Published online Jan 28, 2025. doi: 10.3748/wjg.v31.i4.100401
Revised: October 23, 2024
Accepted: December 2, 2024
Published online: January 28, 2025
Processing time: 136 Days and 22.5 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective intervention for managing complications of portal hypertension, particularly acute variceal bleeding (AVB). While effective in reducing portal pressure and preventing rebleeding, TIPS is associated with a considerable risk of overt hepatic encephalopathy (OHE), a complication that significantly elevates mortality rates.
To develop a machine learning (ML) model to predict OHE occurrence post-TIPS in patients with AVB using a 5-year dataset.
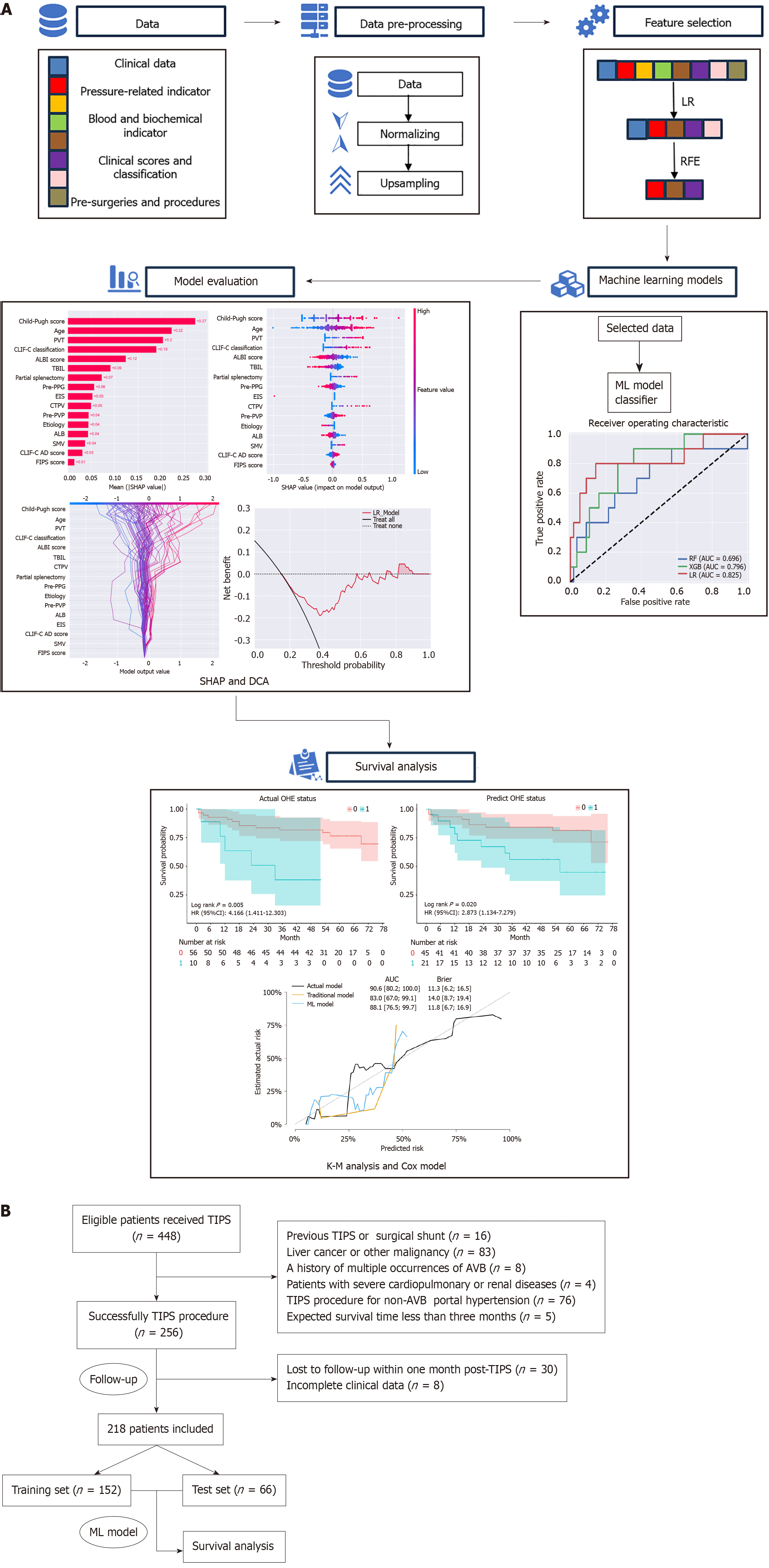
This retrospective single-center study included 218 patients with AVB who underwent TIPS. The dataset was divided into training (70%) and testing (30%) sets. Critical features were identified using embedded methods and recursive feature elimination. Three ML algorithms-random forest, extreme gradient boosting, and logistic regression-were validated via 10-fold cross-validation. SHapley Additive exPlanations analysis was employed to interpret the model’s predictions. Survival analysis was conducted using Kaplan-Meier curves and stepwise Cox regression analysis to compare overall survival (OS) between patients with and without OHE.
The median OS of the study cohort was 47.83 ± 22.95 months. Among the models evaluated, logistic regression demonstrated the highest performance with an area under the curve (AUC) of 0.825. Key predictors identified were Child-Pugh score, age, and portal vein thrombosis. Kaplan-Meier analysis revealed that patients without OHE had a significantly longer OS (P = 0.005). The 5-year survival rate was 78.4%, with an OHE incidence of 15.1%. Both actual OHE status and predicted OHE value were significant predictors in each Cox model, with model-predicted OHE achieving an AUC of 88.1 in survival prediction.
The ML model accurately predicts post-TIPS OHE and outperforms traditional models, supporting its use in improving outcomes in patients with AVB.
Core Tip: This study developed a machine learning (ML) model to predict overt hepatic encephalopathy (OHE) after transjugular intrahepatic portosystemic shunt (TIPS) in patients with acute variceal bleeding (AVB). Utilizing a 5-year retrospective dataset of 218 patients, key features such as Child-Pugh score, age, and portal vein thrombosis were identified. The ML model demonstrated a strong performance, with an area under the curve of 0.825. This ML model effectively predicts post-TIPS OHE, providing a valuable tool for tailoring personalized treatment plans. Its superior performance over traditional models supports its integration into clinical practice to enhance outcomes for patients with AVB undergoing TIPS.
- Citation: Liu DJ, Jia LX, Zeng FX, Zeng WX, Qin GG, Peng QF, Tan Q, Zeng H, Ou ZY, Kun LZ, Zhao JB, Chen WG. Machine learning prediction of hepatic encephalopathy for long-term survival after transjugular intrahepatic portosystemic shunt in acute variceal bleeding. World J Gastroenterol 2025; 31(4): 100401
- URL: https://www.wjgnet.com/1007-9327/full/v31/i4/100401.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i4.100401
Transjugular intrahepatic portosystemic shunt (TIPS) is a widely recognized intervention for managing complications of portal hypertension, particularly in patients suffering from acute variceal bleeding (AVB)[1-3]. Despite TIPS effectively reducing portal pressure and controlling bleeding, it carries a significant risk of overt hepatic encephalopathy (OHE) with an incidence of 10% to 30%[4]. Multiple studies and meta-analyses have demonstrated that post-TIPS hepatic encephalopathy, especially OHE, significantly increases the risk of early mortality in patients. Moreover, post-TIPS OHE and Child-Pugh grade have been recognized as key independent predictors of early mortality[5]. This risk constrains TIPS from being considered first-line treatment for AVB. Accurate prediction of OHE post-TIPS is essential for optimizing patient management and improving clinical outcomes.
Previous studies have developed various predictive models for assessing and stratifying the risk factors for OHE, yet none have been widely adopted in clinical practice[6-8]. Recent advancements in machine learning (ML) have significantly improved the accuracy of clinical outcome predictions by leveraging large datasets and utilizing sophisticated algorithms that can uncover intricate patterns and relationships within the data, enhancing predictive capabilities in medical practice[9]. ML models incorporate diverse variables from clinical, biochemical, and procedural data, thereby enhancing predictive accuracy[10,11]. Various ML algorithms, including logistic regression (LR) and more sophisticated approaches such as extreme gradient boosting (XGBoost), have demonstrated strong potential for improving clinical outcome predictions by capturing essential predictive factors across multiple medical conditions. By utilizing advanced techniques such as recursive feature elimination (RFE) and employing robust evaluation metrics such as the area under the curve (AUC), these models can enhance prediction accuracy. In addition, the integration of model results with preoperative indicators offers valuable insights into patient prognosis following TIPS, contributing to more informed decision-making and personalized treatment strategies.
This study developed an ML model for predicting post-TIPS OHE in patients with AVB using a 5-year longitudinal dataset and assessed the impact of model metrics on patient survival. The model’s predictions are expected to serve as crucial indicators of long-term survival following TIPS.
This retrospective, single-center study was conducted at our institution with approval of the institutional review board, following the principles of the Declaration of Helsinki. Patients who underwent TIPS for AVB between April 2017 and December 2020 were screened. The inclusion criteria were: (1) Liver cirrhosis diagnosis confirmed by clinical evaluation, imaging, or biopsy; and (2) Initial AVB due to portal hypertension. The exclusion criteria were: (1) Previous TIPS or surgical shunt; (2) Repeated occurrences of AVB and related treatments; (3) Liver cancer or other extrahepatic malignancies; (4) TIPS unrelated to AVB; (5) Severe cardiac, pulmonary, or renal insufficiency and expected survival time of less than 3 months; and (6) Loss to follow-up within 1 month post-TIPS (Figure 1). All patients with AVB received treatment with vasoactive drugs, received endoscopic hemostasis as appropriate, and underwent TIPS within 7 days of first onset. After TIPS, all patients were administered lactulose and rifaximin to prevent OHE onset. Within 7 days before the TIPS procedure, demographic and baseline clinical characteristics were collected.
Patients were followed up regularly at specific intervals-1 week, 1 month, 3 months, 6 months, and 1 year after the TIPS procedure-and annually thereafter until death or the end of the follow-up period. Key outcome measures included the occurrence of OHE, defined as the development of clinically apparent hepatic encephalopathy requiring medical intervention, and overall survival (OS), defined as the time from TIPS placement to death from any cause. Follow-up data were collected through clinical visits, imaging studies, and biochemical tests. The occurrence of OHE and other complications was recorded based on standardized diagnostic criteria, and survival status was confirmed through hospital records or follow-up communications.
All procedures were successfully performed by the same interventional radiology team with 5 years to 20 years of experience. Using the Seldinger technique, the right internal jugular vein was punctured successfully, followed by pre-dilation with a 10F dilator. Under guidewire guidance, the RUPS-100 sheath (COOK Medical, Bloomington, IN, United States) was advanced to the inferior vena cava to measure inferior vena cava pressure. The catheter was positioned in the hepatic vein and confirmed by a “smoke” sign. Under fluoroscopic guidance, a puncture needle was introduced to puncture the left or right branch of the portal vein via the hepatic vein through the liver parenchyma. Then, a catheter was advanced into the main portal vein for pressure measurement and contrast imaging to assess portal venous flow and varices. Embolization was performed using coils and/or tissue glue. An exchange for a super-stiff guidewire was done, and the liver parenchymal tract was dilated with a 6 mm balloon. The Viatorr covered stent (Gore Medical, Flagstaff, AZ, United States) was deployed to create a hepatic-portal vein shunt, followed by dilation of the stent with an 8 mm balloon. Follow-up imaging confirmed stent position and shunt function, and pressures in the inferior vena cava and portal vein were remeasured. The right internal jugular vein sheath was removed, and the puncture site was compressed and bandaged securely. The goal was a portosystemic gradient of less than 12 mmHg or a reduction of 50% from the baseline portal pressure gradient for AVB.
A dataset was built using baseline demographic characteristics (sex, age, etiology), along with clinical, biochemical, and procedural data. We employed embedded methods (EMs) with LR as the classifier, along with RFE for feature selection. The selected features were used to train models and predict OHE after TIPS. The data were normalized to standardize the variables to a common scale. Subsequently, the dataset was randomly divided into training and test sets in a ratio of 7:3. To address the issue of the imbalanced sample sizes between patients with and without OHE, we used the synthetic minority oversampling technique (SMOTE) to balance the training dataset. The SMOTE algorithm was implemented using the imblearn package in Python 3.7.13.
In the present study, three common ML algorithms, including random forest classifier, XGBoost, and LR classifier, were developed and validated using the scikit-learn and XGBoost packages in Python 3.7.13 to predict OHE after TIPS. Ten-fold cross-validation was used for model derivation and internal validation. The grid search algorithm was used during the training process for each model to optimize the model’s hyperparameters on the training set as the standard of the AUC of the receiver operating characteristic curve. We integrated SHapley Additive exPlanations (SHAP), a locally interpretable method, to elucidate the optimal model. SHAP applies game theory to explain ML model outputs, linking optimal credit assignment with localized explanations using classical SHAP values. This enabled identification of pivotal features influencing model outputs and provided insights into the decision-making process of the models.
Statistical analyses were performed using RStudio (version 4.3.1). Categorical variables are expressed as numbers and percentages, while continuous variables are summarized as the median with 95% confidence interval (CI) or mean with SD. Baseline characteristics of patients were analyzed using the Student’s t-test, Mann-Whitney U test, and χ2 test. OS in the test sets was estimated using the Kaplan-Meier analysis, and the log-rank test was used to compare OS differences between patients with and without post-TIPS OHE. In Kaplan-Meier analysis, we stratified the patients into OHE and non-OHE groups based on the actual OHE status or predicted OHE status. Covariates were adjusted to meet the proportional hazards, linearity, and independence assumptions of Cox regression. Stepwise Cox regression analysis was conducted, and variables with P < 0.05 were included in further Cox modeling. The final model was validated using the concordance C statistic (known as the C-index) and Brier score. All data were analyzed using two-sided tests, and P < 0.05 was considered statistically significant.
A total of 218 patients were included in the study (male/female: 163/55), with a median age of 52.14 ± 11.96 years at the time of the TIPS procedure. The predominant cause of AVB was portal hypertension resulting from hepatitis B virus infection (70.18%). The median follow-up time was 5 years. During this period, the 5-year survival rate was 78.4% (47/218), while the incidence of OHE was 15.1% (33/218). Detailed demographic data and characteristics of the patients are presented (Table 1).
| Variables | Total, n = 218 | Training set, n = 152 | Test set, n = 66 | P value |
| Age in years | 52.14 ± 11.96 | 51.86 ± 11.93 | 52.80 ± 12.10 | 0.592 |
| OS | 47.83 ± 22.95 | 48.56 ± 22.66 | 46.14 ± 23.69 | 0.475 |
| Etiology | 0.044 | |||
| Hepatitis B virus | 153 (70.18) | 110 (72.37) | 43 (65.15) | |
| Hepatitis C virus | 11 (5.05) | 9 (5.92) | 2 (3.03) | |
| Alcoholism | 24 (11.01) | 15 (9.87) | 9 (13.64) | |
| Autoimmune disease | 7 (3.21) | 7 (4.61) | 0 (0.00) | |
| Other etiologies | 23 (10.55) | 11 (7.24) | 12 (18.18) | |
| HE | 0.856 | |||
| No | 208 (95.41) | 145 (95.39) | 63 (95.45) | |
| Mild | 8 (3.67) | 5 (3.29) | 3 (4.55) | |
| Moderate and severe | 2 (0.92) | 2 (1.32) | 0 (0.00) | |
| Ascites | 0.686 | |||
| No | 73 (33.49) | 54 (35.53) | 19 (28.79) | |
| Mild | 85 (38.99) | 56 (36.84) | 29 (43.94) | |
| Moderate | 59 (27.06) | 41 (26.97) | 18 (27.27) | |
| Severe | 1 (0.46) | 1 (0.66) | 0 (0.00) | |
| PVT | 0.594 | |||
| No | 177 (81.19) | 122 (80.26) | 55 (83.33) | |
| Yes | 41 (18.81) | 30 (19.74) | 11 (16.67) | |
| SMVT | 0.412 | |||
| No | 197 (90.37) | 139 (91.45) | 58 (87.88) | |
| Yes | 21 (9.63) | 13 (8.55) | 8 (12.12) | |
| SVT | 1 | |||
| No | 213 (97.71) | 149 (98.03) | 64 (96.97) | |
| Yes | 5 (2.29) | 3 (1.97) | 2 (3.03) | |
| BCS | 0.318 | |||
| No | 213 (97.71) | 147 (96.71) | 66 (100.00) | |
| Yes | 5 (2.29) | 5 (3.29) | 0 (0.00) | |
| CTPV | 0.085 | |||
| No | 203 (93.12) | 145 (95.39) | 58 (87.88) | |
| Yes | 15 (6.88) | 7 (4.61) | 8 (12.12) | |
| Pressure-related indicators | ||||
| Pre-IVCP | 5.66 ± 4.07 | 5.53 ± 4.09 | 5.97 ± 4.03 | 0.461 |
| Pre-PVP | 28.07 ± 6.62 | 28.05 ± 6.50 | 28.14 ± 6.96 | 0.927 |
| Pre-PPG | 22.41 ± 5.51 | 22.52 ± 5.43 | 22.17 ± 5.73 | 0.665 |
| Post-IVCP | 8.28 ± 4.62 | 8.26 ± 4.65 | 8.33 ± 4.59 | 0.911 |
| Post-PVP | 18.00 ± 5.87 | 18.02 ± 5.78 | 17.94 ± 6.12 | 0.926 |
| Post-PPG | 9.72 ± 3.71 | 9.76 ± 3.73 | 9.61 ± 3.69 | 0.775 |
| Blood and biochemical indicators | ||||
| TBIL | 22.79 ± 15.02 | 22.97 ± 14.75 | 22.38 ± 15.75 | 0.789 |
| ALB | 33.94 ± 5.19 | 33.88 ± 5.32 | 34.10 ± 4.92 | 0.775 |
| CR | 90.51 ± 78.67 | 90.90 ± 73.21 | 89.61 ± 90.59 | 0.911 |
| ALT | 24.93 ± 18.65 | 24.18 ± 17.19 | 26.65 ± 21.71 | 0.37 |
| Prolonged PT | 1.86 ± 2.43 | 1.93 ± 2.30 | 1.69 ± 2.72 | 0.504 |
| INR | 1.30 ± 0.23 | 1.31 ± 0.22 | 1.27 ± 0.24 | 0.187 |
| Sodium | 140.68 ± 12.47 | 140.06 ± 3.41 | 142.11 ± 22.12 | 0.266 |
| PLT | 94.59 ± 77.50 | 89.96 ± 71.21 | 105.24 ± 90.05 | 0.182 |
| WBC | 5.17 ± 3.51 | 5.05 ± 3.29 | 5.46 ± 3.97 | 0.426 |
| Clinical scores and classifications | ||||
| Child-Pugh score | 7.17 ± 1.71 | 7.18 ± 1.75 | 7.15 ± 1.61 | 0.897 |
| Child-Pugh classification | 0.776 | |||
| A | 85 (38.99) | 61 (40.13) | 24 (36.36) | |
| B | 111 (50.92) | 75 (49.34) | 36 (54.55) | |
| C | 22 (10.09) | 16 (10.53) | 6 (9.09) | |
| MELD score | 10.86 ± 3.70 | 11.05 ± 3.62 | 10.42 ± 3.87 | 0.243 |
| MELD classification | 0.54 | |||
| Mild | 103 (47.25) | 67 (44.08) | 36 (54.55) | |
| Moderate | 78 (35.78) | 57 (37.50) | 21 (31.82) | |
| Severe | 37 (16.97) | 27 (18.42) | 9 (13.64) | |
| MELD-Na score | 11.50 ± 4.64 | 11.66 ± 4.33 | 11.14 ± 5.31 | 0.447 |
| ALBI score | -0.95 ± 0.62 | -0.94 ± 0.62 | -0.99 ± 0.61 | 0.591 |
| ALBI classification | 0.343 | |||
| Low risk | 52 (23.85) | 39 (25.66) | 13 (19.70) | |
| High risk | 166 (76.15) | 113 (74.34) | 53 (80.30) | |
| FIPS score | 2.49 ± 1.09 | 2.56 ± 0.98 | 2.34 ± 1.30 | 0.184 |
| CLIF-C AD score | 41.57 ± 10.60 | 42.01 ± 8.10 | 40.55 ± 14.86 | 0.351 |
| CLIF-C AD classification | 0.84 | |||
| Low risk | 147 (67.43) | 101 (66.45) | 46 (69.70) | |
| Intermediate risk | 65 (29.82) | 47 (30.92) | 18 (27.27) | |
| High risk | 6 (2.75) | 4 (2.63) | 2 (3.03) | |
| Previous surgeries and procedures | ||||
| EBL | 0.986 | |||
| No | 195 (89.45) | 136 (89.47) | 59 (89.39) | |
| Yes | 23 (10.55) | 16 (10.53) | 7 (10.61) | |
| Partial splenectomy | 0.629 | |||
| No | 198 (90.83) | 139 (91.45) | 59 (89.39) | |
| Yes | 20 (9.17) | 13 (8.55) | 7 (10.61) | |
| EIS | 0.605 | |||
| No | 211 (96.79) | 146 (96.05) | 65 (98.48) | |
| Yes | 7 (3.21) | 6 (3.95) | 1 (1.52) | |
| GCAE | 1 | |||
| No | 217 (99.54) | 151 (99.34) | 66 (100.00) | |
| Yes | 1 (0.46) | 1 (0.66) | 0 (0.00) | |
| PSE | 0.989 | |||
| No | 213 (97.71) | 148 (97.37) | 65 (98.48) | |
| Yes | 5 (2.29) | 4 (2.63) | 1 (1.52) | |
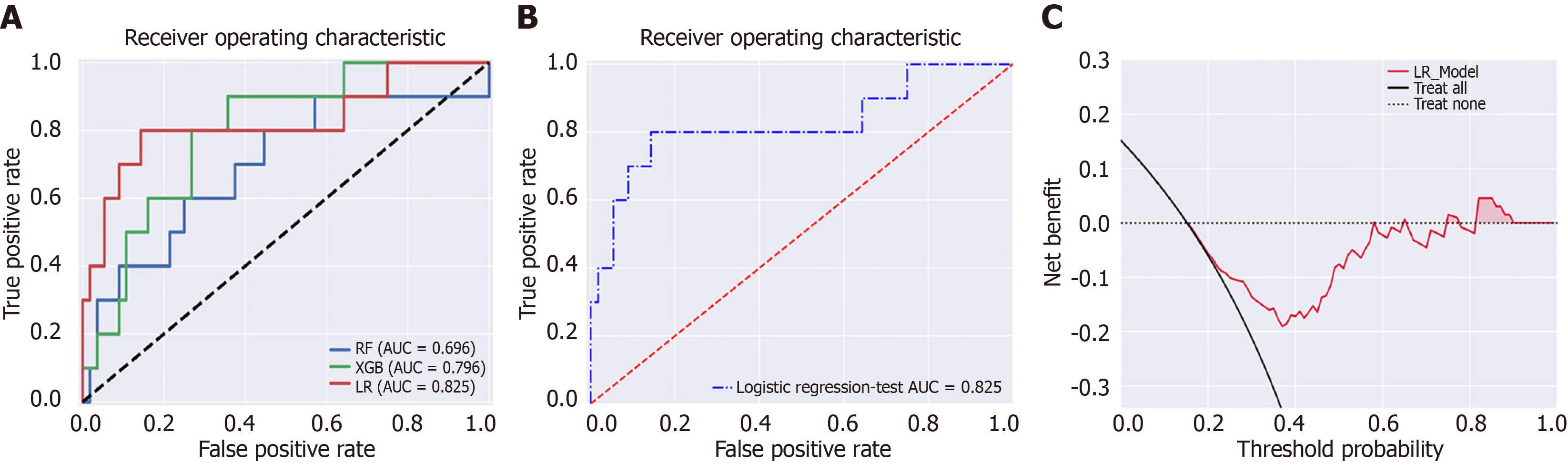
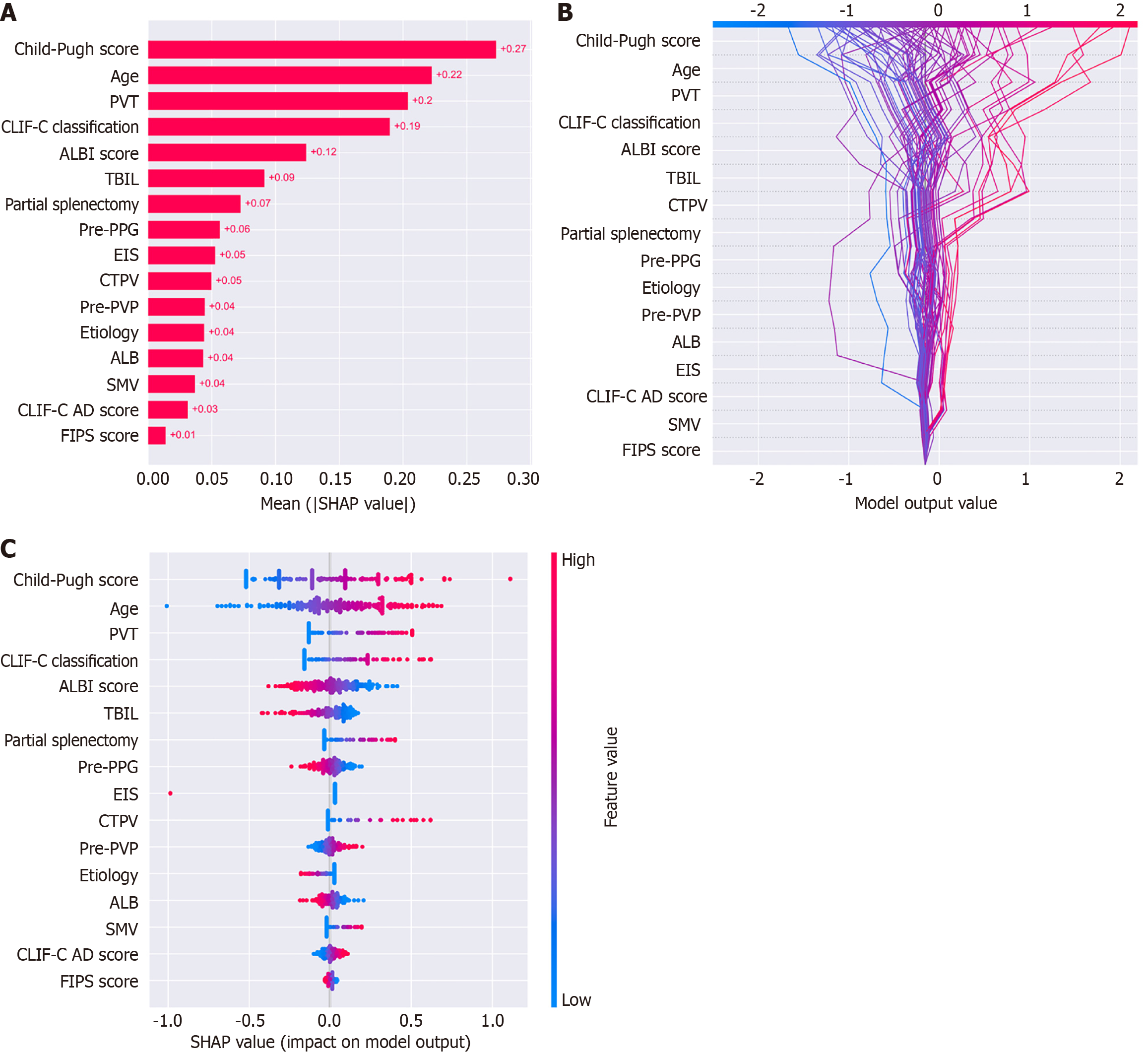
The dataset was randomly divided into a training set (n = 152, 70%) and a test set (n = 66, 30%). In the training set, there were 23 (15.1%) patients with OHE. In the test set, there were 10 (15.1%) patients with OHE. SMOTE algorithm generated 106 cases with OHE in the training set. We employed EMs for feature selection, reducing the initial set of features to 16. Subsequently, we applied RFE to further refine the features down to 9. In the test set, the AUCs for the RF, XGBoost, and LR model were 0.696, 0.796, and 0.825, respectively (Figure 2). The decision curve analysis showed that the LR model provided more net benefits for predicting OHE after TIPS. SHAP algorithm showed that the most important factors were Child-Pugh score, age, and portal vein thrombosis (PVT) (Figure 3).
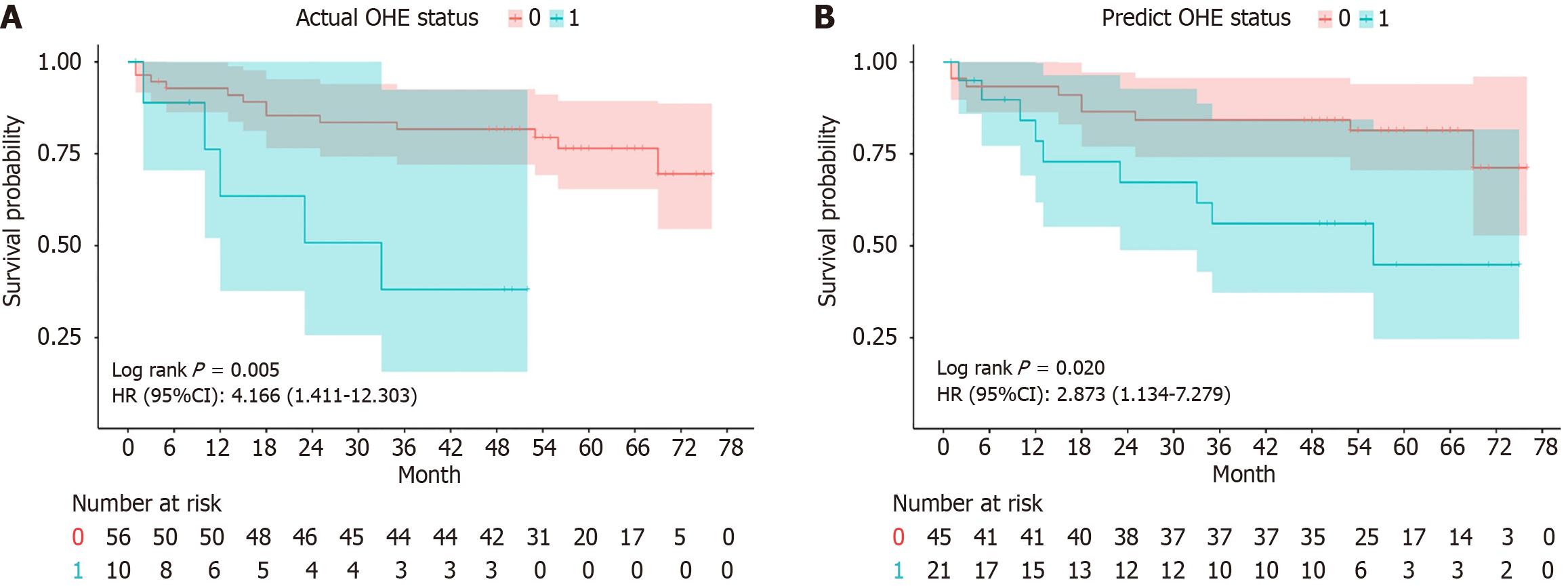
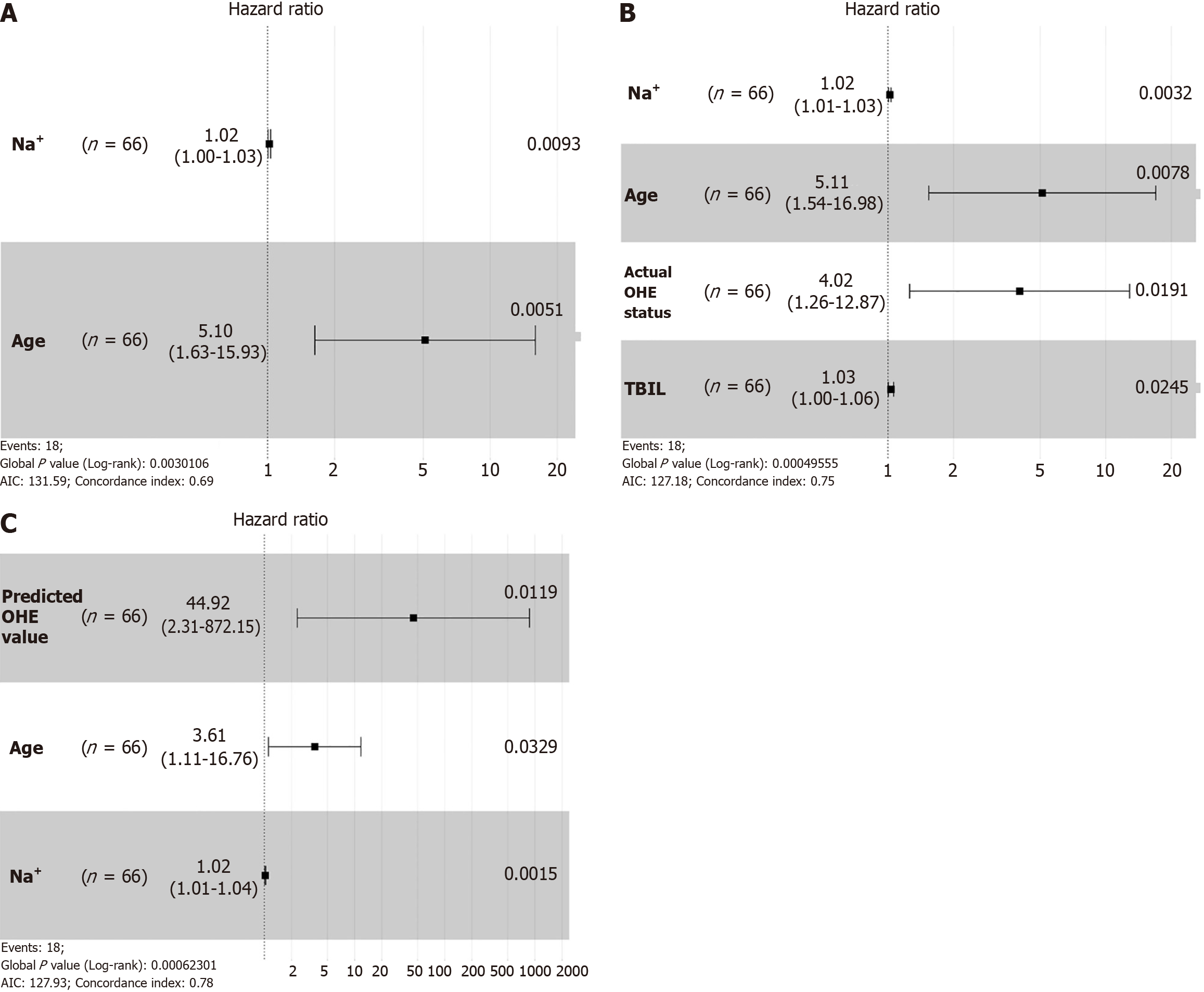
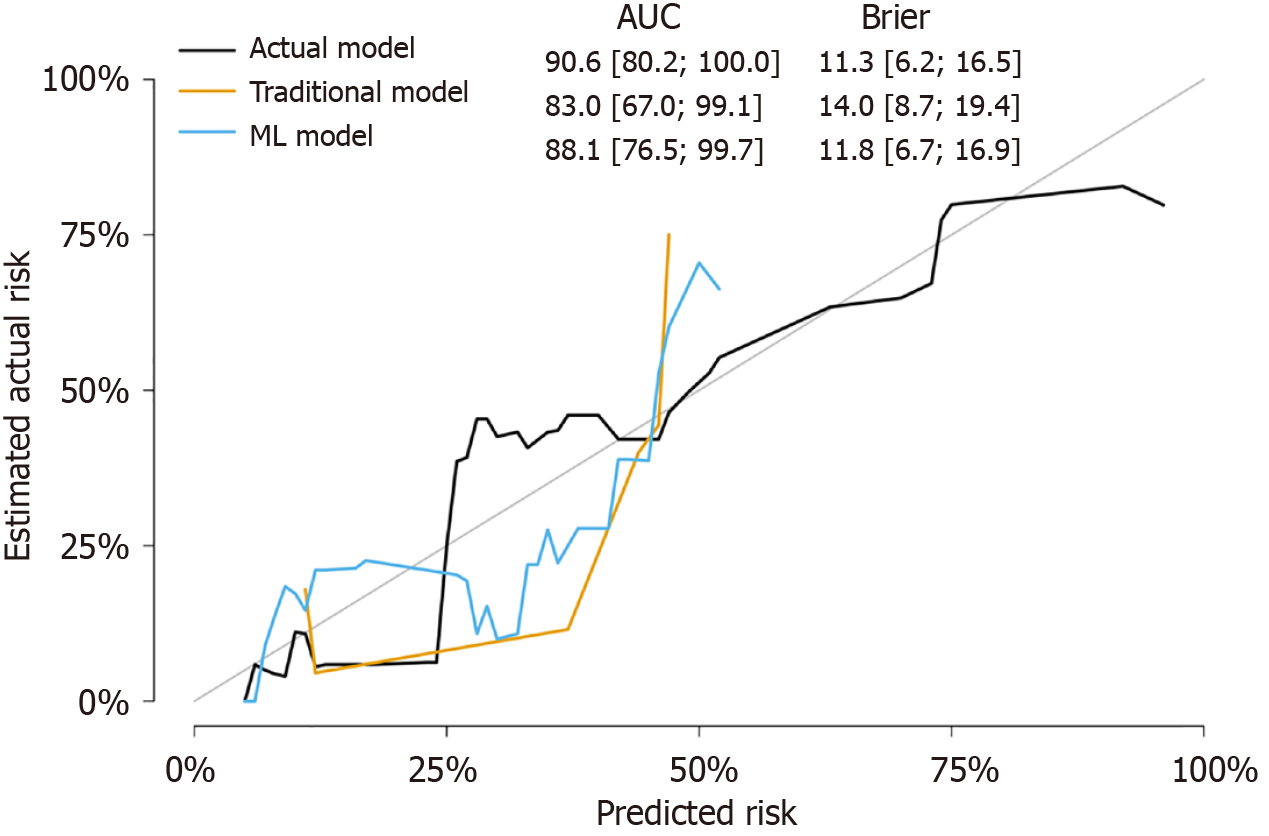
In Kaplan-Meier analysis, the prediction of OHE was based on the LR model, with a threshold set such that patients with a predicted probability greater than 0.5 were classified as predicted OHE status. Patients without post-TIPS OHE had a longer OS than patients with post-TIPS OHE (log-rank test, P = 0.005) (Figure 4A). Similarly, a longer OS was observed for patients with predicted OHE status by the ML model (log-rank test, P = 0.020) (Figure 4B). In multivariate Cox analysis with stepwise selection, the OHE value predicted by the ML model (P = 0.012) is the prognostic factor in the Cox proportional hazards model. As the same time, the actual OHE status was a prognostic factor in the analysis of OS (P = 0.019) (Figure 5 and Supplementary Tables 1-3), the AUCs of the Cox models with the actual OHE status and predicted OHE value were 90.6 (95%CI: 80.2-100.0) and 88.1 (95%CI: 76.5-99.7), respectively, which were very close to each other and higher than the Cox model based on traditional preoperative data (AUC: 83.0, 95%CI: 67.0-99.1) (Figure 6).
We utilized 5-year long-term follow-up data and applied ML methods to successfully establish a predictive model for post-TIPS OHE. Through survival analysis, we observed that the predicted value of OHE was associated with differences in post-TIPS OS among patients, further validating the model’s value. In this study, we employed EMs for feature selection, reducing the initial set of features to 16. Subsequently, we applied RFE to further refine the features down to 9. Our findings indicate that the Child-Pugh score and age emerged as the most critical predictors, followed by PVT, significantly influencing the model’s output (Figure 3).
The Child-Pugh score is a widely used indicator for assessing patients who underwent TIPS preoperatively, serving as a crucial measure of liver function reserve[12,13]. Numerous studies have demonstrated its correlation with both the occurrence of post-TIPS OHE and patient survival[5,6,8]. Patients with poorer liver function have more neurotoxins bypassing the liver and entering the nervous system post-TIPS, increasing the risk of OHE[14]. However, due to the combination of subjective and objective factors in the Child-Pugh score, using it alone to predict post-TIPS OHE is unreliable. In our ML model, although the Child-Pugh score significantly impacts the model, other scoring systems such as the albumin-bilirubin score and chronic liver failure consortium acute decompensation score also play a role. By incorporating different scores and clinical variables, the ML model can better handle the nonlinear relationships between variables compared to traditional statistical models, resulting in more reliable predictions.
With advancing age, gastrointestinal motility decreases, leading to delayed intestinal emptying and higher incidences of constipation and intestinal flora imbalance. Additionally, the aging brain is more sensitive to toxic metabolites such as plasma ammonia[15]. Moreover, aging affects the overall physiological reserve and liver functional capacity, potentially reducing surgical tolerance and increasing risk[16,17]. These findings highlight the need to consider age during preoperative assessments and postoperative care for TIPS patients.
The formation of PVT results from disrupted hepatic blood flow, often accompanied by further liver function impairment[18]. In patients with PVT, the potential influence on post-TIPS OHE can be attributed to the presence of additional portosystemic shunts beyond the TIPS stent, which reduces portal vein blood flow velocity and subsequently affects the central nervous system. Although some studies have reported an association between PVT and OHE, the specific mechanisms underlying this relationship require further investigation[17,19]. Since our enrolled patients experienced AVB, they did not receive anticoagulant therapy either before or after the TIPS procedure, in principle. There is also controversy regarding anticoagulant therapy following TIPS in different guidelines[4,20-22]. Therefore, in AVB patients with PVT, more detailed clinical examination data and multidisciplinary team discussions may be necessary.
In addition to constructing an ML model to predict the status of OHE, we further investigated the prognostic differences between the predicted OHE value from the ML model and the actual OHE status. This analysis was conducted on the test dataset that was split using random seeds. The predicted OHE value was applied to a classification model to evaluate prognostic differences using Kaplan-Meier curves, revealing that both predicted and actual OHE values significantly influenced OS (Figure 4). In further exploring the prognostic factors, the final Cox models consistently identified age and sodium levels as significant factors affecting post-TIPS outcomes. Older patients face higher mortality risks due to reduced physiological reserves and greater susceptibility to OHE complications. Additionally, hyponatremia, which is common in cirrhotic patients, reflects fluid imbalances that can worsen postoperative outcomes. These findings highlight the importance of managing age and sodium levels to improve long-term survival after TIPS. Both the predicted and actual OHE values were equally effective in predicting patient survival, as demonstrated by comparable model performance (AUC: 0.846/0.798, P = 0.368) (Supplementary Figure 1), indicating no significant difference in the performance of the two models in predicting post-TIPS survival. The predicted OHE value was similar to the actual OHE status in predicting patient survival and performed better than traditional preoperative models, demonstrating the reliability and validity of the ML model. In multivariate Cox analysis, both the predicted OHE value and actual OHE status were important prognostic factors in their respective Cox models (Figure 5 and Supplementary Tables 4-6). Although a recent Italian study found that post-TIPS episodic OHE did not increase mortality in patients with cirrhosis[23], our research indicates that post-TIPS OHE is a significant factor affecting patient survival post-TIPS (Figure 5). Long-term follow-up studies from other centers have also shown that post-TIPS OHE is significantly associated with a higher risk of mortality[24-26]. Given the strong prognostic implications of OHE in post-TIPS survival, it is essential for the medical team to thoroughly communicate the risks, diagnosis, and management of OHE with patients and their families.
For the clinical application of our model, predicting the occurrence of OHE after TIPS allows for the individualized treatment of patients. This enables the provision of optimal treatment plans for individual patients, making the treatment of AVB more personalized. Additionally, the predictive model offers a tool for long-term follow-up, guiding decision-making by identifying patients who will benefit the most from the TIPS procedure and allowing for better prevention and treatment measures for potential OHE. This is where our model has an advantage over previous studies that focused on short-term follow-up after TIPS. Furthermore, the predictive performance of our model is superior compared to other models.
This study had several limitations. First, as a retrospective single-center study, there may have been selection bias. Second, although we used survival analysis to further validate our ML model, the relatively small sample size resulted in a small test set, which may have compromised the stability of the results. Additionally, our study focused on predicting OHE and did not consider other complications occurring over a longer period. Lastly, some procedural features were missing in this study, such as specific physical parameters of the stent and the stent implantation angle. Certain potential risk factors, such as sarcopenia[27], which are believed to influence the prognosis of patients undergoing TIPS for AVB, were not included in this study. Future research could incorporate multicenter data and include more clinical course information to further improve the model.
In conclusion, our study demonstrated that our model exhibited high and stable performance in predicting the occurrence of OHE following TIPS in patients with AVB. The predicted OHE value was identified as an independently significant predictor of OS in validation cohorts. The ML model demonstrated promising potential as a computer-assisted diagnostic tool, enabling clinicians to more accurately detect post-TIPS OHE and thus inform and optimize patient treatment plans.
We thank Professor Wang JY for his support and supervision of this study.
| 1. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 2. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 3. | Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia JD, Wei L, Duan ZP, Ling-Hu EQ, Zhuang H. Chinese guidelines on the management of liver cirrhosis (abbreviated version). World J Gastroenterol. 2020;26:7088-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 5. | Bai M, Qi X, Yang Z, Yin Z, Nie Y, Yuan S, Wu K, Han G, Fan D. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Li K, Cheng Y, Zhao R, Jiang H, Zhang L, Tong Y, Li S. Prediction of mortality and overt hepatic encephalopathy undergoing transjugular intrahepatic portosystemic shunt: a retrospective cohort study. Abdom Radiol (NY). 2024;49:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Li J, Feng D, Pang N, Zhao C, Gao L, Liu S, Li L. Controlling nutritional status score as a new indicator of overt hepatic encephalopathy in cirrhotic patients following transjugular intrahepatic portosystemic shunt. Clin Nutr. 2022;41:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Liu J, Zhou C, Wang Y, Yang C, Shi Q, Huang S, Chen Y, Li T, Xiong B. The combination of Child-Pugh score and quantitative CT-based spleen volume could predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. Abdom Radiol (NY). 2021;46:3464-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Gurgitano M, Angileri SA, Rodà GM, Liguori A, Pandolfi M, Ierardi AM, Wood BJ, Carrafiello G. Interventional Radiology ex-machina: impact of Artificial Intelligence on practice. Radiol Med. 2021;126:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Glielmo P, Fusco S, Gitto S, Zantonelli G, Albano D, Messina C, Sconfienza LM, Mauri G. Artificial intelligence in interventional radiology: state of the art. Eur Radiol Exp. 2024;8:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | von Ende E, Ryan S, Crain MA, Makary MS. Artificial Intelligence, Augmented Reality, and Virtual Reality Advances and Applications in Interventional Radiology. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Rajesh S, George T, Philips CA, Ahamed R, Kumbar S, Mohan N, Mohanan M, Augustine P. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26:5561-5596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Vizzutti F, Schepis F, Arena U, Fanelli F, Gitto S, Aspite S, Turco L, Dragoni G, Laffi G, Marra F. Transjugular intrahepatic portosystemic shunt (TIPS): current indications and strategies to improve the outcomes. Intern Emerg Med. 2020;15:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 15. | Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Hassoun Z, Deschênes M, Lafortune M, Dufresne MP, Perreault P, Lepanto L, Gianfelice D, Bui B, Pomier-Layrargues G. Relationship between pre-TIPS liver perfusion by the portal vein and the incidence of post-TIPS chronic hepatic encephalopathy. Am J Gastroenterol. 2001;96:1205-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 475] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Nicoară-Farcău O, Soy G, Magaz M, Baiges A, Turon F, Garcia-Criado A, Barrufet M, Burrel M, Hernández-Gea V, García-Pagán JC. New Insights into the Pathogenesis, Risk Factors, and Treatment of Portal Vein Thrombosis in Patients with Cirrhosis. Semin Thromb Hemost. 2020;46:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Wang J, Deng MJ, Shi PM, Peng Y, Wang XH, Tan W, Wang PQ, Chen YX, Yuan ZL, Ning BF, Xie WF, Yin C. Covert hepatic encephalopathy is associated with aggressive disease progression and poor survival in patients with cirrhosis. J Dig Dis. 2023;24:681-690. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1510] [Article Influence: 503.3] [Reference Citation Analysis (2)] |
| 21. | European Association for the Study of the Liver; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1070] [Article Influence: 267.5] [Reference Citation Analysis (0)] |
| 22. | Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB; Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022;20:1636-1662.e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 23. | Nardelli S, Riggio O, Marra F, Gioia S, Saltini D, Bellafante D, Adotti V, Guasconi T, Ridola L, Rosi M, Caporali C, Fanelli F, Roccarina D, Bianchini M, Indulti F, Spagnoli A, Merli M, Vizzutti F, Schepis F. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J Hepatol. 2024;80:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 24. | Bai M, He CY, Qi XS, Yin ZX, Wang JH, Guo WG, Niu J, Xia JL, Zhang ZL, Larson AC, Wu KC, Fan DM, Han GH. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2014;20:774-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Dissegna D, Sponza M, Falleti E, Fabris C, Vit A, Angeli P, Piano S, Cussigh A, Cmet S, Toniutto P. Morbidity and mortality after transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Bouzbib C, Cluzel P, Sultanik P, Bernard-Chabert B, Massard J, Benosman H, Mallet M, Tripon S, Conti F, Thabut D, Rudler M. Prognosis of patients undergoing salvage TIPS is still poor in the preemptive TIPS era. Clin Res Hepatol Gastroenterol. 2021;45:101593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, Huang S, Chen Y, Wang Y, Li T, Xiong B. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology. 2022;303:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |