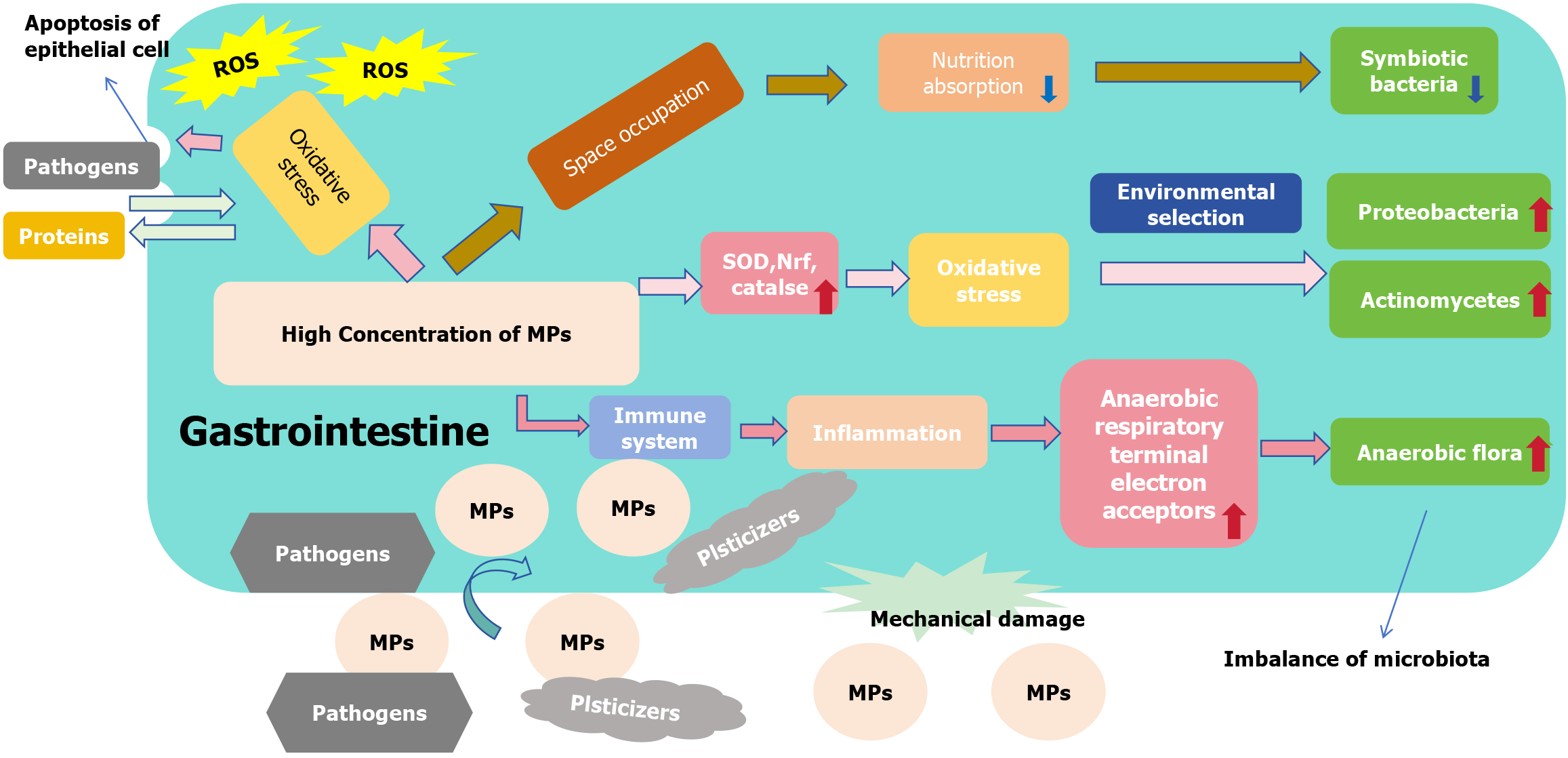
The role of the gastrointestinal tract is varied. In addition to being an important organ for absorbing nutrients, it is an important immune defense barrier in the body[75]. Ibrahim et al[76] reported MPs in colon sections of 11 patients with colorectal cancer after resection, confirming that ingested MPs can remain in the digestive tract. Studies have shown that these particles accumulate over time[62] and that ingestion of MPs can damage the gastrointestinal tract, affecting their normal function[28,51]. Microorganisms in the gastrointestinal tract play an important role in nutrient absorption[77], metabolism[78] and immune defense[79]. Therefore, disrupting the balance between the gastrointestinal flora and the organism will inevitably negatively impact the gastrointestinal tract and the whole organism. Mice fed high concentrations of MPs showed a decrease in the abundance of Parabacteroides and an increase in the abundance of Staphylococcus in the gastrointestinal tract microbiota of mice[58]. Previous studies have reported that patients with ulcerative colitis and irritable bowel syndrome have reduced Parabacteroides simulants in their gut microbiota compared to healthy individuals[80]. Collado et al[81] observed that Staphylococcus overgrowth is related to inflammatory bowel disease, which may be associated with inflammation induced by Staphylococcus superantigens. Therefore, MP-induced changes in Parabacteroides and Staphylococcus have been hypothesized to contribute to intestinal inflammatory diseases[58]. MPs may be associated with intestinal inflammation and related diseases. The altered composition of the flora and dysfunctional ecological balance may be related to various factors (Figure 2). First, the expression of superoxide dismutase (SOD), catalase- and nuclear factor erythroid 2-related factor-signaling pathway-related genes was enhanced in the presence of MPs, which in turn induced an oxidative stress response[82]. The abundance of Actinomycetes and Proteobacteria increased in the oxidized state environment owing to environmental selectivity[83]. Second, MPs stimulate the body’s immune system and induce an inflammatory response of the immune system[84]. In an inflammatory environment, peroxides and nitrogenous substances in the host intestine can produce peroxynitrite, which can be rapidly converted into nitrate[85,86]. Enterobacteriaceae, which may contain enzymes that break down nitrate, proliferate massively in an inflammatory environment[87]. Ultimately, the intestinal microbial balance was disrupted. Third, MPs are difficult to absorb and utilize as inorganic substances, while they can occupy the gastrointestinal space, resulting in reduced nutrient absorption by the body[88], and the normal function of symbiotic flora is also compromised due to the lack of nutrition[89]. Fourth, MPs can act as a carriers of harmful substances owing to their large specific surface area, carrying toxins into the gastrointestinal tract and affecting the microbial composition[90]. Lastly MPs, can abrade the digestive tract and cause mechanical damage as foreign bodies[91], and the growth of bacteria is inevitably affected by the environment in which they survive. The effect of MPs on the gastrointestinal flora should not be underestimated, and its effect on gastrointestinal function should not be ignored.
In addition to their effects on microorganisms in the gastrointestinal tract, MPs can act directly on the gastrointestinal tract. MPs induce oxidative stress to generate large amounts of ROS, mediating apoptosis in epithelial cells[62]. As the first line of defense of the immune system, apoptosis of the epithelial cells of the gastrointestinal mucosa leads to increased permeability, and large proteins and bacteria are free to pass through the intestinal tract, which may result in serious infectious conditions[92]. MPs, owing to their large specific surface area, can easily serve as a carriers to transport pathogens such as bacteria and toxic substances such as plasticizers, into the tract, causing intestinal inflammatory and structural damage[90,93,94]. Increased permeability of the gastrointestinal tract and elevated intestinal absorption of MPs, cause a vicious cycle.
The liver is the largest glandular and parenchymal organ and the most metabolically active organ in the body[95]. As an emerging pollutant, MPs have been reported to accumulate in organisms, particularly in the digestive system. MPs have been observed in liver samples from patients with cirrhosis; however, the exact mechanism is unclear[96]. Another study on MPs in mice tracked the accumulation and distribution of MPs in the liver, intestine, and kidney, and observed that MPs are hepatotoxic, affecting hepatic metabolism and posing a potential risk to mammalian health[70]. MPs have been observed to accumulate in the gills, liver, and intestine of zebrafish, and they can adversely affect the liver by inducing oxidative stress and disrupting lipid and energy metabolism in the liver[97]. These experimental results support that MPs can accumulate in organisms and cause damage, strengthening the detrimental consequences on these organs observed in the in vitro experiments. In a model simulating human exposure to MPs, MPs were reported to accumulate in the liver[98], suggesting that MPs may have an effect on it. We observed that MPs may damage the liver in various ways such as causing hepatocyte damage[99], affecting lipid metabolism processes[26], and even advancing the progression of liver fibrosis[48] through consulting literature.
Hepatocyte damage by MPs manifests in several ways. First, when liver organoids (LOs) were cultured in a polystyrene MP (PS-MP) environment, the number of apoptotic hepatocytes and the diameters of LOs were dose-dependent with PS-MP[100] (the number of apoptotic cells was positively correlated with PS-MP dose, while the diameters of LOs were negatively correlated with PS-MP dose). In addition, study has reported that in a PS-MP environment, the release of aspartate aminotransferase and alanine aminotransferase increases, but their activity decreases, suggesting that PS-MP may induce acute liver injury[100]. This may be related to various mechanisms such as hepatocyte toxicity and oxidative stress caused by MPs. MPs can induce a decrease in the activity of glutathione S-transferase and glutathione and the content of SOD, leading to an antioxidant imbalance and the generation of a large number of ROS[101], which can cause damage to cellular structures such as proteins and DNA.
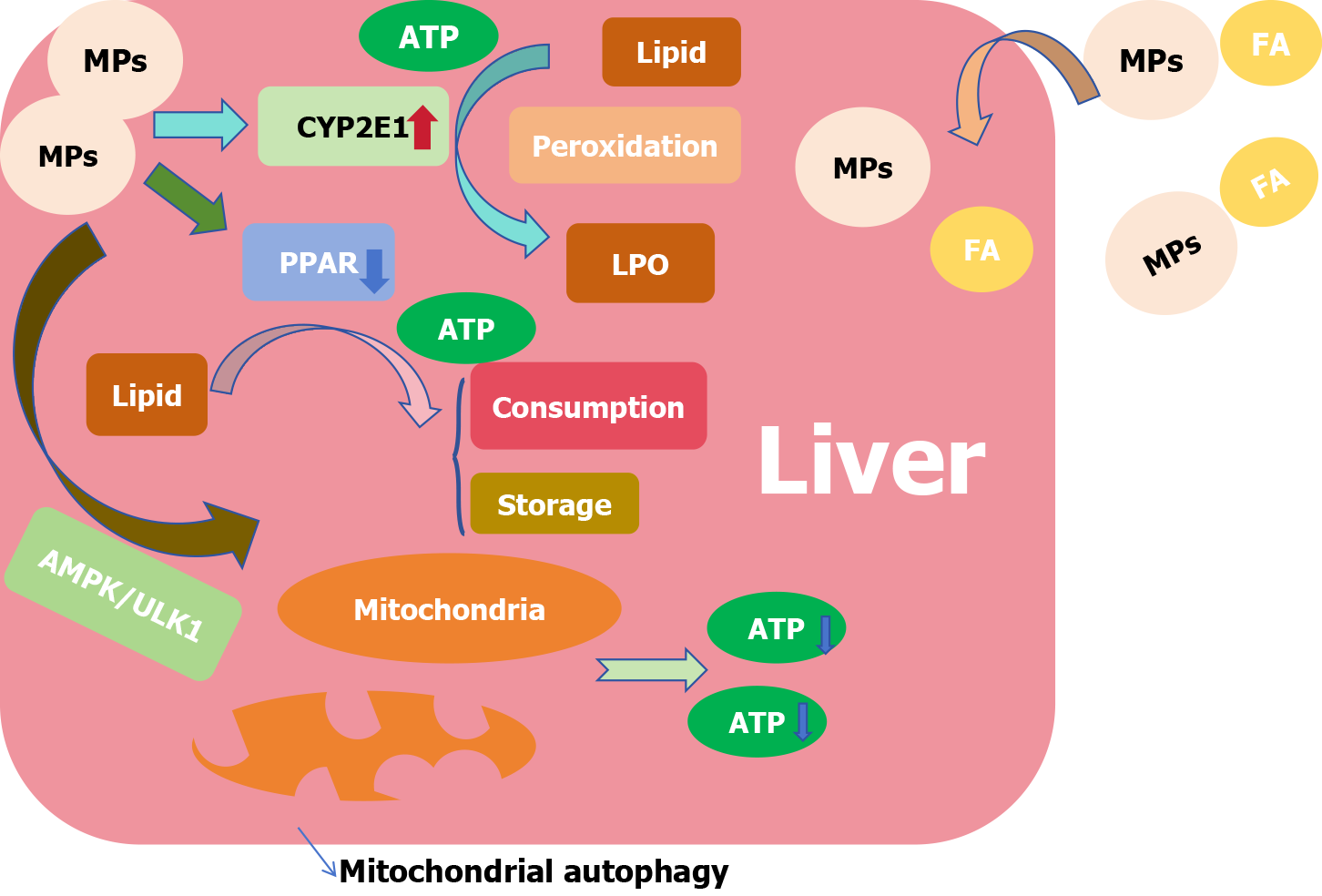
The liver is the hub of fat transport and participates in the regulation of fat metabolism. NAFLD is a clinicopathological syndrome characterized by excessive fat deposition in hepatocytes caused by alcohol and other liver damage factors. Changes in various markers of UDP-glycosyltransferase and sulfotransferase in patients with NAFLD are consistent with LOs cultured in a PS-MP environment, which means that PS-MP may be involved in inducing lipid metabolism disorders[100,102,103]. We observed that the effect of MPs on lipid metabolism in the liver was related to several mechanisms (Figure 3). PS-MP has been reported to increase lipid accumulation in the liver and may act as a carrier to carry fatty acids while endocytosing into the organ and promoting hepatic steatosis[100,104]. At the same time, studies have observed that in a PS-MP environment, the expression of cytochrome P4502E1 in the liver is upregulated, which is considered the main catalyst promoting hepatic steatosis owing to its ability to promote lipid peroxidation[105]. The peroxisome proliferator-activated receptor (PPAR) is closely related to hepatic lipid metabolism, in which PPARα and PPARγ are involved in the consumption of free fatty acids and the uptake and storage of lipids[106,107]. The process of lipid metabolism is impeded by the downregulation of PPAR expression[108] and inhibition of both PPARα and PPARγ[100] in the presence of MPs. In addition, metabolic processes often require the consumption of adenosine triphosphate, while PS-MP can accumulate in mitochondria[109], induce mitochondrial autophagy through the adenosine5’-monophosphate-activated protein kinase/UNC-51-like kinase pathway, inhibit mitochondrial function[100], affect energy supply and inhibit liver metabolic activities.
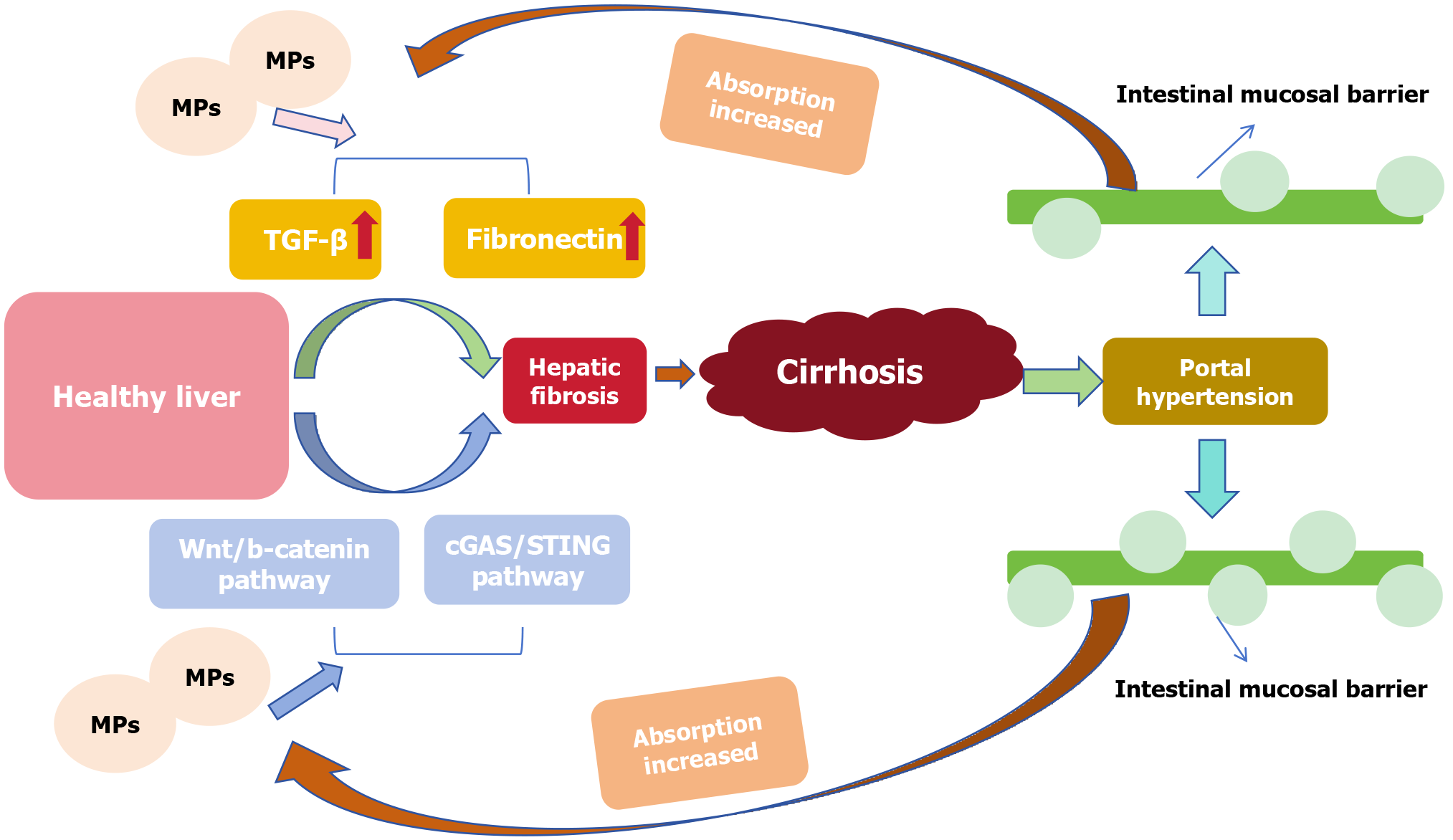
As mentioned before the livers of patients with cirrhosis and other chronic liver diseases accumulated more MPs than those of healthy individuals[96] (Figure 4). The progression of hepatic fibrosis may be related to the enhancement of
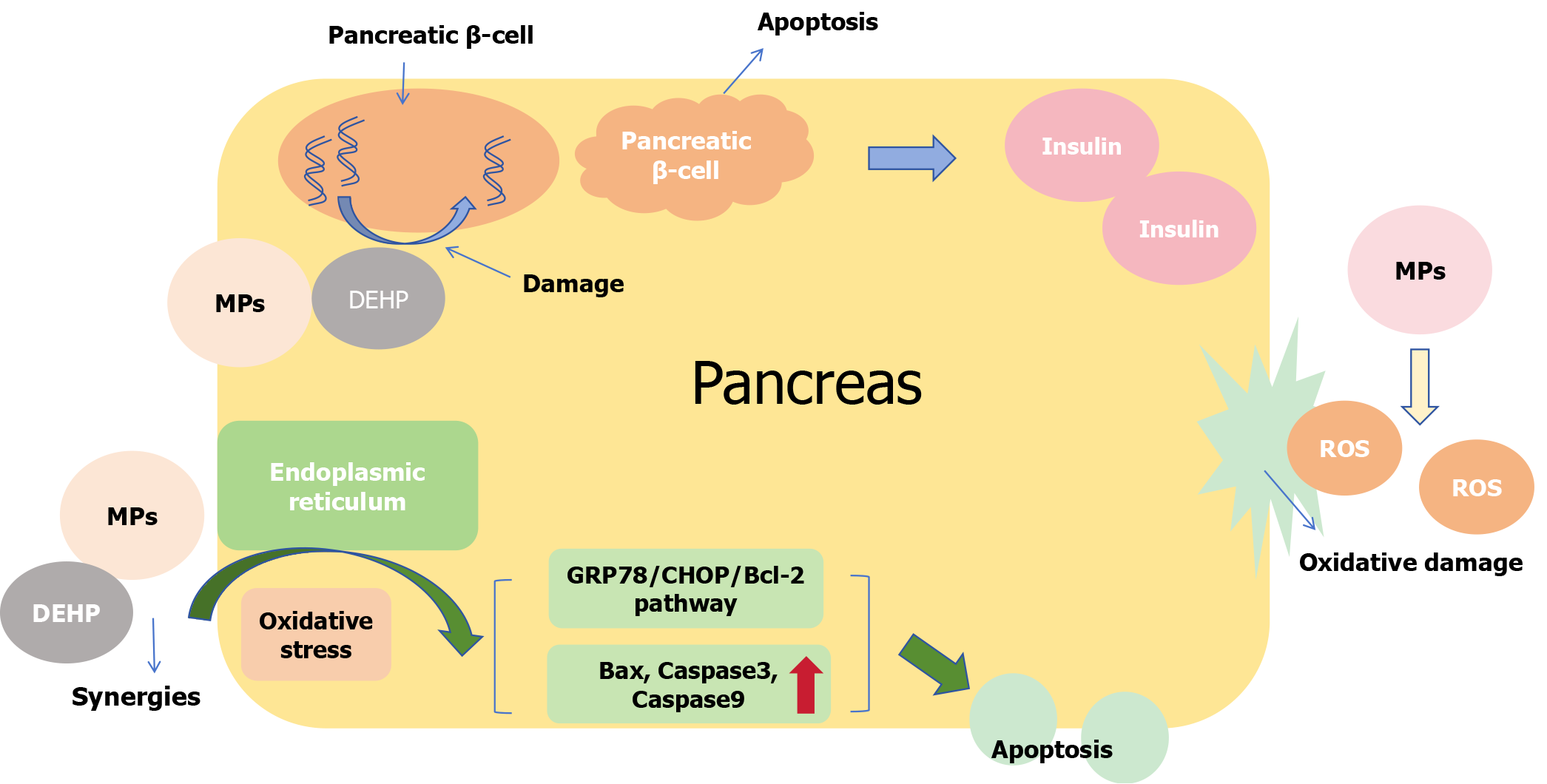
Studies have shown that exposure to MPs increases sugar levels in the blood[27]. The pancreas is an important organ for regulating blood sugar; therefore, we speculate that MPs may have certain effect on the pancreas. MPs were observed to accumulate and cause damage to the pancreas of mice that received MPs orally, accompanied by elevated blood glucose levels[27]. Ducks, such as waterfowl, are at a higher risk of exposure to MPs, which induce inflammation and fibrosis in the pancreas[113]. After reviewing the literature, we have summarized the mechanism of damage to the pancreas by MPs. First, as a type of nanoscale particle, the accumulation of MPs in organs can induce oxidative stress and generate large amounts of ROS[114], which cause direct oxidative damage to pancreatic cells. When exposed to 1 mg/L of MPs, the normal structure of pancreatic tissue disappeared and areas of acinar degeneration necrosis appeared[115]. Second, ROS can cause insulin resistance by interfering with the phosphatidylinositol 3-kinase/protein kinase B pathway, leading to elevated blood glucose[27,116]. Lastly, MPs can activate the gluconeogenic enzyme gene pck1, which reduces the expression domains of insulin-related genes, decreasing insulin levels and increasing blood sugar[117]. MPs can produce synergistic effects, which include promoting ER oxidative stress in pancreatic cells, activating the glucose-regulating protein 78 (GRP78)/C/EBP homologous protein (CHOP)/Bcl-2 pathway, enhancing the expression of mitochondrial apoptosis-related genes such as Bax, caspase3, caspase9 and inducing apoptosis in pancreatic cells (Figure 5)[49]. In addition, because of the large specific surface area of MPs, it is easy to absorb hazardous substances such as DEHP, which is used as a plasticizer of polyvinyl chloride[118]. Pancreatic β-cells are important for insulin secretion, and their DNA can be destroyed by DEHP[119], resulting in impaired function and glucose metabolism[120].
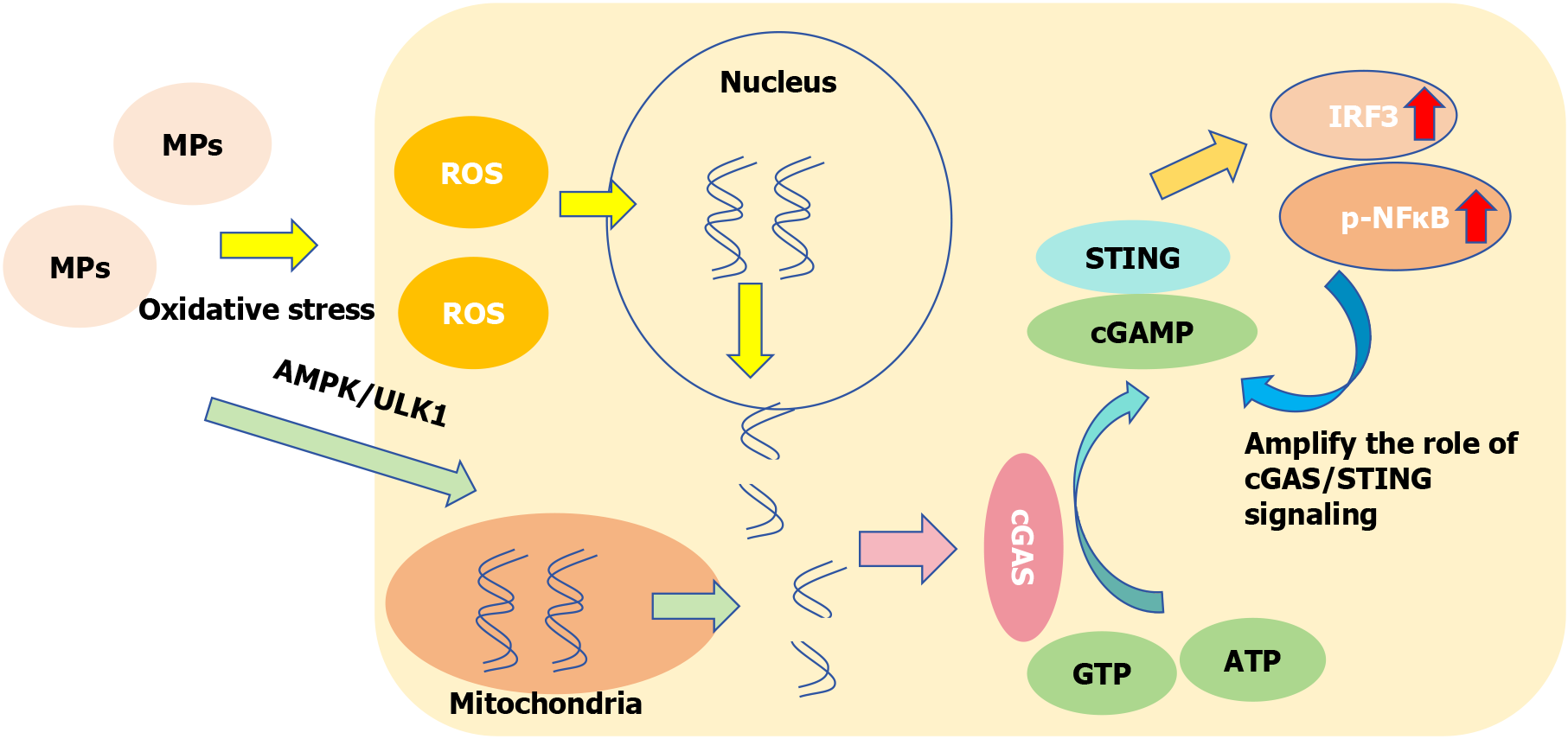
Liver fibrosis refers to the process of hyperplasia and repair of connective tissue caused by various pathogenic factors. If the injury factors persist for a long time, fibrosis will develop into cirrhosis. As an important signaling pathway that can regulate the inflammatory response by controlling the secretion of pro-inflammatory cytokines from cells, the inflammatory repair process is closely related to the cGAS-STING pathway[121]. Figure 6 shows that MPs can penetrate the lipid layer of the cell membrane[122], weaken the barrier effect, facilitate MPs to enter the cell to induce oxidative stress and produce a large amount of ROS[101]. ROS can damage DNA in the nucleus and induce mitochondrial autophagy through the adenosine5’-monophosphate-activated protein kinase/UNC-51-like kinase pathway[100,111], and the damaged DNA fragments enter the cytoplasm. The double-stranded DNA (dsDNA) sensor cGAS interacts with cytoplasmic free dsDNA to catalyze guanosine triphosphate and adenosine triphosphate to form a secondary messenger 2’,3’-cyclic GMP-AMP. Cyclic GMP-AMP binds to the ER receptor protein STING homologous diploid to activate the downstream pathway, and at the same time, the expression of transcription factors interferon regulatory factor 3 and phosphorylated nuclear factor-kappaB increased[48,121,123]. As a transcriptional regulator, nuclear factor-kappaB can amplify the role of cGAS/STING signaling and enhance the inflammatory response[121]. MPs can damage the DNA structure by inducing oxidative stress, and then the free dsDNA activates the cGAS-STING pathway to promote the inflammatory process and liver fibrosis.
Organ tissue damage often leads to repair, that is, fibrosis processes such as liver fibrosis and myocardial fibrosis. Many studies have shown that fibroblast activation and proliferation are related to the Wnt pathway[124]. As a classic Wnt pathway, it is closely related to the inflammatory process of organ and tissue injury and repair[110,111]. Studies have shown that absorbed MPs accumulate easily in the digestive system[11]. MPs induce oxidative stress, producing a large amount of ROS[125], which can cause cell and tissue damage[126]. Oxidative stress can activate the Wnt signaling pathway[127], and experiments have shown that the expression of Wnt, β-catenin, TGF-β and fibronectin in the pathway increases with enhancement of the oxidative stress response[110,111]. Therefore, we believe that MPs can activate the Wnt/β-catenin pathway by inducing oxidative stress and thus promoting fibrosis.
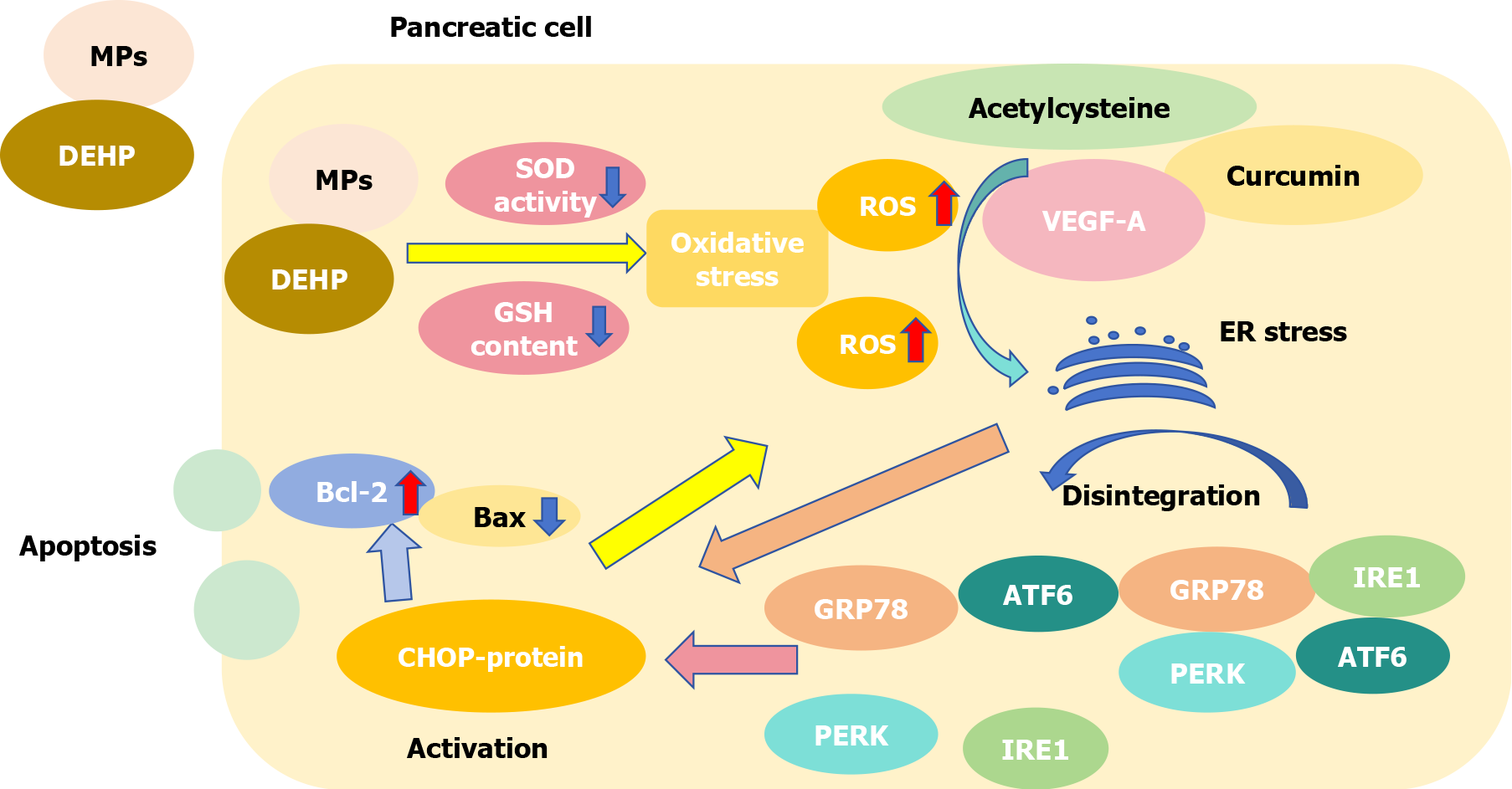
As plasticizers of polyvinyl chloride, DEHP and MPs often occur together harm organisms. The toxicity of DEHP combined with MPs is higher than that of DEHP or MPs alone[49]. DEHP and MPs can reduce SOD activity and glutathione content in pancreatic cells, leading to an imbalance in the oxidation and antioxidant systems, inducing oxidative stress and producing a large amount of ROS[49]. Acetylcysteine, vascular endothelial growth factor A, curcumin and other substances can cause ER stress via ROS[128-130]. As Figure 7 shown, GRP78, a highly conserved chaperone, is mainly located in the ER and combines with inositol-requiring enzyme 1, PKR-like ER kinase and activating transcription factor 6 to form a complex, which disintegrates and then becomes activated after ER stress[131,132]. Disintegrated products promote the activation of CHOP protein[133]. At the same time, ER stress itself can stimulate the activation of CHOP protein[133], while aggravating oxidative stress and promoting the accumulation of ROS[134]. Previous studies have shown that the CHOP protein can decrease the expression of the anti-apoptotic gene Bcl-2 and increase the expression of the pro-apoptotic gene Bax[135,136]. This effect increases mitochondrial membrane permeability, releases Cyt-c into the cytoplasm, activates the caspase pathway, and promotes apoptosis[137]. In other words, the combination of MPs and DEHP can activate the GRP78/CHOP/Bcl-2 pathway to promote the apoptosis of pancreatic cells by inducing oxidative stress.
The most fundamental solution for reducing the impact of MPs on human health is to reduce the intake of MPs. However, owing to the good stability of plastics, low production cost, and good plasticity[138], the production of plastics worldwide is still increasing year by year[139]; thus, the resulting plastic waste is increasing. Therefore, there is an urgent need to develop new environment-friendly materials and suitable degradation methods. At the same time, we should minimize the use of products containing MPs, such as eating more fresh fruits and vegetables and reducing the consumption of processed foods. The principles of prevention and treatment of MP-induced digestive system diseases are the same as those of conventional environmental pollution and digestive system diseases. The diagnosis is based mainly on clinical symptoms and various laboratory and imaging diagnostic techniques. Treatment principles mainly include: Removal of etiology, that is, reducing exposure to and intake of MP products; specific site injury targeted administration to control and improve symptoms; and improving lifestyle, such as smoking cessation, alcohol restriction, active exercises, and eating more fresh food, which can prevent recurrence and improve prognosis.
In addition to the accumulation of MPs in the digestive system, they can easily accumulate in the respiratory system[17], inducing inflammation of lung epithelial cells, which secrete a large number of inflammatory factors such as IL-6 and IL-8, leading to COPD, asthma and other lung diseases[65]. Studies have reported that COPD is highly correlated with α(1)-antitrypsin deficiency[140], and experiments have observed that the expression of α(1)-antitrypsin is significantly reduced by MP exposure[65], further confirming the impact of MPs on the respiratory system. In recent years, studies have been conducted on the presence of MPs in the blood[16]. MPs can enter various tissues and cells through the blood and cause adverse effects, as well as play a role in blood vessels. For example, in 2023, Wu et al[141] first detected MPs in thrombosis. Animal experiments have shown that MPs are related to thrombosis, and different group modifications have different effects[142]. For example, amine group modification can promote thrombosis[143], which may be related to the effect of the amine group and the membrane on the surface of platelets, and then platelets can be connected through the “amine group - MPs” chain[144]. Carboxyl group modified MPs did not promote thrombosis in this study[142]. In addition to causing system-specific effects, small MPs can enter cells through the blood and induce apoptosis and oxidative stress[63,145], which can lead to DNA fragmentation and the production of a large number of ROS, respectively, which may be associated with the cancer development[146]. However, there are currently few studies of MPs in specific cancers at various sites, and we expect that this can be further explored in the future to discover more intrinsic mechanisms and help scientists understand the production of disease.
To better investigate the mechanism of MPs injury to the human digestive system, we should explore more appropriate monitoring methods. For example, we can try to label MPs, observe and record the process of MPs entering the human body through the food chain, and extract parts of the body fluids to understand the distribution of MPs in the human body[19]. Developing and improving existing in vitro simulated organs simulating real-life environments where MPs are exposed and analyzing the physiological process of accumulation and damage in organs[100]. Clarify the toxicity of MPs by identifying their effects on organisms and the compounds to which they are bound[147].
We observed that MPs are a double-edged swords for organisms. The toxic effect of MPs is size-dependent; the smaller the particles, the more toxic they are to organisms[148]. This may be due to the stronger reaction between small-sized MPs and antioxidant enzymes[148], as well as the increased accumulation of organic pollutants, heavy metals and microorganisms in the body[149]. Conversely, larger MPs can act as carriers, carrying harmful substances out of the body and reducing the amount of time they remain in the body[150,151]. In addition, we observed that exposure to MPs alone leads to intestinal damage, but co-exposure to certain antibiotics (e.g., chlortetracycline) reduces intestinal damage by altering the intestinal microbiota[150]. Shang et al[148] reported that the effect of MPs on organisms is biphasic in dose (both low and high concentrations of MPs are less harmful to the organism), and the specific mechanism needs to be further studied. These findings provide new insights for reducing the harmful effects of MPs on the human body.
Minimizing the harm caused by MPs is an important topic that needs to be solved urgently. First, given the size-dependent toxicity of MPs[148,150], we can consider using materials to aggregate MPs; as a result, they are no longer “micro” and the harm caused by the oxidative stress induced by MPs can be reduced. At the same time, large particles can be used as carriers to carry toxic substances (e.g., heavy metals, organic pollutants, and harmful microorganisms) out of the body, reduce their residence time in the gastrointestinal tract[151,152], and minimize hazards to the human body. Second, reasonable and feasible ways to degrade MPs, such as using earthworms to degrade them and the soil environment can be improved after they digest MPs and convert them into organic matter[153]. However, considering the decomposition efficiency and treatment cost issues, it is currently challenging to implement[154], but biodegradation is one area that warrants further exploration, as it is more environmentally friendly and sustainable. Exploring the mechanism of MPs injury and reducing the harm of MPs to human beings requires a lot of time and effort; therefore, it is more half-hearted to solve the problem at the source. Governments in various countries (regions) have begun to gradually improve the relevant laws and regulations on restricting MPs to reduce the reckless discharge of MPs into the natural environment. As early as 2015, the United States passed a law prohibiting the use of microbeads[155]. The European Commission adopted a regulations on September 25, 2023, further amending the restrictions on MPs under the Registration, Evaluation, Authorization, and Restriction of Chemicals Finding new materials that can replace MPs is a topic that deserves our attention, as new environmentally friendly materials can avoid the harmful effects of MPs on the human body. Finally, from each person, we can eat more fresh food and minimize the consumption of take-out and other fast-food products to reduce the intake of MPs[156]. In addition, canvas bags can be used instead of plastic bags to live a green and low-carbon life.
The harmful effects of MPs have been discussed since their discovery in recent years. Although there is a lack of specific mechanisms for the toxic effects of MPs on the human body for ethical reasons, we can conclude that MPs are harmful to the digestive system based on various experiments on marine organisms and mice. In summary, these include damaging the epithelial barrier of the digestive tract, disturbing the ecological balance of the bacterial flora, damaging parenchymal organs such as the liver and pancreas, affecting their normal functioning, and acting as a carrier for toxins to enter the human body, exerting a poisonous effect.
| 1. | Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE. Lost at sea: where is all the plastic? Science. 2004;304:838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2946] [Cited by in RCA: 3581] [Article Influence: 170.5] [Reference Citation Analysis (2)] |
| 2. | Bajt O. From plastics to microplastics and organisms. FEBS Open Bio. 2021;11:954-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46:3060-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2548] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 4. | Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ Sci Technol. 2018;52:1704-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1373] [Article Influence: 196.1] [Reference Citation Analysis (0)] |
| 5. | Zarus GM, Muianga C, Hunter CM, Pappas RS. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ. 2021;756:144010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | van Raamsdonk LWD, van der Zande M, Koelmans AA, Hoogenboom RLAP, Peters RJB, Groot MJ, Peijnenburg AACM, Weesepoel YJA. Current Insights into Monitoring, Bioaccumulation, and Potential Health Effects of Microplastics Present in the Food Chain. Foods. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 7. | Moore CJ. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res. 2008;108:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 964] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 8. | Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Ubeda B, Hernández-León S, Palma AT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML, Duarte CM. Plastic debris in the open ocean. Proc Natl Acad Sci U S A. 2014;111:10239-10244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1545] [Article Influence: 140.5] [Reference Citation Analysis (0)] |
| 9. | Zhang S, Yang X, Gertsen H, Peters P, Salánki T, Geissen V. A simple method for the extraction and identification of light density microplastics from soil. Sci Total Environ. 2018;616-617:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Mani T, Hauk A, Walter U, Burkhardt-Holm P. Microplastics profile along the Rhine River. Sci Rep. 2015;5:17988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 11. | Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013;47:6646-6655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1403] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 12. | Zuri G, Karanasiou A, Lacorte S. Microplastics: Human exposure assessment through air, water, and food. Environ Int. 2023;179:108150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 13. | Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3:e1700782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6592] [Cited by in RCA: 6157] [Article Influence: 769.6] [Reference Citation Analysis (0)] |
| 14. | Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human Consumption of Microplastics. Environ Sci Technol. 2019;53:7068-7074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1109] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 15. | Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann Intern Med. 2019;171:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 16. | Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 1388] [Article Influence: 462.7] [Reference Citation Analysis (0)] |
| 17. | Amato-Lourenço LF, Carvalho-Oliveira R, Júnior GR, Dos Santos Galvão L, Ando RA, Mauad T. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 507] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 18. | Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, Tan BK, Wong CY, Leong CO. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 373] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 19. | Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371:672-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 636] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 20. | Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ Sci Technol. 2008;42:5026-5031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1334] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 21. | Thiele CJ, Grange LJ, Haggett E, Hudson MD, Hudson P, Russell AE, Zapata-Restrepo LM. Microplastics in European sea salts - An example of exposure through consumer choice and of interstudy methodological discrepancies. Ecotoxicol Environ Saf. 2023;255:114782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 22. | Kosuth M, Mason SA, Wattenberg EV. Anthropogenic contamination of tap water, beer, and sea salt. PLoS One. 2018;13:e0194970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 23. | Zha H, Lv J, Lou Y, Wo W, Xia J, Li S, Zhuge A, Tang R, Si N, Hu Z, Lu H, Chang K, Wang C, Si G, Li L. Alterations of gut and oral microbiota in the individuals consuming take-away food in disposable plastic containers. J Hazard Mater. 2023;441:129903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145:1517-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 25. | Okamura T, Hamaguchi M, Hasegawa Y, Hashimoto Y, Majima S, Senmaru T, Ushigome E, Nakanishi N, Asano M, Yamazaki M, Sasano R, Nakanishi Y, Seno H, Takano H, Fukui M. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ Health Perspect. 2023;131:27006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 26. | Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631-632:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 620] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 27. | Fan X, Wei X, Hu H, Zhang B, Yang D, Du H, Zhu R, Sun X, Oh Y, Gu N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere. 2022;288:132607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Yong CQY, Valiyaveettil S, Tang BL. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 29. | Zhang Q, He Y, Cheng R, Li Q, Qian Z, Lin X. Recent advances in toxicological research and potential health impact of microplastics and nanoplastics in vivo. Environ Sci Pollut Res Int. 2022;29:40415-40448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Frias JPGL, Nash R. Microplastics: Finding a consensus on the definition. Mar Pollut Bull. 2019;138:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 861] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 31. | Nizzetto L, Futter M, Langaas S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ Sci Technol. 2016;50:10777-10779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 767] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 32. | Silori R, Shrivastava V, Mazumder P, Mootapally C, Pandey A, Kumar M. Understanding the underestimated: Occurrence, distribution, and interactions of microplastics in the sediment and soil of China, India, and Japan. Environ Pollut. 2023;320:120978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | de Souza Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC. Impacts of Microplastics on the Soil Biophysical Environment. Environ Sci Technol. 2018;52:9656-9665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 683] [Cited by in RCA: 782] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 34. | Jiang X, Chen H, Liao Y, Ye Z, Li M, Klobučar G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ Pollut. 2019;250:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 35. | Gao M, Liu Y, Dong Y, Song Z. Effect of polyethylene particles on dibutyl phthalate toxicity in lettuce (Lactuca sativa L.). J Hazard Mater. 2021;401:123422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ. 2018;619-620:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 765] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 37. | Bodor A, Feigl G, Kolossa B, Mészáros E, Laczi K, Kovács E, Perei K, Rákhely G. Soils in distress: The impacts and ecological risks of (micro)plastic pollution in the terrestrial environment. Ecotoxicol Environ Saf. 2024;269:115807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 38. | Chang X, Fang Y, Wang Y, Wang F, Shang L, Zhong R. Microplastic pollution in soils, plants, and animals: A review of distributions, effects and potential mechanisms. Sci Total Environ. 2022;850:157857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 39. | Zhang Y, Yin K, Wang D, Wang Y, Lu H, Zhao H, Xing M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci Total Environ. 2022;840:156727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 40. | Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 632] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 41. | Sun W, Yan S, Meng Z, Tian S, Jia M, Huang S, Wang Y, Zhou Z, Diao J, Zhu W. Combined ingestion of polystyrene microplastics and epoxiconazole increases health risk to mice: Based on their synergistic bioaccumulation in vivo. Environ Int. 2022;166:107391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 42. | Gonçalves JM, Benedetti M, d'Errico G, Regoli F, Bebianno MJ. Polystyrene nanoplastics in the marine mussel Mytilus galloprovincialis. Environ Pollut. 2023;333:122104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 43. | Ma C, Chen Q, Li J, Li B, Liang W, Su L, Shi H. Distribution and translocation of micro- and nanoplastics in fish. Crit Rev Toxicol. 2021;51:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 44. | Mamun AA, Prasetya TAE, Dewi IR, Ahmad M. Microplastics in human food chains: Food becoming a threat to health safety. Sci Total Environ. 2023;858:159834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 175] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 45. | Curren E, Leaw CP, Lim PT, Leong SCY. Evidence of Marine Microplastics in Commercially Harvested Seafood. Front Bioeng Biotechnol. 2020;8:562760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Bhagat J, Zang L, Nishimura N, Shimada Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci Total Environ. 2020;728:138707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 47. | Makhdoumi P, Hossini H, Pirsaheb M. A review of microplastic pollution in commercial fish for human consumption. Rev Environ Health. 2023;38:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 48. | Shen R, Yang K, Cheng X, Guo C, Xing X, Sun H, Liu D, Liu X, Wang D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. 2022;300:118986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 49. | Wang Y, Zhang Y, Sun X, Shi X, Xu S. Microplastics and di (2-ethylhexyl) phthalate synergistically induce apoptosis in mouse pancreas through the GRP78/CHOP/Bcl-2 pathway activated by oxidative stress. Food Chem Toxicol. 2022;167:113315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Lett Z, Hall A, Skidmore S, Alves NJ. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ Pollut. 2021;291:118190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 51. | Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 52. | Wang S, Chen L, Shi X, Wang Y, Xu S. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ Pollut. 2023;324:121388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 53. | Li X, Feng L, Kuang Q, Wang X, Yang J, Niu X, Gao L, Huang L, Luo P, Li L. Microplastics cause hepatotoxicity in diabetic mice by disrupting glucolipid metabolism via PP2A/AMPK/HNF4A and promoting fibrosis via the Wnt/β-catenin pathway. Environ Toxicol. 2024;39:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 54. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 486] [Article Influence: 162.0] [Reference Citation Analysis (1)] |
| 55. | Zhou B, Yuan Y, Zhang S, Guo C, Li X, Li G, Xiong W, Zeng Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front Immunol. 2020;11:575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 56. | Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 1252] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 57. | Mayer EA, Nance K, Chen S. The Gut-Brain Axis. Annu Rev Med. 2022;73:439-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 58. | Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji X, Zhang Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 377] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 59. | Marušić M, Paić M, Knobloch M, Liberati Pršo AM. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (2)] |
| 60. | Ajoolabady A, Kaplowitz N, Lebeaupin C, Kroemer G, Kaufman RJ, Malhi H, Ren J. Endoplasmic reticulum stress in liver diseases. Hepatology. 2023;77:619-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 190] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 61. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 692] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 62. | Liang B, Zhong Y, Huang Y, Lin X, Liu J, Lin L, Hu M, Jiang J, Dai M, Wang B, Zhang B, Meng H, Lelaka JJJ, Sui H, Yang X, Huang Z. Underestimated health risks: polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part Fibre Toxicol. 2021;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 265] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 63. | Kumar R, Manna C, Padha S, Verma A, Sharma P, Dhar A, Ghosh A, Bhattacharya P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere. 2022;298:134267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 64. | Yedier S, Yalçınkaya SK, Bostancı D. Exposure to polypropylene microplastics via diet and water induces oxidative stress in Cyprinus carpio. Aquat Toxicol. 2023;259:106540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 65. | Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 66. | Hu J, Xu X, Song Y, Liu W, Zhu J, Jin H, Meng Z. Microplastics in Widely Used Polypropylene-Made Food Containers. Toxics. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Celebi Sozener Z, Ozdel Ozturk B, Cerci P, Turk M, Gorgulu Akin B, Akdis M, Altiner S, Ozbey U, Ogulur I, Mitamura Y, Yilmaz I, Nadeau K, Ozdemir C, Mungan D, Akdis CA. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy. 2022;77:1418-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 219] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 68. | Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 69. | Wu X, Chen J, Xu M, Zhu D, Wang X, Chen Y, Wu J, Cui C, Zhang W, Yu L. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J Oral Microbiol. 2017;9:1324725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7:46687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 600] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 71. | Djouina M, Waxin C, Dubuquoy L, Launay D, Vignal C, Body-Malapel M. Oral exposure to polyethylene microplastics induces inflammatory and metabolic changes and promotes fibrosis in mouse liver. Ecotoxicol Environ Saf. 2023;264:115417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (1)] |
| 72. | Lee YY, Roslan NS, Tee V, Koo TH, Ibrahim YS. Climate Change and the Esophagus: Speculations on Changing Disease Patterns as the World Warms. Curr Gastroenterol Rep. 2023;25:280-288. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Murciano-Brea J, Garcia-Montes M, Geuna S, Herrera-Rincon C. Gut Microbiota and Neuroplasticity. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 75. | Cheng LK, O'Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med. 2010;2:65-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Ibrahim YS, Tuan Anuar S, Azmi AA, Wan Mohd Khalik WMA, Lehata S, Hamzah SR, Ismail D, Ma ZF, Dzulkarnaen A, Zakaria Z, Mustaffa N, Tuan Sharif SE, Lee YY. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 77. | Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary Protein and Gut Microbiota Composition and Function. Curr Protein Pept Sci. 2019;20:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 78. | Takeuchi T, Kubota T, Nakanishi Y, Tsugawa H, Suda W, Kwon AT, Yazaki J, Ikeda K, Nemoto S, Mochizuki Y, Kitami T, Yugi K, Mizuno Y, Yamamichi N, Yamazaki T, Takamoto I, Kubota N, Kadowaki T, Arner E, Carninci P, Ohara O, Arita M, Hattori M, Koyasu S, Ohno H. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. 2023;621:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 167] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 79. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2249] [Article Influence: 281.1] [Reference Citation Analysis (1)] |
| 80. | Noor SO, Ridgway K, Scovell L, Kemsley EK, Lund EK, Jamieson C, Johnson IT, Narbad A. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010;10:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 82. | Qu M, Xu K, Li Y, Wong G, Wang D. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci Total Environ. 2018;643:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 83. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1183] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 84. | von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46:11327-11335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1013] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 85. | Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 328] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 1701] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 87. | Winter SE, Bäumler AJ. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 2014;5:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 88. | Possatto FE, Barletta M, Costa MF, do Sul JA, Dantas DV. Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Mar Pollut Bull. 2011;62:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 89. | Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front Immunol. 2018;9:1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 90. | Wang Y, Zhao Y, Liang H, Ma C, Cui N, Cao H, Wei W, Liu Y. Single and combined effects of polyethylene microplastics and acetochlor on accumulation and intestinal toxicity of zebrafish (Danio rerio). Environ Pollut. 2023;333:122089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 91. | Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environ Pollut. 2013;178:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2435] [Cited by in RCA: 2264] [Article Influence: 188.7] [Reference Citation Analysis (0)] |
| 92. | Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 865] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 93. | Harrison JP, Schratzberger M, Sapp M, Osborn AM. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014;14:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 309] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 94. | Karuppasamy PK, Ravi A, Vasudevan L, Elangovan MP, Dyana Mary P, Vincent SGT, Palanisami T. Baseline survey of micro and mesoplastics in the gastro-intestinal tract of commercial fish from Southeast coast of the Bay of Bengal. Mar Pollut Bull. 2020;153:110974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 95. | Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl). 2008;92:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 653] [Article Influence: 38.4] [Reference Citation Analysis (1)] |
| 96. | Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, Püschel K, Huber S, Fischer EK. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 292] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 97. | Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ Sci Technol. 2016;50:4054-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1282] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 98. | Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. Lifetime Accumulation of Microplastic in Children and Adults. Environ Sci Technol. 2021;55:5084-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 273] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 99. | Mu Y, Sun J, Li Z, Zhang W, Liu Z, Li C, Peng C, Cui G, Shao H, Du Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 2022;291:132944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 100. | Cheng W, Li X, Zhou Y, Yu H, Xie Y, Guo H, Wang H, Li Y, Feng Y, Wang Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci Total Environ. 2022;806:150328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 101. | Lee MT, Lin WC, Yu B, Lee TT. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - A review. Asian-Australas J Anim Sci. 2017;30:299-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 102. | Dubaisi S, Barrett KG, Fang H, Guzman-Lepe J, Soto-Gutierrez A, Kocarek TA, Runge-Morris M. Regulation of Cytosolic Sulfotransferases in Models of Human Hepatocyte Development. Drug Metab Dispos. 2018;46:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Jamwal R, Barlock BJ. Nonalcoholic Fatty Liver Disease (NAFLD) and Hepatic Cytochrome P450 (CYP) Enzymes. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 744] [Cited by in RCA: 654] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 105. | Aljomah G, Baker SS, Liu W, Kozielski R, Oluwole J, Lupu B, Baker RD, Zhu L. Induction of CYP2E1 in non-alcoholic fatty liver diseases. Exp Mol Pathol. 2015;99:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 107. | Lee YJ, Ko EH, Kim JE, Kim E, Lee H, Choi H, Yu JH, Kim HJ, Seong JK, Kim KS, Kim JW. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci U S A. 2012;109:13656-13661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 108. | Zhang X, Wen K, Ding D, Liu J, Lei Z, Chen X, Ye G, Zhang J, Shen H, Yan C, Dong S, Huang Q, Lin Y. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ Int. 2021;151:106452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 109. | Huang Y, Liang B, Li Z, Zhong Y, Wang B, Zhang B, Du J, Ye R, Xian H, Min W, Yan X, Deng Y, Feng Y, Bai R, Fan B, Yang X, Huang Z. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part Fibre Toxicol. 2023;20:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 110. | An R, Wang X, Yang L, Zhang J, Wang N, Xu F, Hou Y, Zhang H, Zhang L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 111. | Li Z, Zhu S, Liu Q, Wei J, Jin Y, Wang X, Zhang L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. 2020;265:115025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 112. | El-Ashmawy NE, Al-Ashmawy GM, Fakher HE, Khedr NF. The role of WNT/β-catenin signaling pathway and glutamine metabolism in the pathogenesis of CCl(4)-induced liver fibrosis: Repositioning of niclosamide and concerns about lithium. Cytokine. 2020;136:155250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Sun J, Su F, Chen Y, Wang T, Ali W, Jin H, Xiong L, Ma Y, Liu Z, Zou H. Co-exposure to PVC microplastics and cadmium induces oxidative stress and fibrosis in duck pancreas. Sci Total Environ. 2024;927:172395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 114. | Brand W, Peters RJB, Braakhuis HM, Maślankiewicz L, Oomen AG. Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology. 2020;14:985-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 115. | Hamed M, Soliman HAM, Badrey AEA, Osman AGM. Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis niloticus) early juveniles. Tissue Cell. 2021;71:101512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 116. | Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 117. | Brun NR, van Hage P, Hunting ER, Haramis AG, Vink SC, Vijver MG, Schaaf MJM, Tudorache C. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun Biol. 2019;2:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 118. | Wowkonowicz P, Kijeńska M, Koda E. Potential environmental risk assessment of di-2-ethylhexyl phthalate emissions from a municipal solid waste landfill leachate. PeerJ. 2021;9:e12163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | She Y, Jiang L, Zheng L, Zuo H, Chen M, Sun X, Li Q, Geng C, Yang G, Jiang L, Liu X. The role of oxidative stress in DNA damage in pancreatic β cells induced by di-(2-ethylhexyl) phthalate. Chem Biol Interact. 2017;265:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 120. | Rajesh P, Balasubramanian K. Gestational exposure to di(2-ethylhexyl) phthalate (DEHP) impairs pancreatic β-cell function in F1 rat offspring. Toxicol Lett. 2015;232:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Dunphy G, Flannery SM, Almine JF, Connolly DJ, Paulus C, Jønsson KL, Jakobsen MR, Nevels MM, Bowie AG, Unterholzner L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol Cell. 2018;71:745-760.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 470] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 122. | Rossi G, Barnoud J, Monticelli L. Polystyrene Nanoparticles Perturb Lipid Membranes. J Phys Chem Lett. 2014;5:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 123. | Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 1294] [Article Influence: 323.5] [Reference Citation Analysis (0)] |
| 124. | Huang GR, Wei SJ, Huang YQ, Xing W, Wang LY, Liang LL. Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. World J Gastroenterol. 2018;24:4178-4185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 125. | Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Zhou B, Souissi S, Lee SJ, Lee JS. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ Sci Technol. 2016;50:8849-8857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 764] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 126. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1091] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 127. | Andersson-Sjöland A, Karlsson JC, Rydell-Törmänen K. ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling. Lab Invest. 2016;96:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 128. | Hu W, Xia M, Zhang C, Song B, Xia Z, Guo C, Cui Y, Jiang W, Zhang S, Xu D, Fang J. Chronic cadmium exposure induces epithelial mesenchymal transition in prostate cancer cells through a TGF-β-independent, endoplasmic reticulum stress induced pathway. Toxicol Lett. 2021;353:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, Zhang H, Xiao X, Wang K, Wang N. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. 2019;234:17690-17703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 130. | Zeng Y, Du Q, Zhang Z, Ma J, Han L, Wang Y, Yang L, Tao N, Qin Z. Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch Biochem Biophys. 2020;694:108613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 131. | Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1031] [Cited by in RCA: 1084] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 132. | Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1840] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 133. | Lew QJ, Chu KL, Lee J, Koh PL, Rajasegaran V, Teo JY, Chao SH. PCAF interacts with XBP-1S and mediates XBP-1S-dependent transcription. Nucleic Acids Res. 2011;39:429-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1536] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 135. | Ghosh AP, Klocke BJ, Ballestas ME, Roth KA. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One. 2012;7:e39586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 136. | Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1130] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 137. | Wang TS, Coppens I, Saorin A, Brady NR, Hamacher-Brady A. Endolysosomal Targeting of Mitochondria Is Integral to BAX-Mediated Mitochondrial Permeabilization during Apoptosis Signaling. Dev Cell. 2020;53:627-645.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 138. | Rodrigues MO, Abrantes N, Gonçalves FJM, Nogueira H, Marques JC, Gonçalves AMM. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ Toxicol Pharmacol. 2019;72:103239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 139. | Zhao X, Boruah B, Chin KF, Đokić M, Modak JM, Soo HS. Upcycling to Sustainably Reuse Plastics. Adv Mater. 2022;34:e2100843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 140. | Tuder RM, Janciauskiene SM, Petrache I. Lung disease associated with alpha1-antitrypsin deficiency. Proc Am Thorac Soc. 2010;7:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Wu D, Feng Y, Wang R, Jiang J, Guan Q, Yang X, Wei H, Xia Y, Luo Y. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res. 2023;49:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 127] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 142. | Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med. 2002;166:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 143. | Zhu X, Wang C, Duan X, Liang B, Genbo Xu E, Huang Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ Int. 2023;171:107662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 144. | Smyth E, Solomon A, Vydyanath A, Luther PK, Pitchford S, Tetley TD, Emerson M. Induction and enhancement of platelet aggregation in vitro and in vivo by model polystyrene nanoparticles. Nanotoxicology. 2015;9:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 145. | Zhu K, Jia H, Sun Y, Dai Y, Zhang C, Guo X, Wang T, Zhu L. Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: Combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ Int. 2020;145:106137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 146. | Rubio L, Marcos R, Hernández A. Potential adverse health effects of ingested micro- and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J Toxicol Environ Health B Crit Rev. 2020;23:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 147. | Xu JL, Lin X, Wang JJ, Gowen AA. A review of potential human health impacts of micro- and nanoplastics exposure. Sci Total Environ. 2022;851:158111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 148. | Shang X, Lu J, Feng C, Ying Y, He Y, Fang S, Lin Y, Dahlgren R, Ju J. Microplastic (1 and 5 μm) exposure disturbs lifespan and intestine function in the nematode Caenorhabditis elegans. Sci Total Environ. 2020;705:135837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 149. | Zheng Q, Cui L, Liao H, Junaid M, Li Z, Liu S, Gao D, Zheng Y, Lu S, Qiu J, Wang J. Combined exposure to polystyrene nanoplastics and bisphenol A induces hepato- and intestinal-toxicity and disturbs gut microbiota in channel catfish (Ictalurus punctatus). Sci Total Environ. 2023;891:164319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 150. | Liu B, Yu D, Ge C, Luo X, Du L, Zhang X, Hui C. Combined effects of microplastics and chlortetracycline on the intestinal barrier, gut microbiota, and antibiotic resistome of Muscovy ducks (Cairina moschata). Sci Total Environ. 2023;887:164050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 151. | Hu Y, Lin S, Tang J, Li Y, Wang X, Jiang Y, Zhang H, Wang B. Effects of microplastics and lead exposure on gut oxidative stress and intestinal inflammation in common carp (Cyprinus carpio L.). Environ Pollut. 2023;327:121528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 152. | Scopetani C, Cincinelli A, Martellini T, Lombardini E, Ciofini A, Fortunati A, Pasquali V, Ciattini S, Ugolini A. Ingested microplastic as a two-way transporter for PBDEs in Talitrus saltator. Environ Res. 2018;167:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 153. | Khaldoon S, Lalung J, Maheer U, Kamaruddin MA, Yhaya MF, Alsolami ES, Alorfi HS, Hussein MA, Rafatullah M. A Review on the Role of Earthworms in Plastics Degradation: Issues and Challenges. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Khan ML, Hassan HU, Khan FU, Ghaffar RA, Rafiq N, Bilal M, Khooharo AR, Ullah S, Jafari H, Nadeem K, Siddique MAM, Arai T. Effects of microplastics in freshwater fishes health and the implications for human health. Braz J Biol. 2023;84:e272524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 155. | Auta HS, Emenike CU, Fauziah SH. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ Int. 2017;102:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1304] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 156. | Siddiqui SA, Bahmid NA, Salman SHM, Nawaz A, Walayat N, Shekhawat GK, Gvozdenko AA, Blinov AV, Nagdalian AA. Migration of microplastics from plastic packaging into foods and its potential threats on human health. Adv Food Nutr Res. 2023;103:313-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |