Published online Aug 14, 2025. doi: 10.3748/wjg.v31.i30.109465
Revised: June 17, 2025
Accepted: July 21, 2025
Published online: August 14, 2025
Processing time: 86 Days and 0.5 Hours
Poorly cohesive gastric carcinomas are classified based on the proportion of signet-ring cell carcinoma (SRCC) components. In surgically resected gastric cancer, SRCC is diagnosed when the signet-ring cell (SRC) component constitutes ≥ 50% of the entire tumor, whereas poorly cohesive carcinoma (PCC) not other
To investigate how the proportion of SRCC affects tumor pathology, clinical outcomes, and prognosis and treatment decision-making.
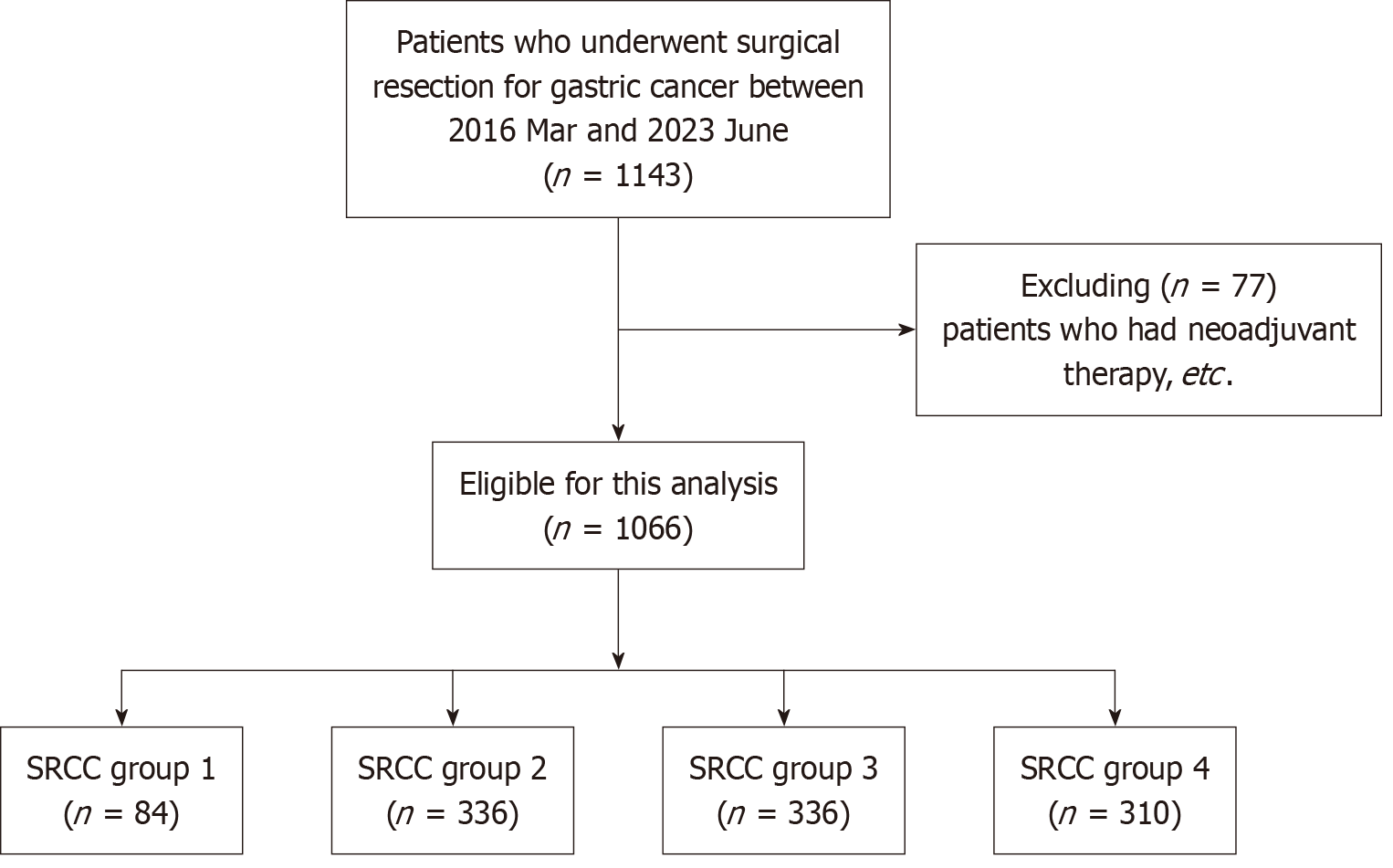
This retrospective study included 1066 patients with PCC who underwent gastric cancer surgery at Seoul National University Bundang Hospital from 2016 to 2023. Patients were classified into four groups based on the SRCC proportion: < 10%, ≥ 10% and < 50%, ≥ 50% and < 90%, and ≥ 90%. Clinicopathological and molecular data were compared between the groups. The correlation between SRCC proportion and pathological factors associated with indications for endoscopic resection in patients with early-stage gastric cancer (EGC) was analyzed.
A higher SRCC proportion was associated with smaller tumor size, lower tumor stage pathological tumor-node-metastasis, and reduced rates of lymphatic, vascular, and neural invasion (P < 0.001). Notably, the ≥ 90% SRCC group exhibited the highest recurrence-free survival (P = 0.0072) and overall survival (P = 0.0002). In EGC, lower SRCC rates were correlated with increased ulceration, larger tumor size, and deeper submucosal invasion (P < 0.001).
Higher SRCC proportions in the PCC correlate with lower tumor aggressiveness and improved prognosis. Its role in EGC should be validated as a factor influencing therapeutic strategies, including endoscopic submucosal dissection.
Core Tip: A higher proportion of signet-ring cell carcinoma (SRCC) in poorly cohesive gastric carcinoma indicates less aggressive tumor behavior, reflected by smaller size, lower stage, and reduced lymphovascular invasion. Patients with ≥ 90% SRCC show excellent recurrence-free and overall survival. In early gastric cancer, lower SRCC proportions are linked to ulceration, larger size, and deeper invasion. These findings highlight the prognostic value of SRCC proportion and its potential to guide therapeutic strategies, including endoscopic resection.
- Citation: Kim C, Lee HS, Na HY, Kwon HJ, Lee JA, Suh YS, Kang SH, Kim HH, Ahn SH, Oh HJ. Prognostic value of signet-ring cell carcinoma proportion in undifferentiated gastric cancer: Implications for endoscopic treatment decisions. World J Gastroenterol 2025; 31(30): 109465
- URL: https://www.wjgnet.com/1007-9327/full/v31/i30/109465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i30.109465
Gastric carcinoma is the sixth leading cause of cancer-related mortality in Korea[1]. Gastric carcinoma exhibits morphological diversity, even within a single tumor. Diffuse-type gastric carcinoma (DGC) is a major subtype of gastric cancer, according to the Lauren classification[2].
The incidence of DGC has been increasing, accounting for approximately one-third of all gastric cancers, whereas the overall incidence of gastric cancer has decreased over the past few decades[3]. DGC exhibits clinical and pathological characteristics distinct from those of intestinal-type gastric cancer (IGC), with patients with DGC typically being younger than those with IGC. Despite its increasing incidence, the etiology of DGC remains unclear, and its prognostic factors are poorly defined. A previous meta-analysis reported that patients with DGC have a poorer prognosis than those with the intestinal type, regardless of tumor stage[4]. However, several studies have demonstrated that the prognosis of gastric signet ring cell carcinoma (SRCC) varies by stage; SRCC is associated with a better prognosis than non-SRCC tumors in the early stage, but a worse prognosis in the advanced stage[5,6].
According to the World Health Organization (WHO) classification, poorly cohesive carcinoma (PCC), a major type of DGC, is divided into two histological subtypes: SRCC and PCC not otherwise specified (PCC-NOS). The morphological features of SRCC include prominent intracytoplasmic mucin and eccentrically placed nuclei. SRCC is diagnosed when the signet-ring cell (SRC) component constitutes more than 50% of the entire tumor, whereas PCC-NOS is diagnosed when the proportion of the SRC component is less than 50% of the entire tumor. Several studies have demonstrated the different biological behaviors of PCC-NOS and SRCC. Nonetheless, given that these cell types often coexist and the final histological diagnosis is typically determined based on whether the SRCC proportion exceeds 50% or not, their distinct prognostic impact is often obscured.
Developed in the 1990s, endoscopic resection is usually considered the first treatment option for early-stage gastric cancer (EGC) owing to its less invasive nature compared with that of surgical resection[7]. Endoscopic submucosal dissection (ESD) or endoscopic mucosal resection is suitable for EGC with a significantly low possibility of lymph node metastasis. The fifth Japanese gastric cancer treatment guidelines recommend distinctive restrictive conditions for endoscopic resection based on the differentiation grade[8]. According to these guidelines, only expanded indications exist for DGC [cT1a, ≤ 2 cm, ulcer (-)][9,10]. Despite being included under the expanded indications for endoscopic resection, undifferentiated-type gastric carcinoma is rarely treated with endoscopic resection in clinical practice. In Korea, the recommendation grade for ESD of undifferentiated EGC is weak. In Japan, it is also included in the expanded non-absolute indications. In Europe, gastrectomy is recommended for DGC, with treatment decisions individualized based on patient age, comorbidities, and preferences through a multidisciplinary approach[8,11,12].
ESD enables curative treatment without the need for surgery in selected cases, while preserving gastric function and reducing morbidity. Recent studies have shown that, even in lesions larger than 2 cm, undifferentiated-type EGCs can achieve favorable outcomes if R0 resection is obtained[13,14]. Although recurrence may be more frequent after ESD than surgery, overall survival (OS) is comparable, emphasizing the importance of appropriate case selection and follow-up[15].
In patients with unresectable advanced gastric cancer, targeted therapies, such as anti-human epidermal growth factor receptor 2 (HER2) antibodies and immune checkpoint inhibitors targeting programmed death-ligand 1 (PD-L1), have been established as the standard first-line treatments for those exhibiting HER2 amplification or PD-L1 overexpression. However, DGC exhibits a significantly lower frequency of HER2 overexpression compared with intestinal and mixed types, with reported rates of 6%, 34%, and 20%, respectively[16]. Similarly, PD-L1 expression is less frequently observed in DGC than in other subtypes[17]. Consequently, anti-HER2 therapy and immune checkpoint inhibitors are used less frequently for DGC, predominantly including PCC. Considering the limited availability of effective therapeutic options for DGC, investigating the histological characteristics that may affect prognosis is of clinical importance.
SRCC histology has been suggested as an independent prognostic factor for gastric cancer. Recent findings by Kong et al[18] have highlighted the association between SRCC histology and poor prognosis, particularly with regard to recurrence in patients with node-negative gastric cancer. Their study suggested that incorporating SRCC histological analysis with the American Joint Committee on Cancer (AJCC) staging system could enhance the prediction of recurrence rate. Conversely, other studies have indicated that mixed-type gastric cancer may have a poorer prognosis than DGC, with a higher propensity for lymph node metastasis[19]. In this context, the proportion of SRCC or PCC-NOS compo
Therefore, this study aimed to analyze the relationship between the SRCC proportion and clinicopathological factors, as well as prognosis, and to evaluate the effect of SRCC proportion on these outcomes. In this study, the presence of lymph node metastasis in EGC was examined according to SRCC proportion to determine whether indications for endoscopic resection in DGC should be adjusted based on the SRCC proportion.
A retrospective analysis was conducted using a prospectively maintained database of patients who underwent gastric cancer surgery at Seoul National University Bundang Hospital, Korea, from March 2016 to June 2023. A total of 1143 patients diagnosed with PCC according to the WHO classification were included. Among them, 77 patients who received neoadjuvant treatment, underwent gastrectomy after ESD, or had multiple gastric cancers were excluded. Ultimately, 1066 patients were enrolled in this study (Figure 1).
This study was approved by the Ethics Committee of Seoul National University Bundang Hospital (No. B-2403-888-303). The requirement for obtaining informed consent from patients was waived owing to the retrospective nature of this study.
Electronic medical records were reviewed to collect data on clinical factors such as sex, age, recurrence, and mortality. Pathological reports were also reviewed for tumor size; tumor (T), node (N), and metastasis (M) categories; along with lymphatic, vascular, and perineural invasion. The presence of ulcers, submucosal invasion, and invasion depth in EGC were also recorded. Immunohistochemical and molecular factors associated with therapeutic options, such as HER2 expression, microsatellite status, Epstein-Barr virus (EBV) in situ hybridization, and PD-L1 score, were assessed. Clinicopathological data were collected from medical records during July 2023 to Jan 2024.
All slides with tumor involvement were independently reviewed by pathologist for diagnostic confirmation. For EGC, one section was prepared for every 0.4 cm, and all tumor areas were submitted for slide preparation. In contrast, for advanced gastric cancer, only representative sections were submitted, with an average of one section per 1 cm. Considering the tumor size in our cohort (4.15 cm ± 3.85 cm; Table 1), an average of seven slides per case were reviewed.
| Total (n = 1066) | Group 1 (SRCC < 10%) (n = 84) | Group 2 (10% ≤ SRCC < 50%) (n = 336) | Group 3 (50% ≤ SRCC < 90%) (n = 336) | Group 4 (SRCC ≥ 90%) (n = 310) | 1P value | |
| Age | 56.2 ± 11.98 | 54.82 ± 12.35 | 57.04 ± 12.39 | 57.29 ± 11.71 | 54.46 ± 11.52 | 0.0064 |
| Largest size (cm) | 4.15 ± 3.85 | 7.22 ± 4.63 | 5.08 ± 4.16 | 3.92 ± 3.61 | 2.56 ± 2.51 | < 0.001 |
| Sex | ||||||
| F | 600 (56.29) | 39 (46.43) | 197 (58.63) | 184 (54.76) | 180 (58.06) | 0.1883 |
| M | 466 (43.71) | 45 (53.57) | 139 (41.37) | 152 (45.24) | 130 (41.94) | |
| pT | ||||||
| T1a | 435 (40.81) | 4 (4.76) | 52 (15.48) | 144 (42.86) | 235 (75.81) | < 0.001 |
| T1b | 214 (20.08) | 10 (11.9) | 79 (23.51) | 93 (27.68) | 32 (10.32) | |
| T2 | 86 (8.07) | 7 (8.33) | 44 (13.1) | 19 (5.65) | 16 (5.16) | |
| T3 | 97 (9.1) | 12 (14.29) | 50 (14.88) | 27 (8.04) | 8 (2.58) | |
| T4a | 205 (19.23) | 47 (55.95) | 94 (27.98) | 46 (13.69) | 18 (5.81) | |
| T4b | 29 (2.72) | 4 (4.76) | 17 (5.06) | 7 (2.08) | 1 (0.32) | |
| AGC or EGC | ||||||
| AGC | 417 (39.12) | 70 (83.33) | 205 (61.01) | 99 (29.46) | 43 (13.87) | < 0.001 |
| EGC | 649 (60.88) | 14 (16.67) | 131 (38.99) | 237 (70.54) | 267 (86.13) | |
| pN (AJCC) | ||||||
| N0 | 764 (71.67) | 27 (32.14) | 193 (57.44) | 264 (78.57) | 280 (90.32) | < 0.001 |
| N1 | 62 (5.82) | 6 (7.14) | 33 (9.82) | 12 (3.57) | 11 (3.55) | |
| N2 | 74 (6.94) | 17 (20.24) | 31 (9.23) | 18 (5.36) | 8 (2.58) | |
| N3a | 65 (6.1) | 14 (16.67) | 30 (8.93) | 16 (4.76) | 5 (1.61) | |
| N3b | 101 (9.47) | 20 (23.81) | 49 (14.58) | 26 (7.74) | 6 (1.94) | |
| pN (2nd Japanese classification) | ||||||
| N0 | 756 (72.34) | 27 (33.33) | 189 (57.98) | 263 (78.98) | 277 (90.82) | < 0.001 |
| N1 | 143 (13.68) | 31 (38.27) | 65 (19.94) | 30 (9.01) | 17 (5.57) | |
| N2 | 132 (12.63) | 20 (24.69) | 67 (20.55) | 36 (10.81) | 9 (2.95) | |
| N3 | 14 (1.34) | 3 (3.7) | 5 (1.53) | 4 (1.2) | 2 (0.66) | |
| pM | ||||||
| M0 | 1039 (97.47) | 78 (92.86) | 323 (96.13) | 331 (98.51) | 307 (99.03) | 0.0026 |
| M1 | 27 (2.53) | 6 (7.14) | 13 (3.87) | 5 (1.49) | 3 (0.97) | |
| Lymphatic invasion | 258 (24.2) | 46 (54.76) | 112 (33.33) | 71 (21.13) | 29 (9.35) | < 0.001 |
| Venous invasion | 108 (10.13) | 20 (23.81) | 46 (13.69) | 33 (9.82) | 9 (2.9) | < 0.001 |
| Neural invasion | 392 (36.77) | 67 (79.76) | 189 (56.25) | 99 (29.46) | 37 (11.94) | < 0.001 |
| HER2 | ||||||
| -/3 | 579 (54.32) | 52 (61.9) | 193 (57.44) | 176 (52.38) | 158 (50.97) | 0.3751 |
| 1 +/3 | 364 (34.15) | 24 (28.57) | 109 (32.44) | 117 (34.82) | 114 (36.77) | |
| 2 +/3 | 111 (10.41) | 6 (7.14) | 32 (9.52) | 37 (11.01) | 36 (11.61) | |
| 3 +/3 | 12 (1.13) | 2 (2.38) | 2 (0.6) | 6 (1.79) | 2 (0.65) | |
| EBV | ||||||
| Negative | 1059 (99.34) | 83 (98.81) | 333 (99.11) | 336 (100) | 307 (99.03) | 0.3428 |
| Positive | 7 (0.66) | 1 (1.19) | 3 (0.89) | 0 (0) | 3 (0.97) | |
| Recurrence | 23 (2.16) | 2 (2.38) | 14 (4.17) | 7 (2.08) | 0 (0) | 0.0041 |
| Death | 55 (5.16) | 9 (10.71) | 22 (6.55) | 20 (5.95) | 4 (1.29) | < 0.001 |
| PD-L1 (n = 63) | ||||||
| Negative (CPS < 1) | 27 (42.86) | 6 (46.15) | 15 (41.67) | 5 (41.67) | 1 (50) | 0.9884 |
| Positive (CPS ≥ 1) | 36 (57.14) | 7 (53.85) | 21 (58.33) | 7 (58.33) | 1 (50) | |
| MSI | ||||||
| MSI-H | 9 (0.84) | 1 (1.19) | 5 (1.49) | 2 (0.6) | 1 (0.32) | 0.3854 |
| MSS/MSI-L | 1057 (99.16) | 83 (98.81) | 331 (98.51) | 334 (99.4) | 309 (99.68) | |
All pathological slides of the primary gastric cancer tissue from each patient were reviewed by two experienced pathologists (Kim C and Oh HJ). The proportion of SRCC was determined by consensus, based on the average proportion of SRCC cells observed across all available slides for each case. SRCC cells were defined according to the WHO classification as tumor cells containing intracytoplasmic mucin and eccentrically displaced nuclei, typically with mild nuclear atypia. PCC cells that did not meet these criteria were classified as PCC-NOS.
Based on the WHO definition, a 50% threshold was initially applied to distinguish SRCC from PCC-NOS. Additionally, recent studies suggested distinct tumor behavior when the SRCC proportion was either very low or very high[20,21]. Accordingly, patients were categorized into four groups according to SRCC proportion: < 10%, 10% ≤ SRC < 50%, 50% ≤ SRC < 90%, and ≥ 90%.
Between-group comparisons were conducted using Fisher’s test, χ2 test, Jonckheere-Terpstra test, Kruskal-Wallis rank sum test, and Mann-Whitney U test. OS and recurrence-free survival (RFS) were analyzed using the Kaplan-Meier method. All analyses were performed using R version 3.6.3, with statistical significance set at P < 0.05.
Patients with PCC were categorized into four groups according to the SRCC proportion: Group 1 (< 10%, n = 84); Group 2 (10% ≤ SRCC < 50%, n = 336); Group 3 (50% ≤ SRCC < 90%, n = 336); Group 4 (≥ 90%, n = 310) (Figure 2 and Table 1).
The mean value of the largest tumor size decreased from group 1 to group 4, with group 1 having the largest mean tumor size (7.22 mm ± 4.63 mm), followed by group 2 (5.08 mm ± 4.16 mm), group 3 (3.92 mm ± 3.61 mm), and group 4 (2.56 mm ± 2.51 mm) (P < 0.001). We confirmed no significant multicollinearity between tumor size and SRCC proportion (Spearman’s = 0.40, P < 0.001; variance inflation factor = 1.39). Sex distribution differed across the groups, although the difference was not statistically significant (P = 0.1883). The proportion of the T1a category progressively increased from group 1 to group 4 in the pathological T category (pT). Specifically, T1a tumors were found in 4 (4.76%) patients in group 1, 52 (15.48%) patients in group 2, 144 (42.86%) patients in group 3, and 235 (75.81%) patients in group 4. Conversely, the proportion of patients in advanced pT categories, such as pT3 and pT4, decreased from group 1 to 4. Similarly, the pathological N category (pN) showed significant differences among groups. The proportion of pN0 tumors according to the AJCC classification increased from group 1 (32.14%) to group 4 (90.32%) (P < 0.001). According to the Japanese classification, the proportion of pN0 categories also increased gradually from group 1 (33.33%) to group 4 (90.82%) (P < 0.001). The pathological M category (pM) also showed significant differences among the four groups. In the pM1 category, group 1 had six patients (7.14%), whereas group 4 had three patients (0.97%). Group 1 tumors showed more frequent lymphatic, venous, and neural invasions than those in other groups. These invasions showed a marked decrease from group 1 to group 4 (P < 0.001). Molecular features such as HER2 status, EBV in situ positivity, microsatellite instability (MSI), and PD-L1 expression showed no significant differences among the groups (Table 1).
These invasions showed a marked decrease from group 1 to group 4 (P < 0.001). Molecular features such as HER2 status, EBV in situ positivity, MSI, and PD-L1 expression showed no significant differences among the groups (Table 1).
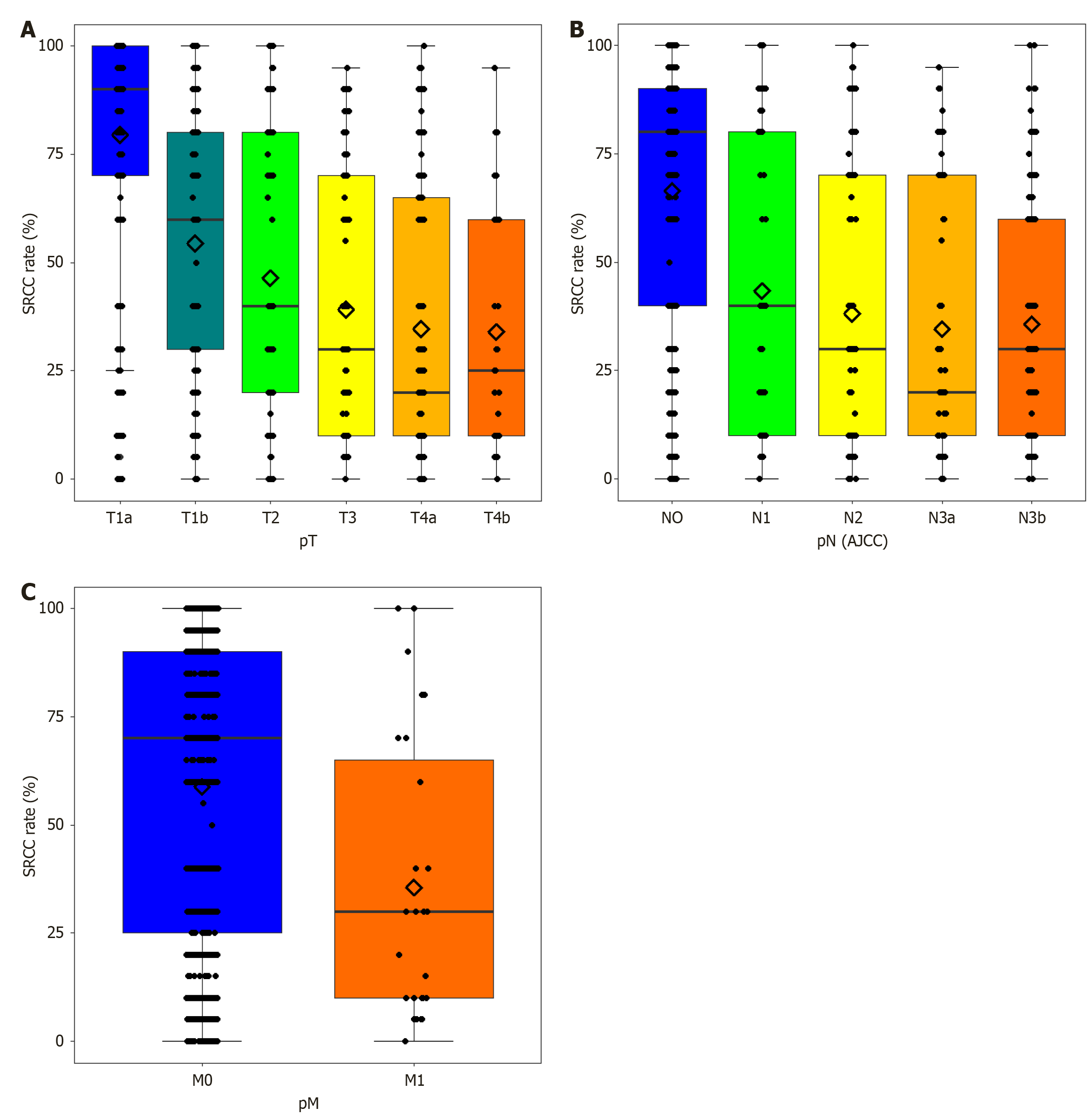
The SRCC rates differed significantly among the pT, pN, and pM categories (P < 0.0001) (Figure 3). The mean SRCC rates for pT1a, pT1b, pT2, pT3, pT4a, and pT4b were 79.48%, 54.35%, 46.4%, 39.07%, 34.61%, and 33.97%, respectively (Supplementary Table 1). The mean SRCC rate demonstrated a statistically significant decrease with an advanced T category (P < 0.001) (Figure 3A). The mean SRCC rates for pN0, pN1, pN2, pN3a, and pN3b were 66.37%, 43.31%, 38.11%, 34.54%, and 35.69% (Supplementary Table 2). Lower pN according to both the AJCC and 2nd Japanese classification stages was associated with higher SRCC rates (P < 0.001) (Figure 3B). DGC with distant metastasis (pM1) had lower SRCC rates (35.56%) than DGC without distant metastasis (pM0) (58.81%) (P = 0.0012; Figure 3C, Supplementary Table 3).
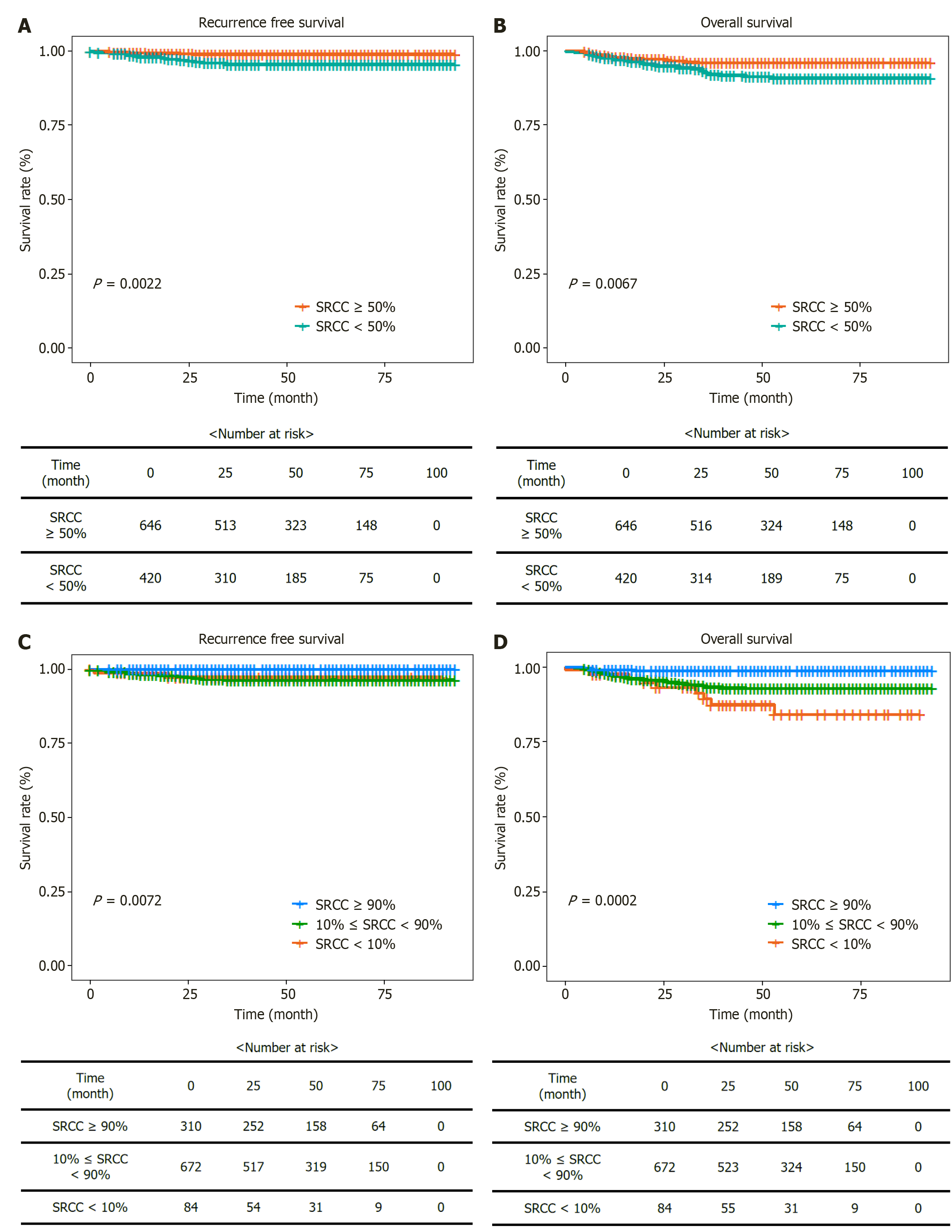
The recurrence rates among the SRCC groups were 2.38% in group 1, 4.17% in group 2, 2.08% in group 3, and 0% in group 4 (Table 1). The Kaplan-Meier survival curve for RFS in the two SRCC groups (< 50% and ≥ 50%) indicated statistically significant differences in RFS probabilities (P = 0.0022; Figure 4). The death rates were 10.71% in group 1, 6.55% in group 2, 5.95% in group 3, and 1.29% in group 4, with group 4 showing the lowest death rate and better OS than that of the other groups (P < 0.001) (Table 1). The Kaplan-Meier survival curve for OS among the three SRCC groups (Figure 4) demonstrated differences in OS probabilities (P = 0.0002), with group 4 exhibiting markedly better outcome compared to that of the other groups.
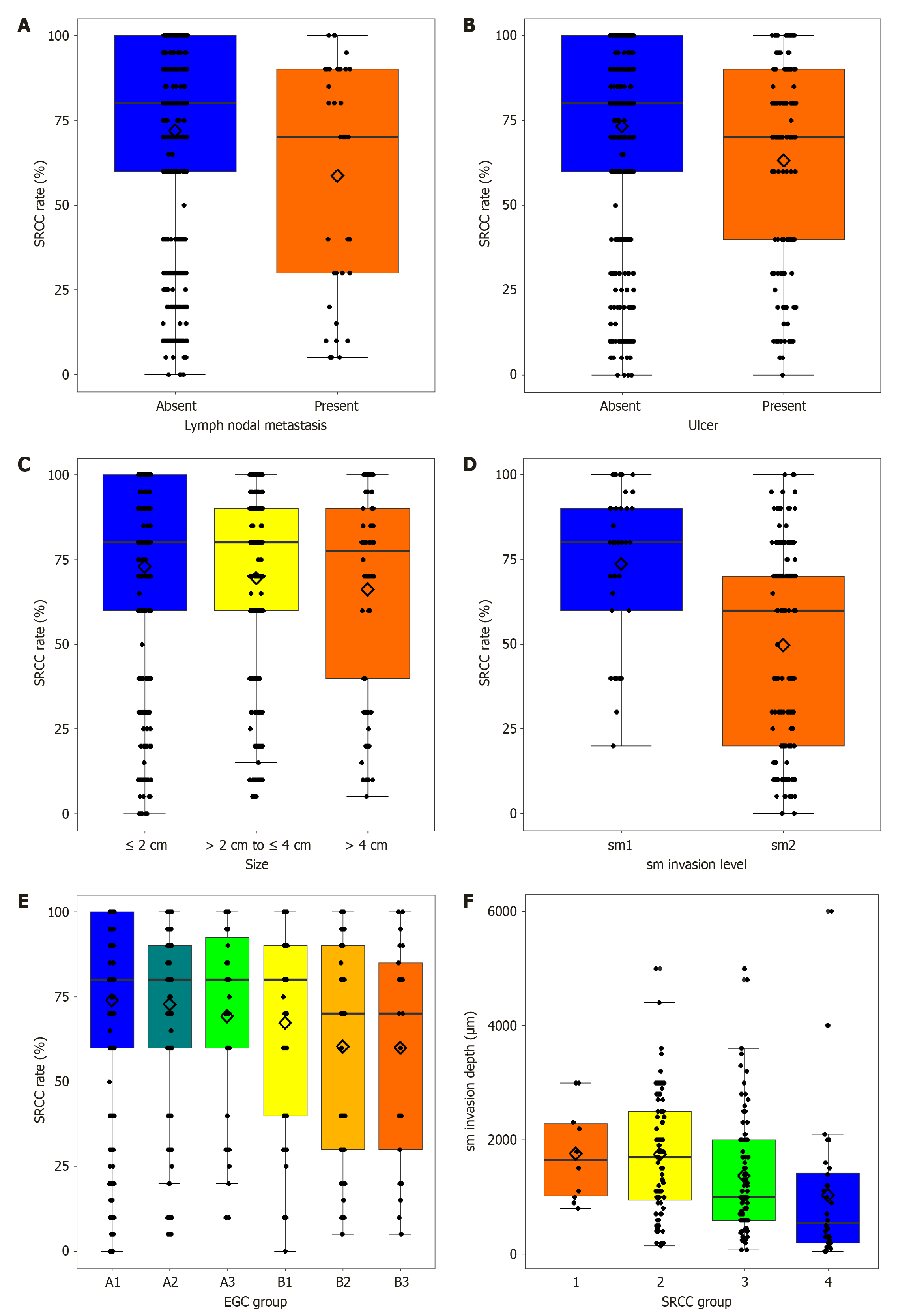
Relationship of SRCC rates with lymph nodal metastasis, ulceration, and tumor size in EGC: In EGC, the SRCC proportion was higher in patients with lymph node metastasis than in those without lymph node metastasis (P = 0.018) (Figure 5A, Supplementary Table 4). The presence of ulcers in patients with EGC was associated with a lower SRCC proportion than in those without ulcers (P < 0.001) (Figure 5B and Supplementary Table 5). The tumor size (largest diameter) of EGC showed a negative correlation with SRCC rates (P < 0.001; Figure 5C and Supplementary Table 6).
Furthermore, the SRCC proportion was analyzed by categorizing patients with EGC according to ulcer and tumor size. Patients were divided into six groups based on the combination of ulcer and tumor size: Ulcer (-), size ≤ 2 cm (A1); Ulcer (-) 2 cm < size ≤ 4 cm (A2); Ulcer (-), size > 4 cm (A3); Ulcer (+), size ≤ 2 cm (B1); Ulcer (+), 2 cm < size ≤ 4 cm (B2); and Ulcer (+), size > 4 cm (B3). This categorization was derived from the Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition) for ESD criteria and Horiuchi et al’s study[22], which suggested ESD indications for undifferentiated-type carcinoma[9,22]. When we evaluated the SRCC proportion across these groups, significant differences were observed (P = 0.0025; Figure 5D). For patient groups without ulcer (A groups), the mean SRCC proportion was 74.04% in those with tumors ≤ 2 cm (A1), 72.83% in those with tumors between 2 cm and 4 cm (A2), and 69.30% in those with tumors > 4 cm (A3). For patient groups with ulcers (B groups), the mean SRCC proportion was 67.32% in those with tumors ≤ 2 cm (B1), 60.36% in those with tumors between 2 and 4 cm (B2), and 60.00% in those with tumors > 4 cm (B3) (Table 2).
| EGC subgroup | Number | SRCC rates (%) (mean) | SD |
| A1: Ulcer (-), size ≤ 2 cm | 317 | 74.0379 | 29.5714 |
| A2: Ulcer (-), 2 cm < size ≤ 4 cm | 157 | 72.8344 | 26.568 |
| A3: Ulcer (-), size > 4 cm | 43 | 69.3023 | 28.3175 |
| B1: Ulcer (+), size ≤ 2 cm | 56 | 67.3214 | 29.7102 |
| B2: Ulcer (+), 2 cm < size ≤ 4 cm | 56 | 60.3571 | 31.0989 |
| B3: Ulcer (+), size > 4 cm | 21 | 60 | 32.4808 |
Lymph node metastasis rates were further analyzed based on tumor size, ulcer presence, and SRCC proportion to explore their combined prognostic significance (Table 3). Tumors smaller than 2 cm demonstrated a lymph node meta
| Variable | Total | Lymph node metastasis | Proportion with 95%CI |
| Size ≤ 2 cm | 372 | 11 | 0.03 (0.01-0.05) |
| 2 cm < size ≤ 4 cm | 213 | 13 | 0.06 (0.03-0.09) |
| Size > 4 cm | 64 | 12 | 0.19 (0.09-0.28) |
| Ulcer (-) | 516 | 18 | 0.03 (0.02-0.05) |
| Ulcer (+) | 133 | 18 | 0.14 (0.08-0.19) |
| SRCC ≥ 50% | 504 | 21 | 0.04 (0.02-0.06) |
| SRCC < 50% | 145 | 15 | 0.10 (0.05-0.15) |
Odds ratios were calculated to quantify the impact of these factors on poor prognosis (Table 4). Tumor size > 2 cm was associated with an odds ratio of 2.64 (95%CI: 1.25-5.58, P = 0.01), ulcer presence with an odds ratio of 3.25 (95%CI: 1.59-6.62, P = 0.001), and SRCC < 50% with an odds ratio of 2.08 (95%CI: 1.01-4.28, P = 0.046).
| OR | P value | 95%LCI for OR | 95%UCI for OR | |
| Size > 2 cm | 2.6436 | 0.0107 | 1.2532 | 5.5764 |
| Ulcer present | 3.2474 | 0.0012 | 1.5922 | 6.623 |
| SRCC rate < 50% | 2.0816 | 0.0461 | 1.0127 | 4.2787 |
Relationship between submucosal invasion depth and SRCC proportion in pT1b EGC: In pT1b EGC, the depths of submucosal invasion were 1760 μm, 1747 μm, 1374 μm, and 1033 μm for groups 1-4, respectively (P = 0.022; Figure 5E and Supplementary Table 7). T1b EGC was categorized into sm1 (< 500 μm) and sm2 (≥ 500 μm) groups based on the depth of submucosal invasion. SRCC rate of pT1b tumors was 73.66% in the sm1 group and 49.77% in the sm2 group, with a statistically significant difference (P < 0.001; Figure 5F and Supplementary Table 8).
Gastric carcinoma, particularly DGC, presents significant clinical challenges in Korea owing to its rising incidence despite a general decline in gastric carcinoma cases[3]. PCC usually exhibits a better prognosis in EGC and a poorer prognosis in advanced stages. PCC-NOS exhibits poorer outcomes than differentiated-type GC, whereas SRCC has shown better outcomes than differentiated-type gastric carcinoma in many previous studies[23,24]. These distinct prognostic results may be due to the biological differences between SRCC and PCC-NOS.
In our study, we stratified patients into four groups based on the SRCC proportion: < 10% (group 1), 10% ≤ SRCC < 50% (group 2), 50% ≤ SRCC < 90% (group 3), and ≥ 90% (group 4). Statistical analyses revealed significant differences between the SRCC groups. Tumor size, pT, pN, and pM categories, and lymphatic, venous, and perineural invasions exhibited statistically significant differences among the SRCC groups. As the SRCC proportion decreased, tumor size increased (P < 0.001). The largest tumor diameter was observed in group 1 (< 10% SRCC, 7.22 cm) and the smallest in group 4 (≥ 90% SRCC, 2.56 cm). The pathological tumor-node-metastasis stage advanced as the SRCC rate decreased. In group 1, the most frequent pT category was pT4a (55.95%), whereas pT1a predominated in group 4 (75.81%). The proportion of EGC relative to the total tumor increased with increasing SRCC proportions. This trend was similar for all pN categories. The nodal metastasis rates gradually decreased as SRCC proportion increased. Group 4 had the highest proportion of pN0 tumors, indicating a lower likelihood of lymphatic spread and a less aggressive tumor phenotype. Our study demonstrated that a higher SRCC proportion was associated with a reduced incidence of nodal metastasis, reinforcing the idea that the SRCC proportion could be a predictive prognostic factor in determining treatment strategies. These results are consistent with those of previous studies, where gastric cancer with a high SRCC proportion exhibited less aggressive behavior in EGC[25,26].
Lymphatic, venous, and perineural invasions were also correlated with SRCC rates. Tumors with a higher SRCC proportion exhibited significantly lower rates of lymphatic invasion. Venous and perineural invasions exhibited similar trends. PCC-NOS rates were positively correlated with aggressive biological factors.
Moreover, we examined the association of EBV, MSI, HER2 status, and PD-L1 expression with SRCC proportion and found no significant differences across the SRCC groups.
Survival analysis demonstrated that a higher SRCC proportion was correlated with better OS and RFS. Group 4 exhibited no recurrence and had the lowest mortality rate among the groups, highlighting the prognostic value of the SRCC proportion. DGC with SRCC proportion ≥ 50% showed higher OS than those with < 50%. The lowest SRCC group (< 10%) had the worst OS, whereas the highest SRCC group (≥ 90%) demonstrated the most favorable outcome. RFS and OS imply the importance of incorporating the SRCC proportion into prognostic models to enhance the accuracy of survival predictions and guide treatment decisions more effectively.
These distinct clinical outcomes may reflect fundamental biological differences between SRCC and PCC-NOS components. Through our extensive histopathological review, we observed that in EGCs, PCC-NOS components often localized at the invasive front, whereas SRCC components remained more centrally situated (Supplementary Figure 1A). In advanced gastric cancers, desmoplastic reactions were more commonly associated with PCC-NOS than with SRCC (Supplementary Figure 1B). These patterns suggest that PCC-NOS may possess more aggressive invasive potential than SRCC. Previous research on hereditary diffuse gastric cancer also supports this, describing SRCC as a more differentiated phenotype and PCC-NOS as exhibiting stem cell-like features[27]. In addition, prior studies have proposed that epithelial-mesenchymal transition-like processes drive the progression from early to advanced stages in DGC[6]. In future studies, we aim to investigate this hypothesis further using single-cell transcriptomic analyses to delineate gene expression profiles of SRCC and PCC-NOS cells across disease stages. This may uncover specific molecular drivers underlying the differences in tumor behavior based on SRCC proportion.
A detailed analysis of EGC revealed significant correlations between the SRCC proportion and lymph node metastasis, which necessitated endoscopic resection instead of surgical resection. The SRCC rate was lower among patients with EGC with lymph node metastasis than among those in the EGC group without lymph node metastasis (Figure 5A). The SRCC rate in EGC without nodal metastasis was 71.19% (± 28.85%), higher than the SRCC rate of 58.61% (± 34.01%) observed in EGC with nodal metastasis (P = 0.0269; Supplementary Table 4). Therefore, EGC with a SRCC rate exceeding 70% is associated with a lower probability of lymph node metastasis. This suggests that a higher SRCC proportion may be a protective factor against lymphatic spread, which is a critical determinant of EGC prognosis.
In this study, the correlation between the SRCC rates of pure undifferentiated-type EGC and the risk factors associated with the ESD indication guidelines was assessed. Lower SRCC proportion was correlated with ulceration, larger tumor size, and deeper submucosal invasion in EGC (Figure 5). EGCs with ulceration had lower mean SRCC rates (63.23% ± 30.71%) than those without ulcers (73.25% ± 28.58%) (P < 0.001; Supplementary Table 5), suggesting that higher SRCC components may be linked to a reduced likelihood of ulcer formation. In EGC, tumors larger than 4 cm had an SRCC rate of 42.03% (± 32.42%), compared with 58.61% (± 32.93%) for tumors between 2 cm and 4 cm, and 71.59% (± 30.27%) for tumors smaller than 2 cm (P < 0.001; Supplementary Table 6). The depth of submucosal invasion in pT1b EGC was significantly correlated with the SRCC proportion. Significant differences in submucosal invasion depth were observed among the SRCC groups (P = 0.0217; Supplementary Table 7). Tumors with a lower SRCC proportion tended to have deeper submucosal invasion, indicative of a more aggressive phenotype.
Tumor size, presence of ulcers, and SRCC proportion were significantly correlated with lymph node metastasis in patients with EGC. Tumors with SRCC proportion < 50% showed a higher rate of lymph node metastasis than those with SRCC proportion ≥ 50% (10% vs 4%). Larger tumor size (> 2 cm) and ulcer presence were associated with increased rates of nodal metastasis, emphasizing the importance of these variables in predicting prognosis (Table 3).
Table 4 reinforces the role of SRCC as a prognostic factor by evaluating the odds ratios for poor prognostic features. Tumors > 2 cm, ulcer-positive cases, and those with a low SRCC proportion (< 50%) exhibited significantly higher odds ratios for poor prognosis. This highlights the SRCC proportion as a potential risk factor for predicting aggressive behavior in undifferentiated-type EGC. Although SRCC alone may not currently warrant modifications to existing ESD guidelines, its integration with other established factors, such as tumor size and ulceration, could enhance the accuracy of risk stratification models. The model’s predictive performance for lymph node metastasis in EGC was evaluated using the area under the receiver operating characteristic curve (AUC). The baseline model, which included tumor size and the presence of ulcers, demonstrated an AUC of 0.714, indicating a moderate predictive ability (Supplementary Figure 2A). When the SRCC proportion was included as an additional variable, the AUC increased to 0.7297 (Supplementary Figure 2B). Although the improvement in the AUC was modest, this suggests that incorporating the SRCC proportion enhanced the predictive accuracy of the model for lymph node metastasis in EGC.
In our stratified analysis, patients were divided into six groups based on the presence of ulcers and tumor size, according to ESD indications (Table 2). The A1 group was included under the extended indication, irrespective of whether it exhibited pure or mixed undifferentiated-type histology. A2 was included in the “new extended criteria” when it consisted of only pure undifferentiated-type tumors according to the further expanded guidelines[22]. The SRCC proportion differed significantly between the groups (P = 0.0025; Figure 5E and Table 2).
These findings underscore the prognostic value of the SRCC proportion as an indicator of tumor aggressiveness and its potential utility in refining clinical decision-making processes. Future research should focus on calculating hazard ratios to further elucidate the relationship between SRCC proportion and adverse outcomes and to validate these findings through multicenter studies with larger cohorts. Moreover, integrating the SRCC proportion into comprehensive risk models could improve the precision of treatment guidelines and optimize patient management strategies.
The endoscopic complete resection of undifferentiated-type resection for assessment (eCURA) system is widely used to evaluate the likelihood of lymph node metastasis in patients with EGC, incorporating variables such as tumor size, ulceration, vertical margin, deep submucosal invasion (≥ 500 μm), and lymphovascular invasion. In this study, we identified the SRCC proportion as a significant determinant of lymph node metastasis and overall tumor aggressiveness. A higher SRCC rates may serve as a prognostic indicator in EGC. Specifically, EGC with SRCC < 50% exhibited higher rates of nodal metastasis and poorer prognostic features than those with SRCC ≥ 50% (Tables 3 and 4). Given these findings, we propose that the SRCC proportion could serve as an additional determinant within the eCURA system, enhancing its predictive accuracy. Incorporating the SRCC proportion may refine the risk stratification for patients with EGC, providing a more comprehensive evaluation of tumor behavior and informing treatment strategies, particularly in cases where endoscopic resection is being considered.
The retrospective design and single-center database may limit the generalizability of our findings. Multicenter prospective studies with larger populations should be conducted to validate the results of this study and explore the role of SRCC proportion in greater detail. Its integration into the eCURA system as a scoring factor also requires further investigation, particularly through comparative studies to evaluate the relative weight of the SRCC proportion against established risk factors. Additionally, understanding the biology of SRC and NOS components through single-cell transcriptomics to identify actionable genes is essential for future research.
Our study suggests that the SRCC proportion is a critical prognostic factor in DGC. A higher SRCC proportion is associated with smaller tumor size, less advanced T stages, fewer nodal or distant metastases, and reduced lymphatic, venous, and neural invasion. Particularly, the SRCC proportion in EGC should be validated as a significant factor in therapeutic approaches, including ESD.
| 1. | Jung KW, Kang MJ, Park EH, Yun EH, Kim HJ, Kong HJ, Im JS, Seo HG. Prediction of Cancer Incidence and Mortality in Korea, 2023. Cancer Res Treat. 2023;55:400-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 2. | LAUREN P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4321] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 3. | Assumpção PP, Barra WF, Ishak G, Coelho LGV, Coimbra FJF, Freitas HC, Dias-Neto E, Camargo MC, Szklo M. The diffuse-type gastric cancer epidemiology enigma. BMC Gastroenterol. 2020;20:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Petrelli F, Berenato R, Turati L, Mennitto A, Steccanella F, Caporale M, Dallera P, de Braud F, Pezzica E, Di Bartolomeo M, Sgroi G, Mazzaferro V, Pietrantonio F, Barni S. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2017;8:148-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Graziosi L, Marino E, Natalizi N, Donini A. Prognostic Survival Significance of Signet Ring Cell (SRC) Gastric Cancer: Retrospective Analysis from a Single Western Center. J Pers Med. 2023;13:1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Monster JL, Kemp LJS, Gloerich M, van der Post RS. Diffuse gastric cancer: Emerging mechanisms of tumor initiation and progression. Biochim Biophys Acta Rev Cancer. 2022;1877:188719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW, Lee SH, Bae JH, Chung H, Choi KD, Park JC, Lee H, Kwak MS, Kim B, Lee HJ, Lee HS, Choi M, Park DA, Lee JY, Byeon JS, Park CG, Cho JY, Lee ST, Chun HJ. [Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer]. Korean J Gastroenterol. 2020;75:264-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1332] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 9. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 593] [Article Influence: 296.5] [Reference Citation Analysis (2)] |
| 10. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 307] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 11. | Lee A, Chung H. Endoscopic Resection of Undifferentiated-type Early Gastric Cancer. J Gastric Cancer. 2020;20:345-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 13. | Bae JY, Ryu CB, Lee MS, Dua KS. Long-term outcomes of endoscopic submucosal dissection for undifferentiated type early gastric cancer over 2 cm with R0 resection. World J Gastrointest Endosc. 2024;16:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Lee GH, Lee E, Park B, Roh J, Lim SG, Shin SJ, Lee KM, Noh CK. Long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated intramucosal gastric cancer regardless of size. World J Gastroenterol. 2022;28:840-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Gong EJ, Kim DH, Ahn JY, Jung KW, Lee JH, Choi KD, Song HJ, Lee GH, Jung HY, Kim HS, Lee IS, Kim BS, Yoo MW, Oh ST, Yook JH, Kim BS. Comparison of long-term outcomes of endoscopic submucosal dissection and surgery for esophagogastric junction adenocarcinoma. Gastric Cancer. 2017;20:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 871] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 17. | Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng J, Yuan J, Chen R, Li Y, Ge Z, Ji J, Zhang L, Wang J, Li Z, Lai Y, Hu Y, Li Y, Li Y, Gao J, Chen L, Xu J, Zhang C, Jung SY, Choi JM, Jain A, Liu M, Song L, Liu W, Guo G, Gong T, Huang Y, Qiu Y, Huang W, Shi T, Zhu W, Wang Y, He F, Shen L, Qin J. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 18. | Kong P, Wu R, Yang C, Geng Q, Liu J, Chen S, Liu X, Ye M, He W, Yang Q, Xia L, Xu D. Prognostic Impact of the Signet Ring Cell Type in Node-Negative Gastric Cancer. Sci Rep. 2016;6:26313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Roviello F, Marano L, Ambrosio MR, Resca L, D'Ignazio A, Petrelli F, Petrioli R, Costantini M, Polom K, Macchiarelli R, Biviano I, Marrelli D. Signet ring cell percentage in poorly cohesive gastric cancer patients: A potential novel predictor of survival. Eur J Surg Oncol. 2022;48:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 22. | Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Kumagai K, Ohashi M, Sano T, Fujisaki J. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, Ahn JB, Kim H, Chung HC, Rha SY, Noh SH, Jeung HC. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg. 2017;265:946-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Drubay V, Nuytens F, Renaud F, Adenis A, Eveno C, Piessen G. Poorly cohesive cells gastric carcinoma including signet-ring cell cancer: Updated review of definition, classification and therapeutic management. World J Gastrointest Oncol. 2022;14:1406-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 25. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (3)] |
| 26. | Yu C, Yang J, Li H, Wang J, Jin K, Li Y, Zhang Z, Zhou J, Tang Y. Prognostic prediction and treatment options for gastric signet ring cell carcinoma: a SEER database analysis. Front Oncol. 2024;14:1473798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Barber ME, Save V, Carneiro F, Dwerryhouse S, Lao-Sirieix P, Hardwick RH, Caldas C, Fitzgerald RC. Histopathological and molecular analysis of gastrectomy specimens from hereditary diffuse gastric cancer patients has implications for endoscopic surveillance of individuals at risk. J Pathol. 2008;216:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |