Published online Jun 14, 2025. doi: 10.3748/wjg.v31.i22.108815
Revised: May 10, 2025
Accepted: May 26, 2025
Published online: June 14, 2025
Processing time: 49 Days and 16.3 Hours
Intermittent fasting (IF), particularly time-restricted feeding (TRF), is increasingly popular has gained popularity for weight loss, yet management, but its effects impact on gut health remain unclear. Remains inadequately understood. This study explores how investigated the effects of TRF effects on intestinal health and explored the underlying mechanisms.
To assess the effects of IF on intestinal health, elucidate the mechanisms involved.
Mice were divided into two groups: Normal control (NC) and IF. The IF group underwent TRF, while the NC group had unrestricted access to food. Body weight, fecal characteristics, and intestinal morphology were analyzed. Colon tissue, serum, and fecal samples were collected for histological analysis, enzyme-linked immunosorbent assay, flow cytometry, 16S rRNA sequencing, and meta
IF significantly affected body weight and intestinal morphology, compromised the intestinal barrier, increased pro-inflammatory cytokines, and heightened gut immune activation (P < 0.05). It also disrupted the gut microbiota, promoting pro-inflammatory bacteria, reducing anti-inflammatory metabolites, and elevating pro-inflammatory metabolites (P < 0.05). Indoleacrylic acid (IAA) supplementation notably alleviated intestinal inflammation and reversed immune dysfunction induced by IF (P < 0.05).
Prolonged IF exacerbates intestinal inflammation by impairing gut barrier integrity and disrupting microbial homeostasis. However, IAA supplementation effectively mitigates fasting-induced intestinal inflammation and immune imbalance, suggesting its potential as a therapeutic agent.
Core Tip: This study demonstrates that long-term intermittent fasting (IF) compromises intestinal barrier function, alters the metabolite profile (reducing anti-inflammatory compounds and increasing pro-inflammatory ones), and induces dysbiosis (increasing pathogenic bacteria while decreasing short-chain fatty acid-producing bacteria), which collectively promote Th17-driven inflammation. Exogenous indoleacrylic acid supplementation restores barrier integrity, suppresses Th17 activation, and enhances interleukin-10 expression, highlighting its therapeutic promise and emphasizing the need for a reevaluation of the long-term safety of IF due to its potential gut-disrupting effects.
- Citation: Fu R, Zhang P, Zhang JW, Hong Y, Chen B, Cao GD. Intermittent fasting exacerbates colon inflammation by promoting Th17 cell differentiation through inhibition of gut microbiota-derived indoleacrylic acid. World J Gastroenterol 2025; 31(22): 108815
- URL: https://www.wjgnet.com/1007-9327/full/v31/i22/108815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i22.108815
The global increase in metabolic syndrome and obesity has spurred significant interest in dietary interventions, with intermittent fasting (IF) emerging as a widely adopted strategy[1]. IF may be a method of preventing diseases or extending lifespan by reducing weight[2], has fueled debates over the translational relevance of IF, especially regarding its unintended gastrointestinal effects. This study examined time-restricted feeding (TRF), a specific form of IF, to explore its dual impacts on colonic inflammation, microbial ecology, and mucosal immunity, thereby resolving conflicting preclinical findings and clarifying the role of tryptophan-derived metabolites in mediating these effects.
Due to its simplicity and the lack of complex calorie counting, IF has gained widespread popularity, greatly improved dietary tolerance[3]. However, studies have shown that prolonged intermittent diet can impair beta cell maturation and function in adolescent mice[4], and there are also studies reporting that 12 weeks IF intervention in pregnant mice can make their offspring susceptible to liver steatosis and obesity[5]. This paradox suggests that the biological effects of IF exhibit substantial individual variability and may present potential risk periods, necessitating further mechanistic exploration. Depending on the fasting schedule and frequency, IF can be classified into various types: TRF, in which food is consumed within a specific window (typically 8-10 hours) each day, with fasting during the remaining hours; Alternate-day fasting, characterized by complete or partial fasting on alternate days; The 5:2 IF regimen, where normal eating is maintained for five days and calorie restriction occurs on two non-consecutive days[6]; and The fasting-mimicking diet, which involves low-calorie, low-protein food intake designed to simulate the effects of fasting[7]. During fasting, blood glucose and liver glycogen levels are gradually depleted, shifting energy metabolism from glucose to fat-derived ketone bodies and free fatty acids[8]. These fatty acids and glycerol become primary energy sources, with the liver converting fatty acids into ketones to supply energy to the brain. Ketone bodies not only play a pivotal role in maintaining health and delaying aging but also promote the production of proteins involved in pathways such as poly (adenosine diphosphate-ribose) polymerase 1[9], nicotinamide adenine dinucleotide[10], peroxisome proliferator-activated receptor gamma coactivator 1-alpha[11], and peroxisome proliferator-activated receptor alpha[12], enhancing glucose regulation and improving cardiovascular health, including reductions in blood pressure and heart rate.
Colon inflammation refers to an inflammatory response in the colonic mucosa and underlying tissues, driven by complex interactions among genetic, immune, and environmental factors[13]. Over the past decade, inflammatory bowel disease (IBD) has emerged as a significant global public health challenge[14]. Patients with IBD commonly experience symptoms such as abdominal pain, diarrhea, and bloody stools, with severe cases leading to complications like intestinal perforation and obstruction, which profoundly impact patients' quality of life[15]. Although the precise cause of IBD remains unclear, research indicates a close association between intestinal inflammation and factors such as impaired intestinal barrier function[16], dysbiosis of the gut microbiota[17], and immune system abnormalities[18]. Disruption of the intestinal barrier leads to increased permeability, allowing bacteria and toxins to more easily infiltrate the intestinal lamina propria, activating immune cells and triggering inflammation. Moreover, the composition and functionality of the gut microbiota play a critical role in modulating intestinal inflammation. Beneficial bacteria, such as Bifidobacteria and Lactobacilli, produce anti-inflammatory metabolites like short-chain fatty acids (SCFAs), which inhibit pathogen growth and help maintain gut health[19]. In contrast, pathogenic bacteria, including enteroinvasive Escherichia coli and Clostridium difficile, can damage the intestinal barrier and initiate inflammatory responses[20]. The gut microbiota also metabolizes dietary tryptophan into bioactive indoles, such as indoleacrylic acid (IAA), which activate the aryl hydrocarbon receptor in intestinal epithelial cells and innate lymphoid cells. This signaling promotes interleukin (IL)-22 production, enhances mucus layer integrity through upregulation of MUC2 expression, and suppresses pro-inflammatory Th17/Th1 responses.
To explore the underlying mechanisms, this study used TRF as the primary intervention. By employing a long-term IF mouse model, changes in colon health-including the progression of intestinal inflammation, alterations in gut microbiota, disruptions in intestinal barrier function, and shifts in gut immune regulation-were systematically examined. In summary, this study provides new scientific insights and theoretical frameworks for understanding the mechanisms by which IF induces intestinal inflammation.
SPF-grade male BALB/c mice (4 weeks old) were provided by the Anhui Laboratory Animal Center for this study. All mice were healthy, first-time users, and housed in a controlled environment with a temperature range of 22 °C-26 °C and appropriate humidity. They were kept in transparent plastic cages with sterile wood shavings as bedding and maintained on a 12-hour light/dark cycle. A one-week acclimatization period preceded the experiment. All procedures were approved by the Animal Ethics Committee of Anhui Medical University. Sample size was determined based on power analysis using GPower 3.1 (effect size = 2.2, α = 0.05, power = 0.8), resulting in a minimum of 4 animals per group; 5 animals were used to ensure statistical reliability
After the acclimatization period, 10 mice were randomly selected and divided into two groups: A normal control group (NC group) and an experimental group (IF group), using a random number table. Each group consisted of 5 mice, with no significant difference in body weight between them. The NC group received a standard diet, while the IF group underwent TRF (8 hours per day). The experiment lasted 6 weeks, during which body weight was recorded daily and fecal characteristics were monitored. At the conclusion of the study, the mice were anesthetized and euthanized. Serum, fecal samples, and tissues from the cecum, colon, heart, lungs, liver, spleen, stomach, and kidneys were collected and weighed, and the intestinal length was compared.
For tissue processing, the samples were initially rinsed in 0.9% sodium chloride solution to remove blood and residual contents. After blotting dry, tissues were fixed in 4% paraformaldehyde for 24 hours. After fixation, tissues were washed with phosphate-buffered saline (PBS) to remove any remaining fixative and subjected to a graded ethanol dehydration series (30-60 minutes per step). The tissues were then immersed in a mixture of absolute alcohol and a clearing agent for 1 hour before being transferred to a pure clearing agent for further processing. After clearing, the tissue was infiltrated with molten paraffin until fully saturated. The tissue was then embedded at 63 °C in a preheated embedding station, ensuring the absence of air bubbles. After cooling, the paraffin blocks were labeled. The blocks were mounted on a microtome and trimmed, and 4 μm thick sections were cut. The tissue slices were then baked to ensure secure adhesion to glass slides.
During the staining of paraffin sections, the sections were initially immersed in xylene (I) and xylene (II) for 10 minutes each to completely remove paraffin and prevent drying. After dewaxing, the sections underwent sequential hydration in anhydrous ethanol, 95%, 85%, and 70% ethanol, each for 5 minutes. Following hydration, the sections were washed three times with PBS, with each wash lasting 5 minutes. Hematoxylin staining solution was then applied dropwise for 10 minutes, followed by rinsing with distilled water and differentiation with 1% hydrochloric acid ethanol for a few seconds. The sections were then rinsed again with double-distilled water and treated with a bluing agent (such as ammonia water) to yield a blue color in the cell nuclei. Subsequently, the sections were rinsed with distilled water, and an eosin staining solution was applied dropwise for 3 minutes to stain the cytoplasm pink. After staining, the sections were dehydrated in 80% ethanol for 5 seconds, 95% ethanol for 2 minutes, and anhydrous ethanol (I, II) for 2 minutes each. Following dehydration, the sections were cleared in xylene (I, II) for 4 minutes each. After air-drying, the sections were mounted with neutral gum, ensuring no air bubbles. The sections were then examined under a microscope, with high-resolution images captured at both low magnification (10 ×) and high magnification (40 ×) to document tissue morphology and structure. Tissue damage was evaluated using a modified Dieleman scoring system, which assesses inflammation severity, extent, and epithelial damage. Each parameter was scored from 0 to 4, with higher scores indicating more severe histopathological changes. The total score ranged from 0 to 12. All sections were scored in a blinded manner by two independent observers.
In the slice staining process, sections were first deparaffinized in xylene (I) for 15 minutes and xylene (II) for 25 minutes, followed by gradual hydration in anhydrous ethanol, 90%, 80%, and 70% ethanol, and then washed three times with distilled water, each wash lasting 3 minutes. The sections were then stained by soaking in alcian blue solution for 15 minutes, followed by three washes with distilled water for 3 minutes each. After staining, the sections were oxidized in 1% periodic acid solution for 5 minutes, rinsed with tap water for 3 minutes, and washed twice with distilled water for 3 minutes each. The sections were then immersed in Schiff’s staining solution for 15 minutes and rinsed under running water for 10 minutes. For nuclear counterstaining, the sections were stained with hematoxylin for 1 minute, rinsed with running water, differentiated in 1% hydrochloric acid ethanol for 5 seconds, and blued with Scott’s bluing solution for 3 minutes, followed by a final rinse with running water for 3 minutes. After staining, the sections were dehydrated sequentially in 70%, 80%, 95% ethanol, and anhydrous ethanol and then cleared in xylene for 2 minutes per step. The sections were air-dried, mounted with neutral gum, and allowed to solidify. Finally, the sections were examined under a microscope, with high-resolution images captured at both low (10 ×) and high magnifications (40 ×).
Inflammatory cytokine expression levels in colonic tissues were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Jiangsu Province, China). The kit included antibodies for mouse tumor necrosis factor (TNF)-α (MB-2868A), IL-1β (MB-2776A), IL-6 (MB-2899A), and IL-10 (MB-1736A). Colonic tissue was homogenized, and the procedure strictly adhered to the kit’s instructions. Following the addition of the stop solution, optical density values were measured at 450 nm using a microplate reader (BioTek, United States). Cytokine expression levels were calculated using a standard curve.
For the preparation of a spleen single-cell suspension for flow cytometry analysis, the spleen was placed in a glass dish with 1000 μL of PBS. The tissue was disrupted using a sieve and syringe to achieve complete fragmentation, followed by rinsing the sieve with 1 mL of PBS. The resulting homogenate was transferred to a 5 mL Eppendorf tube, ensuring the cell suspension was fully collected. The suspension was centrifuged at 1200 rpm for 5 minutes, and the supernatant was discarded, retaining the cell pellet. Red blood cells were lysed by adding 2 mL of red blood cell lysis buffer, followed by a 15-minute incubation. After centrifugation at 1200 rpm for 5 minutes, the supernatant was discarded, and the white blood cell pellet was retained. The cells were then resuspended in 1 mL of PBS, centrifuged at 1200 rpm for 5 minutes, and the supernatant was discarded. This washing step was repeated three times. Following washing, the cells were transferred to a flow cytometry tube, blocked with an Fc receptor blocking agent, and stained with PB450-cluster of differentiation (CD) 45, fluorescein isothiocyanate (FITC)-CD4, and PE-CD25 antibodies. After incubation at 37 °C for 30 minutes, the cells were washed with PBS, fixed, and permeabilized before adding PE/cyanine 7-IL-17A antibody and incubating again at 37 °C for 30 minutes. After washing, the cells were resuspended in a cell staining buffer. Flow cytometry analysis was performed using a Beckman coulter instrument (United States), and the data were analyzed using FlowJo software (Tree Star, Ashland).
During immunofluorescence staining, the tissue sections were initially deparaffinized in xylene I and II for 15 minutes each. This was followed by a gradual hydration series through ethanol concentrations (100%, 95%, 90%, 80%, 70%, and 50%) for 5 minutes each, and a final rinse in distilled water for 5 minutes. Antigen retrieval was performed by immersing the sections in sodium citrate buffer (potential of hydrogen = 6.0) and microwaving on high heat for 8 minutes. After resting for 7 minutes, the sections were microwaved again on medium-low heat for 8 minutes. After cooling to room temperature, the sections were washed three times with PBS, 5 minutes each. Endogenous peroxidase activity was quenched by applying 3% hydrogen peroxide solution dropwise, followed by incubation in the dark at room temperature for 10 minutes. After three PBS washes, 5 minutes each, tissue sections were circled using an immunohistochemistry pen, and 5% goat serum or bovine serum albumin blocking solution was applied to block non-specific binding at room temperature for 30 minutes. After blocking, sections were incubated overnight (16-18 hours) at 4 °C with diluted primary antibody. Following incubation, sections were washed with PBS three times, 5 minutes each, and incubated with FITC-labeled secondary antibody at room temperature in the dark for 2 hours. After additional PBS washes, the sections were mounted with an anti-fade mounting medium containing 4’,6-Diamidino-2’-phenylindole (DAPI), covered with a coverslip, and stored in the dark. After standing at room temperature for 2 hours, the sections were observed under a fluorescence microscope (e.g., Leica), and images were captured using the appropriate excitation wavelengths (FITC: 488 nm, DAPI: 358 nm) and saved for analysis.
To isolate naive CD4 + T cells, mouse spleens were processed following the literature method. CD4 + T cells were enriched from splenocytes using a CD4 + T cell isolation kit (Miltenyi, 130-104-454, Germany) and LS columns (Miltenyi, 130-042-401). The sorted cells were cultured in 24-well plates with Roswell Park Memorial Institute 1640 medium (containing 10% fetal bovine serum and 1% penicillin-streptomycin) for 5 days. Cells were stimulated with 10 μg/mL anti-CD3 antibody (Bio X Cell, BE0001-1) and 10 μg/mL anti-CD28 antibody (Bio X Cell, BE0015-1). For Th17 differentiation, the culture medium was supplemented with 40 ng/mL IL-6, 1 ng/mL transforming growth factor-β1, 40 ng/mL IL-23, 20 μg/mL anti-IL-4 antibody (Bio X Cell, BE0045), and 20 μg/mL anti-interferon-γ antibody (Bio X Cell, BE0055).
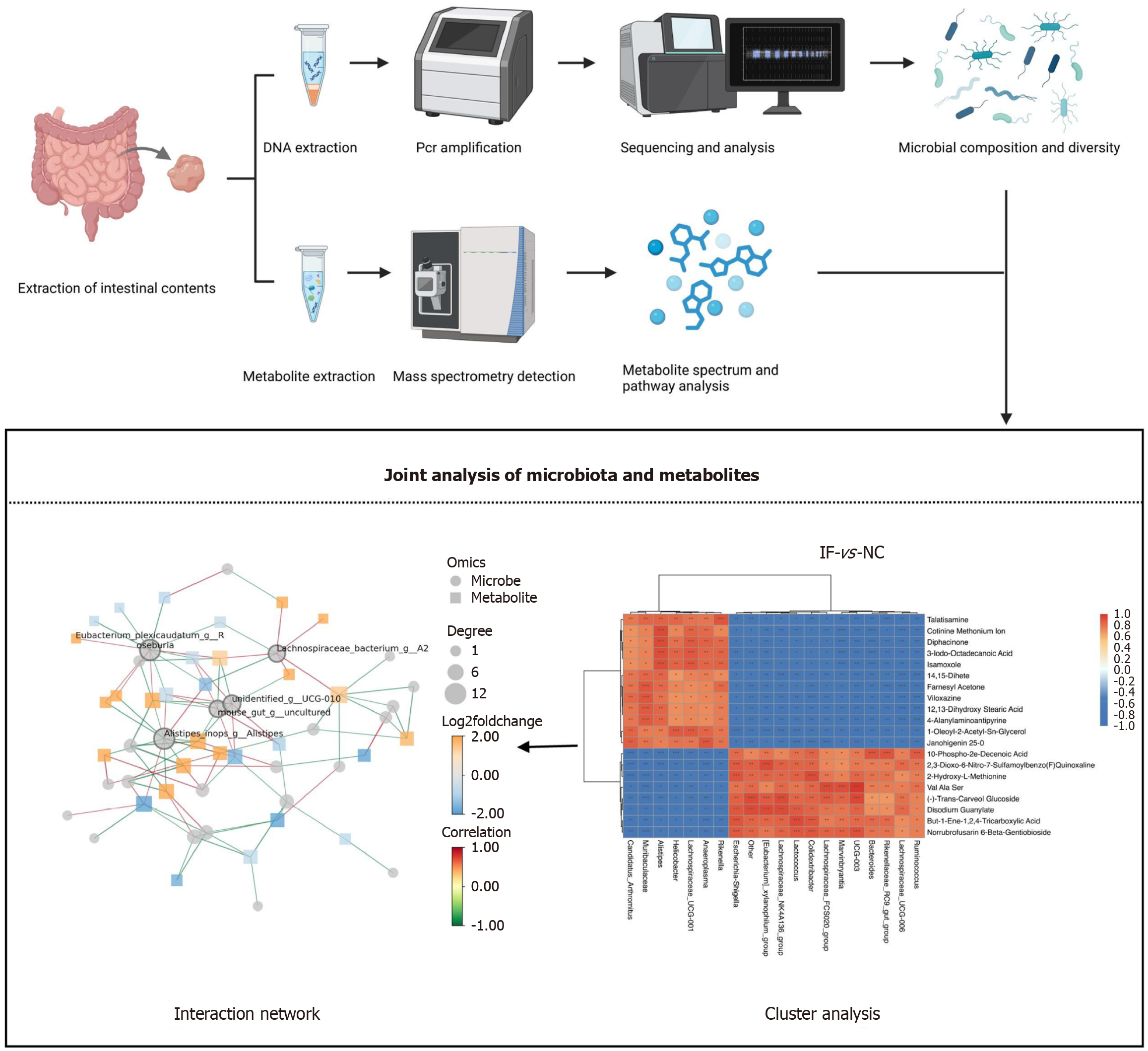
Fecal samples were processed randomly for analysis. Data were processed using SubstraTIQ QI software (v2.3), with parameters set to a precursor tolerance of 5 ppm, product tolerance of 10 ppm, and a production threshold of 5%. Compounds were identified by comparison with databases such as HMDB, LipidMap (V2.3), and Metlin. Positive and negative ion mode data matrices were constructed, and principal component analysis (PCA) was performed using R software to evaluate sample distribution and stability. Further analysis using orthogonal partial least squares discriminant analysis (OPLS-DA) and partial least squares discriminant analysis identified metabolite group differences. Metabolites with variable importance in the projection (VIP) > 1.0 and P < 0.05, based on the OPLS-DA model VIP values, were selected, and group differences were validated using two-tailed t-tests.
In the gut microbiota analysis, fecal samples were collected under sterile conditions, and bacterial DNA was extracted using the Tiangen Fecal DNA extraction kit (DP712). DNA purity and concentration were assessed via agarose gel electrophoresis, and the DNA was diluted to 1 ng/μL for further use. Polymerase chain reaction (PCR) amplification of the 16S rRNA V4 region was performed using barcoded specific primers and the Phusion high-fidelity master mix. The amplification products were analyzed via 2% agarose gel electrophoresis, purified, and quantified using magnetic beads, and target bands were recovered through equal mixing. A sequencing library was constructed using the TruSeq DNA PCR-free kit, and its quality was evaluated with Qubit, Agilent Bioanalyzer 4150, and Q-PCR. Libraries that met quality standards were sequenced on the Illumina NovaSeq 6000 platform. After sequencing, raw data were processed using Fastp, FLASH, and Vsearch for filtering, concatenation, and merging to generate valid tags. Clean sequences were obtained through operational taxonomic units clustering and amplicon sequence variant denoising, followed by species annotation and multiple sequence alignment. Differences in community structure were analyzed using alpha and β diversity metrics, with functional prediction and network analysis conducted thereafter.
In RNA sequencing experiments, total RNA was extracted from colon tissue using TRIzol reagent. RNA concentration and purity (A260/A280 ratio) were assessed using the Nanodrop ND-2000 system, and RNA integrity was evaluated using the Agilent Bioanalyzer 4150, ensuring high-quality RNA. Message RNA (mRNA) was isolated from 1 μg of total RNA using the ABclonal mRNA sequencing Lib prep kit, enriched and fragmented using dT magnetic beads, and converted into double-stranded complementary DNA, which was subsequently ligated with adapters. The sequencing library was prepared through PCR amplification. After library construction, PCR products were purified using the AMPure XP system, and the quality of the library was assessed with the Agilent 4150 Bioanalyzer. Libraries meeting quality standards were sequenced on either the NovaSeq 6000 or MGISEQ-T7 platforms. Gene expression quantification and differential analysis were performed on the sequencing data using R or DESeq2 software packages.
Experimental data were analyzed using GraphPad Prism 10.1 and presented as mean ± SEM. Group comparisons were performed using t-tests or one-way analysis of variance, with a significance level of P < 0.05. For multiple group comparisons, appropriate multiple comparison methods were employed to ensure the reliability of the results.
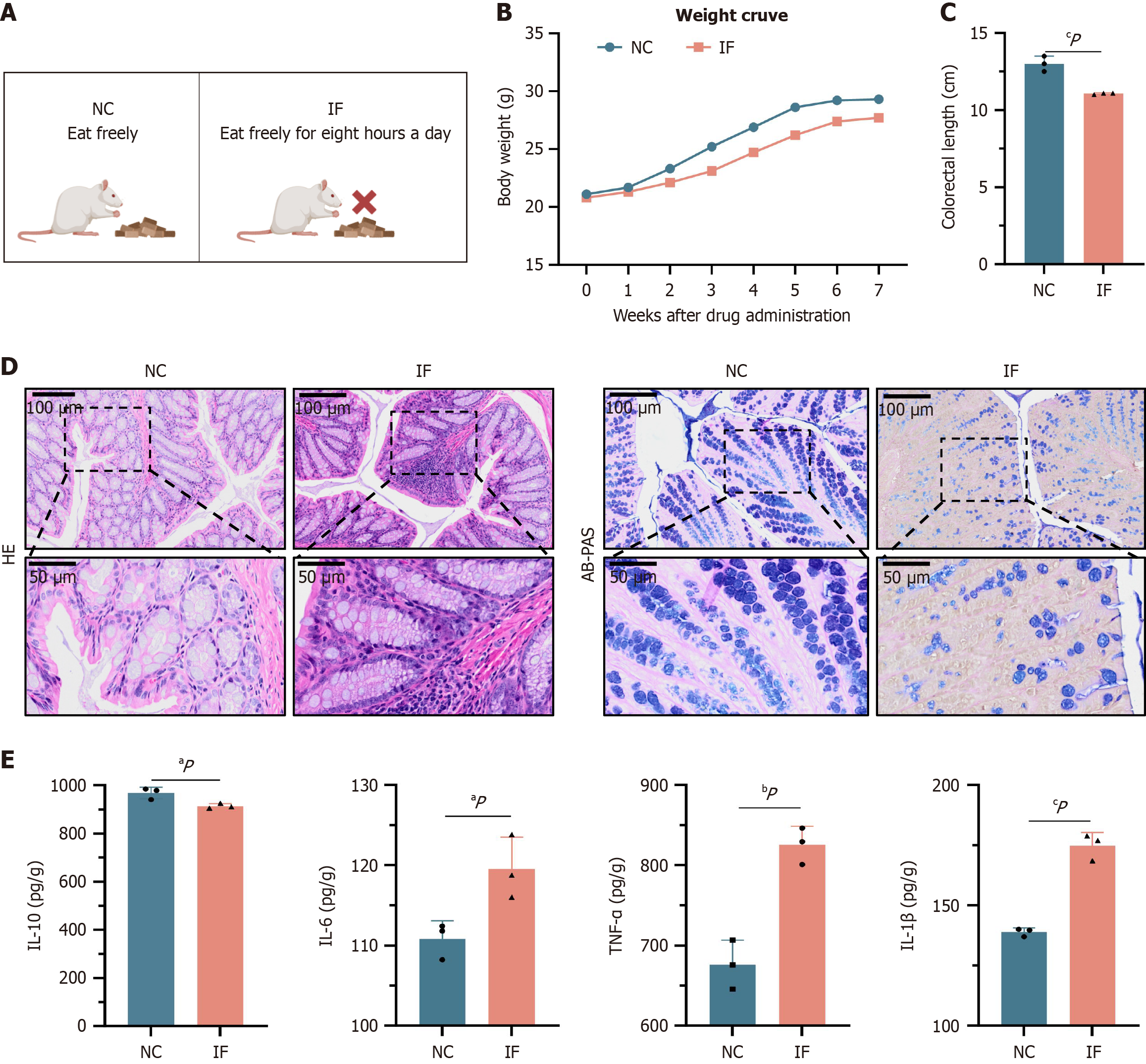
Long-term restrictive dieting can lead to various health issues. To explore these effects, an animal experiment was conducted using the TRF method. Mice were assigned to two groups: An IF group (IF group, with food access limited to 8 hours per day) and a normal diet control group (NC group) (Figure 1A). Prior to the experiment, no significant difference in body weight was observed between the two groups (approximately 21 g on average), ensuring proper group allocation. Body weight changes were monitored daily throughout the experiment. After six weeks, results showed that body weight gain in the IF group was significantly lower than that of the NC group, likely due to intestinal inflammation and nutritional absorption disturbances induced by restrictive dieting (Figure 1B).
Post-experiment analysis of colonic specimens revealed that the colon length in the IF group was significantly shorter than in the NC group (P < 0.05), suggesting potential alterations in the gut microbiota (Figure 1C). Histological examination of colonic sections by hematoxylin and eosin (HE) staining showed extensive inflammatory cell infiltration in the colonic tissues of the IF group, indicating pronounced intestinal inflammation. To further assess tissue damage, Dieleman scoring was performed on HE-stained sections. Based on assessments of inflammation severity, extent, and epithelial damage, the IF group exhibited a significantly higher total histological score (8 points) compared to the NC group (2 points). These results suggest that IF substantially worsened colonic tissue damage. Additionally, alcian blue-periodic acid-Schiff staining (AB-PAS) staining revealed a marked reduction in the number of goblet cells and mucus secretion in the colonic mucosa of the IF group, resulting in a diminished mucus coverage area, which indicates compromised intestinal barrier function (Figure 1D).
To evaluate the extent of inflammation, ELISA was used to measure the expression levels of both pro-inflammatory and anti-inflammatory cytokines. The results demonstrated a significant increase in the levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the IF group, while the expression of the anti-inflammatory cytokine IL-10 was significantly reduced (P < 0.05) (Figure 1E). In conclusion, IF may exacerbate intestinal inflammation and impair colonic health by disrupting the gut microbiota and damaging the intestinal barrier.
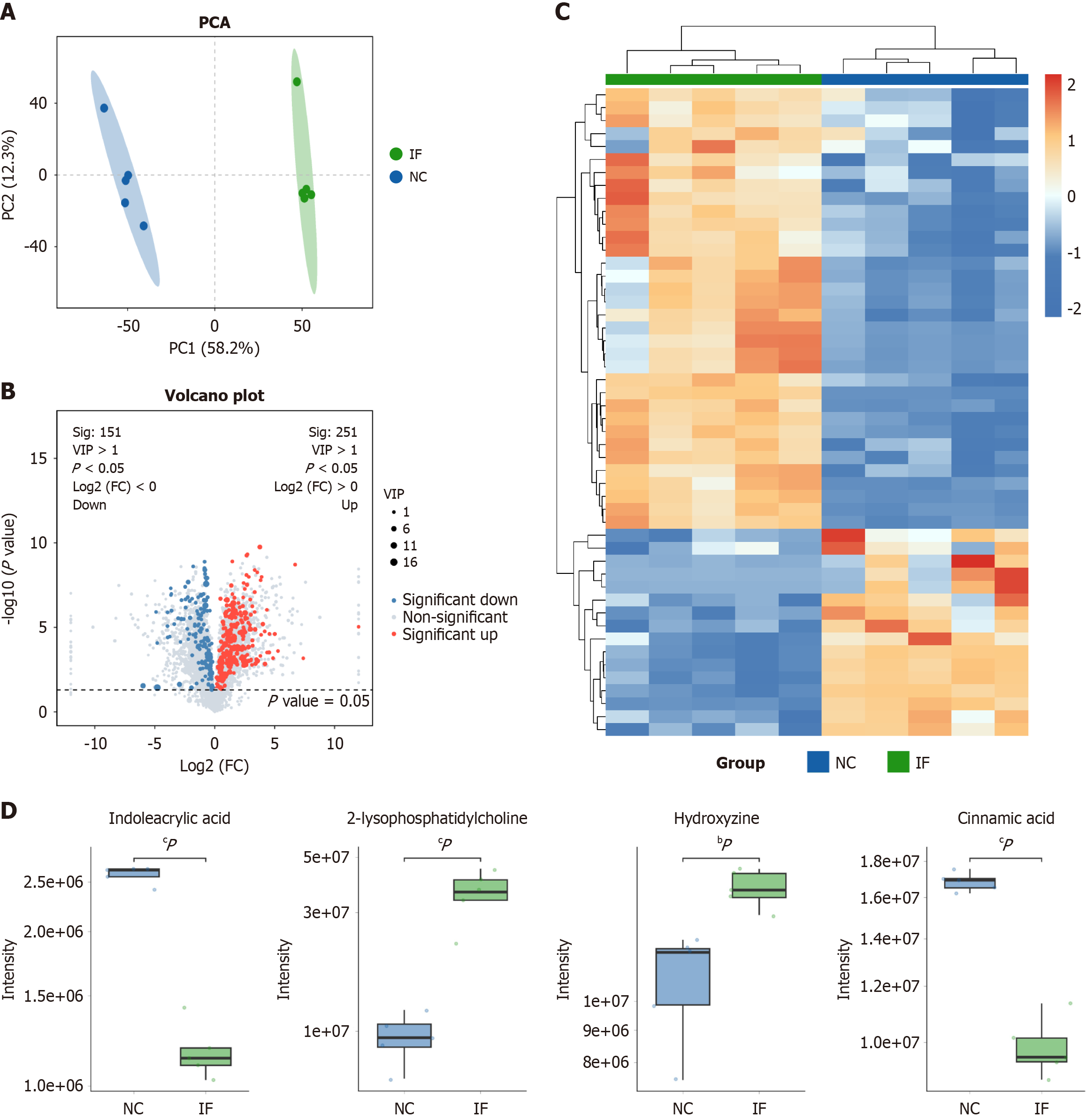
Restrictive diets have been shown to exacerbate colonic inflammation, but the specific mechanisms remain unclear. To investigate whether dietary patterns influence inflammatory changes via metabolic pathways, non-targeted metabolomic sequencing was conducted on intestinal contents from both the NC and IF groups. PCA and OPLS-DA analyses revealed a clear separation between the two groups (NC group in blue, IF group in green) on the PCA plot (principal component 1 = 58.2%, principal component 2 = 12.3%), indicating significant metabolic differences between them (Figure 2A).
Using ultra performance liquid chromatography-mass spectrometry/mass spectrometry technology, a total of 402 metabolites were detected, and a volcano plot was generated to visually identify differential metabolites. Of these, 251 metabolites were upregulated (red) and 151 were downregulated (blue) (Figure 2B). Differential metabolites were further screened based on VIP ≥ 1.00 and fold change ≥ 2 or ≤ 0.5, followed by hierarchical clustering and heatmap generation. The heatmap displayed notable differences in metabolite expression patterns between the IF and NC groups, with orange indicating high expression and blue low expression (Figure 2C).
Additionally, boxplot analysis of selected metabolites, including IAA, 2-lysophosphatidylcholine, cinnamic acid, and hydroxyzine, revealed significant distribution differences between the IF (green) and NC (blue) groups (Figure 2D). These results demonstrate substantial differences in gene expression and metabolite distribution between the two groups, suggesting that restrictive dieting may exacerbate colonic inflammation through alterations in metabolic pathways. Further experimental studies were conducted to validate the biological significance of these differences.
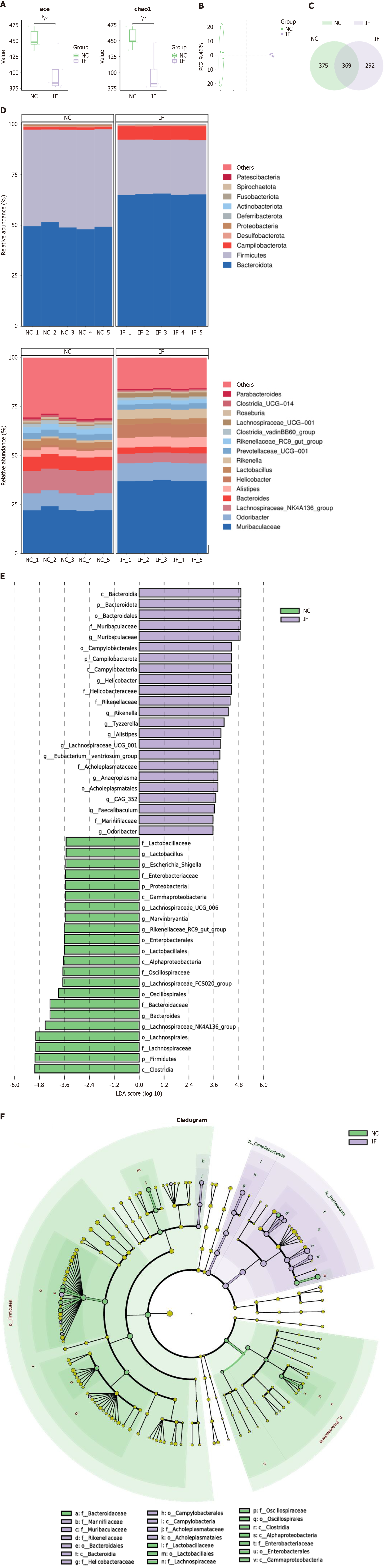
To further investigate the effects of restrictive dieting on the gut microbiota, 16S rRNA sequencing was performed on the intestinal contents of mice in both the NC and IF groups (n = 5). Alpha diversity was assessed using the abundance-based coverage estimator and Chao1 indices, revealing a statistically significant difference between the groups. These results suggest that the restrictive diet notably impacted both the richness and evenness of the gut microbiota (Figure 3A). Additionally, PCA analysis demonstrated a significant difference in beta diversity between the groups, indicating substantial changes in the composition of the gut microbiota (Figure 3B).
Venn diagram analysis identified 369 shared species between the NC and IF groups, with 375 unique species in the NC group and 292 unique species in the IF group (Figure 3C). At the phylum level, the abundance of Firmicutes decreased in the IF group, while Campilobacterota and Bacteroidota abundances increased, potentially reflecting inflammation or stress-related changes (Figure 3D). At the genus level, the abundances of Rikenella and Muribaculaceae were significantly higher in the IF group, suggesting that restrictive dieting may induce low-grade inflammation or alter gut barrier function, fostering the proliferation of inflammation-associated microbiota (Figure 3D).
Lefse analysis highlighted microbial community differences between the two groups, with linear discriminant analysis scores revealing characteristic microbial taxa in each group (Figure 3E). The phylogenetic tree and sample clustering analysis provided a clear depiction of the microbiota composition differences between the NC (green) and IF (purple) groups (Figure 3F). These results from 16S rRNA sequencing indicate that restrictive dieting substantially altered the gut microbiota composition, likely contributing to gut microecological imbalances and associated inflammatory responses.
Additionally, a joint analysis of non-targeted metabolomics and 16S rRNA sequencing was conducted. Cluster analysis revealed the expression levels of microbes and metabolites in different samples, with red indicating high expression and blue indicating low expression, highlighting the differences between the samples. This analysis provided a comprehensive view of microbial and metabolite changes under different conditions. An interaction network analysis further explored the relationships between microbes and metabolites, helping to understand their roles in the ecosystem. The IF group intervention notably altered the association pattern between intestinal microbiota and metabolites. Key microbiota, such as Alistipes and Unidentified_g_UCG-010, formed strong correlations with various metabolites. These changes may be linked to the increased abundance of Bacteroidetes and decreased abundance of Firmicutes, which may influence host metabolism by regulating metabolic pathways, such as fatty acid metabolism (Figure 4).
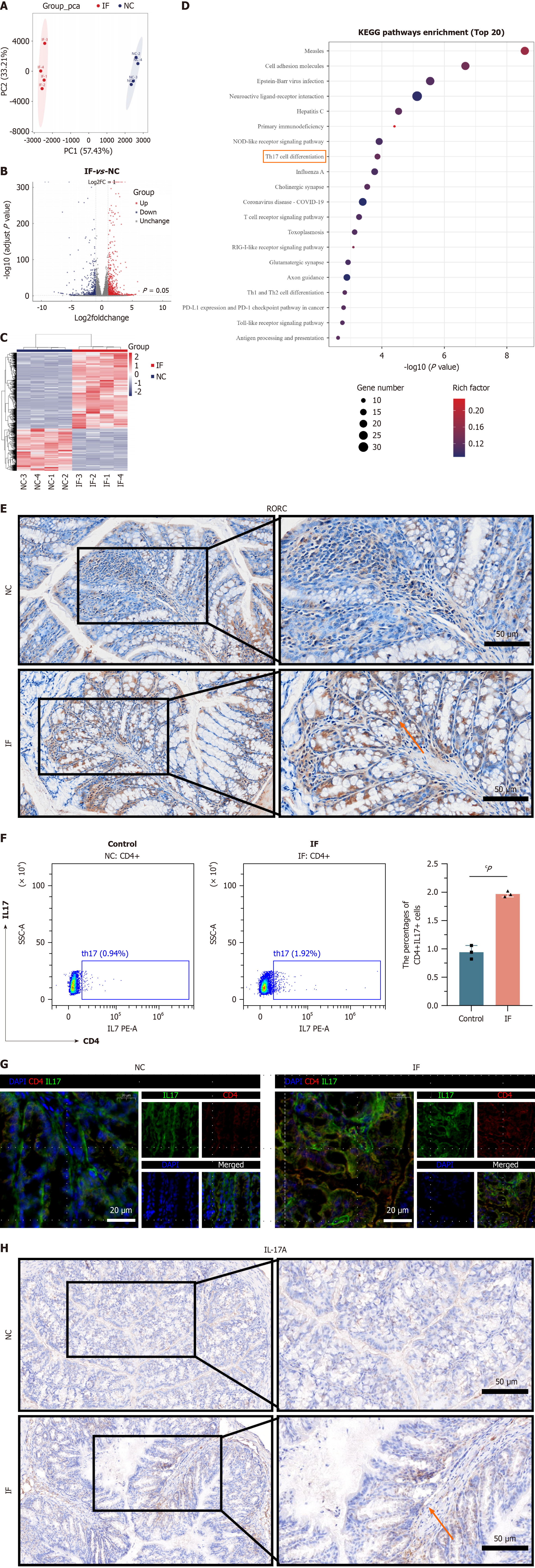
To elucidate the molecular mechanism by which restrictive dieting exacerbates intestinal inflammation, RNA sequencing was performed on the colonic tissues of mice from the NC and IF groups. PCA analysis revealed a clear separation between the IF group (red) and the NC group (blue) on principal component 1 (57.43%) and principal component 2 (33.21%), indicating significant differences between the groups and confirming good sample reproducibility (Figure 5A). Differential gene analysis identified 1131 upregulated genes (red) and 630 downregulated genes (blue) (Figure 5B), with a heatmap visualizing the expression patterns and further highlighting the significant differences in gene expression between the two groups (Figure 5C).
Kyoto Encyclopedia of Genes and Genomes enrichment analysis revealed significant enrichment of immune-related pathways, such as the “T cell receptor signaling pathway” and “Th17 cell differentiation”, suggesting that restrictive dieting may exacerbate intestinal inflammation by modulating immune responses (Figure 5D). Previous studies have established a link between the expression of the key gene RORC and the “Th17 cell differentiation” pathway. Immunohistochemical staining of intestinal tissues showed significantly higher RORC expression in the IF group compared to the NC group (Figure 5E).
To further investigate, the content of CD4 + IL17A + (Th17) cells in the spleen was assessed using flow cytometry. Results revealed a significantly higher proportion of Th17 cells in the IF group compared to the NC group (Figure 5F). Additional immunofluorescence and histochemical staining confirmed that the expression of CD4 and IL17 was significantly increased in the colonic tissues of the IF group (Figure 5G and H). These results collectively suggest that restrictive dieting exacerbates intestinal inflammation by upregulating RORC expression and promoting Th17 cell differentiation.
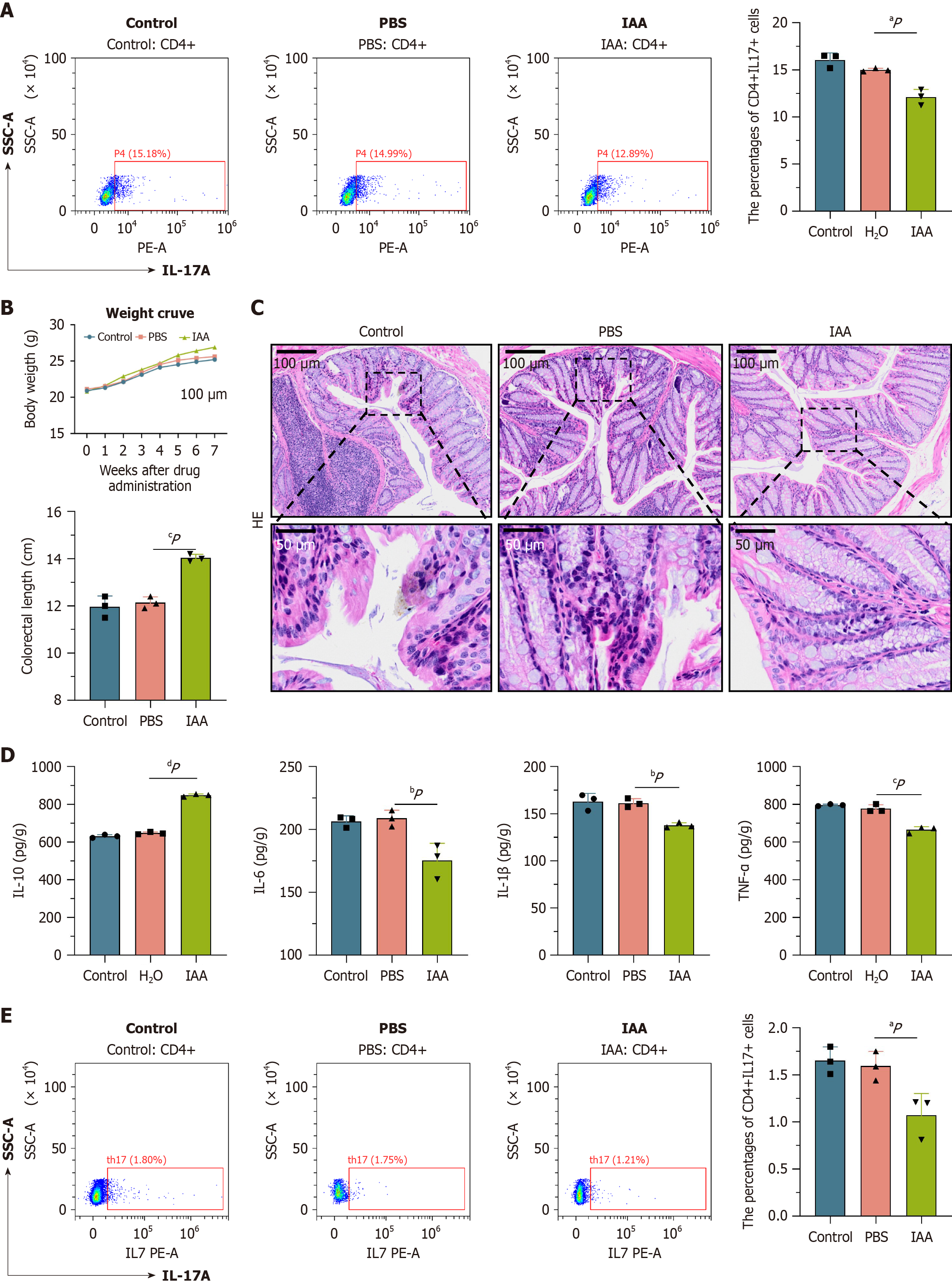
In line with untargeted metabolomics results, IAA content in the IF group was significantly lower than in the NC group. Given the known regulatory effects of IAA on intestinal inflammation[20], it was hypothesized that the reduced IAA content might exacerbate inflammation by promoting Th17 cell differentiation. To test this hypothesis, in vitro experiments were conducted using CD4 + primary cells extracted from the spleens of mice. The cells were divided into three groups: Control (blank control), PBS (PBS added during Th17 induction), and IAA (IAA added during Th17 induction). Flow cytometry analysis revealed that the proportion of Th17 cells in the IAA group was significantly lower than in both the control and PBS groups, suggesting that IAA may inhibit Th17 cell differentiation (Figure 6A).
To further validate these findings, a second cohort of mice was acquired for the animal experiments. The same mouse model used in the prior study was applied, and the animals were allocated to the control, PBS, and IAA groups. Throughout the six-week experimental period, mice in the IAA group demonstrated significantly greater body weight compared to those in the control and PBS groups. Dissection revealed a notable increase in colon length in the IAA group (P < 0.05) (Figure 6B). HE staining of colonic tissue sections showed a marked reduction in inflammatory cell infiltration in the IAA group relative to the control and PBS groups (Figure 6C). To assess the degree of inflammation in each group, Dieleman scoring was performed on the HE-stained sections, evaluating severity, extent, and epithelial damage. Histological scores were 11 for the control group, 10 for the PBS group, and 2 for the IAA group, indicating that IAA treatment significantly alleviated colon tissue damage. ELISA results further revealed that the levels of pro-inflammatory cytokines TNF-α, TNF-β, and IL-6 in the IAA group were substantially lower than those in the control and PBS groups, while the anti-inflammatory cytokine IL-10 was significantly elevated (Figure 6D). Additionally, spleens from the second cohort of mice were harvested, and flow cytometry was employed to quantify the proportion of CD4 + IL17A + (Th17) cells. A significant reduction in Th17 cell proportion was observed in the IAA group compared to both the control and PBS groups, consistent with the in vitro findings (Figure 6E).
In summary, reduced IAA levels exacerbate intestinal inflammation by inducing overexpression of the RORC gene and promoting Th17 cell differentiation, whereas IAA supplementation effectively inhibits Th17 differentiation and mitigates inflammatory responses.
As history progresses, advancements in science and technology have significantly improved material well-being. In certain regions, however, excessive material wealth has fostered a culture of extravagance and waste, contributing to the rising prevalence of obesity. In response, many individuals have turned to fasting methods as a rapid and seemingly effective means of weight loss. Among these, IF has gained notable attention as a distinct dietary pattern[21]. This approach not only aids in weight management but also enhances glucose and lipid metabolism, with some evidence suggesting anti-aging benefits[22]. Recent research has increasingly focused on the effects of IF on gut health, particularly its potential to influence intestinal inflammation by modulating gut immunity, oxidative stress, and microbiota balance[23]. While previous studies suggest that IF may alleviate symptoms of certain conditions[24], it could trigger or exacerbate intestinal inflammation, particularly when fasting periods and food choices are poorly managed[25]. Drawing on these experimental findings, this study examined the potential mechanisms through which IF influences intestinal inflammation, offering new insights for future research in this area.
BALB/c mice were selected for this study due to their characteristic Th2 cell-dominated immune response, which is marked by elevated IL-4/IL-13 levels. In contrast, human IBD typically exhibits a mixed Th1/Th2/Th17 immune profile. IL-4 is known to inhibit retinoic acid receptor-related orphan receptor-γt expression via the signal transducer and activator of transcription-6 signaling pathway, theoretically limiting Th17 cell differentiation. However, the observed presence of significant Th17 cell responses in BALB/c mice suggests that a specific research factor (TRF) may override the inhibitory effects of Th2 cells on Th17 differentiation, potentially through strong stimuli such as specific microbiota or metabolites. Indeed, differences in gut microbiota composition between BALB/c and C57BL/6 mice exist, with BALB/c mice showing relatively lower levels of segmented filamentous bacteria (SFB), a variation that may affect baseline Th17 cell levels. Consequently, TRF might indirectly promote Th17 cell differentiation by reshaping the gut microbiota, for example, by increasing SFB abundance. The decision to use BALB/c mice also stems from their immune characteristics, which more closely resemble those of certain patients with IBD. Additionally, the immune system and gut microbiota of 4-week-old mice, which correspond to the pre-pubertal stage in humans, are still immature and highly plastic. This developmental state offers a unique opportunity to examine the early programming effects of TRF on immune system development, as opposed to investigating the regulation of a fully matured immune system. Intervening during this juvenile period may have lasting metabolic and immunological consequences by influencing immune cell differentiation (e.g., modulating the Th17/Treg balance) or gut microbiota colonization patterns, thus simulating the long-term effects of TRF in a short-term experimental context. In contrast, similar studies in adult mice would likely require longer intervention periods to observe comparable effects. Therefore, 4-week-old male BALB/c mice were ultimately chosen as the experimental subjects.
This study examined the impact of TRF on the immune response in the mouse colon. The immune system plays a critical role in gut health, with the gut serving as an immune organ containing a distinct population of immune cells, including intestinal epithelial cells, dendritic cells, and T cells, all of which are essential in maintaining and protecting gut immunity[26-28]. After prolonged intermittent feeding, significant differences were observed in body weight and intestinal length between the experimental group and the control group. Subsequent histological analysis, including HE staining and AB-PAS staining of intestinal sections observed under electron microscopy, revealed damage to the intestinal epithelial cells in the IF group, with more pronounced disruption of the intestinal villus structure compared to the NC group. ELISA experiments further showed a significant increase in the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), indicating activation of the gut immune response, which may be closely associated with the onset of intestinal inflammation. Excessive immune activation has been well-documented in various IBDs, particularly Crohn’s disease and ulcerative colitis. These findings suggest that intermittent feeding may impair the intestinal epithelial barrier, increasing intestinal permeability and facilitating the invasion of harmful substances and pathogens, which could, in turn, trigger an inflammatory response[29].
Furthermore, intestinal metabolic changes are pivotal in regulating immune responses and maintaining gut health. Previous research has highlighted a strong link between gut metabolite composition and diversity with the gut immune environment and its reaction to external stimuli[30]. Non-targeted metabolomics analysis of fecal samples from the mice revealed significant differences in metabolites between the two groups. Notably, metabolites such as IAA, 2-lysophosphatidylcholine, cinnamic acid, and hydroxyzine showed marked differences between the IF and NC groups. In the IF group, the anti-inflammatory metabolites IAA and cinnamic acid were significantly reduced, while the pro-inflammatory metabolites 2-lysophosphatidylcholine and hydroxyzine were significantly elevated. The combined effects of these metabolites appear to negatively impact intestinal barrier function. The damage to the intestinal barrier observed under intermittent feeding conditions may be linked to cellular stress during the hunger-feeding cycle[31], particularly the cellular damage associated with rapid metabolism and regeneration of intestinal cells after feeding[32]. Impaired intestinal barrier function is a precursor to intestinal inflammation, as it facilitates the entry of endotoxins and bacteria into the intestinal wall, potentially activating the innate immune system and triggering a chronic inflammatory state. Disruption of the intestinal barrier is a common feature of many intestinal diseases, including IBD and intestinal infections.
Dysbiosis of the gut microbiota is a key factor in the onset of intestinal inflammation[33]. To explore which microbiota may influence the intestines of IF group mice, 16S rRNA sequencing was performed to investigate the potential impact of intermittent feeding on gut microbiota composition. Intermittent diet significantly altered both the diversity and composition of the gut microbiota. Notably, the abundance of certain pathogenic bacteria, such as Rikenella and Muribaculaceae, increased substantially in the intestines of experimental mice. This dysbiosis not only compromises intestinal barrier function but may also influence the gut immune response through changes in microbial products, such as SCFAs and other bacterial metabolites[34]. SCFAs, produced by gut probiotics, are critical for maintaining gut health, modulating immune responses, and inhibiting the growth of pathogenic microorganisms[35]. The intermittent diet may reduce SCFA synthesis by altering the gut microbiota composition, thereby disrupting the gut immune environment and promoting inflammation. To further investigate the specific effects of altered gut microbiota on immune cells, flow cytometry was employed to analyze immune cell subpopulations in the intestine. Our findings (Figure 5) demonstrated that intermittent diet increased the proportion of T cells, particularly Th17 cells, in the intestinal mucosa. Th17 cells are essential in gut immune responses[36], and their excessive activation is closely linked to the development of various intestinal inflammatory diseases[37]. Intermittent feeding may facilitate the differentiation of Th17 cells by modifying the interactions between the gut microbiota and the immune system, thereby exacerbating intestinal inflammation. However, the precise mechanisms remain unclear. It is likely that the increased differentiation of Th17 cells intensifies the local immune response, potentially leading to an overactivation of the gut immune system and further aggravating intestinal inflammation. Maintaining an intact intestinal barrier is crucial for preserving gut immune tolerance[38].
To validate our findings, this study intragastrically administered IAA, a metabolite absent in the IF group mice, and assessed its effects. ELISA analysis of colon samples revealed significantly lower levels of pro-inflammatory cytokines (TNF-α, TNF-β, and IL-6) in the IAA-supplemented group compared to the control groups, while the anti-inflammatory cytokine IL-10 was notably elevated. Additionally, the Th17 cell content was significantly reduced in mice receiving IAA, providing further evidence that long-term intermittent feeding induces changes in gut microbiota, triggering intestinal metabolic alterations. By regulating the “Th17 cell differentiation” pathway, intermittent diet may influence the onset of inflammatory responses.
In conclusion, this study investigated the impact of long-term intermittent diet on the development of intestinal inflammation in mice, examining factors such as intestinal barrier damage, increased inflammatory cytokines, alterations in intestinal metabolites and microbiota, and the pathways involved in inflammation. However, several limitations exist. While the data indicate significant correlations between changes in microbiota composition, abnormal IAA metabolism, Th17 cell activation, and colitis phenotypes, certain experimental gaps must be addressed: The lack of direct validation through antibiotic intervention to assess microbiota depletion’s effect on the IAA-Th17 axis, the absence of germ-free (GF) animal models to exclude confounding effects of commensal microbiota, and the failure to establish direct causal evidence through microbiota transplantation. These gaps prevent us from ruling out the possibility that TRF may influence IAA metabolism via microbiota-independent pathways, such as direct effects on intestinal epithelial cells or immune cells. Additionally, the progression of colitis itself may alter microbiota structure and metabolite profiles, potentially affecting the findings. Future studies should adopt a three-arm experimental design combining TRF intervention with antibiotic treatment (conventional housing, antibiotic-treated, and GF models), along with microbiota transplantation, to further clarify the relationships among microbiota, host metabolism, and immune responses. This would offer more robust evidence for the proposed causal model. Moreover, future research should explore the effects of different intermittent diet patterns (such as IF, TRF, etc.) on gut health. A critical area of investigation will be balancing the metabolic benefits of intermittent diets with the risk of intestinal inflammation. Studies should also consider individual differences, such as genetic background, gut microbiota diversity, and lifestyle factors, when examining the effects of intermittent diet. New intervention strategies, including the combined use of antioxidants, probiotics, and intestinal barrier repair agents, may offer therapeutic approaches to mitigate intestinal inflammation induced by intermittent diet.
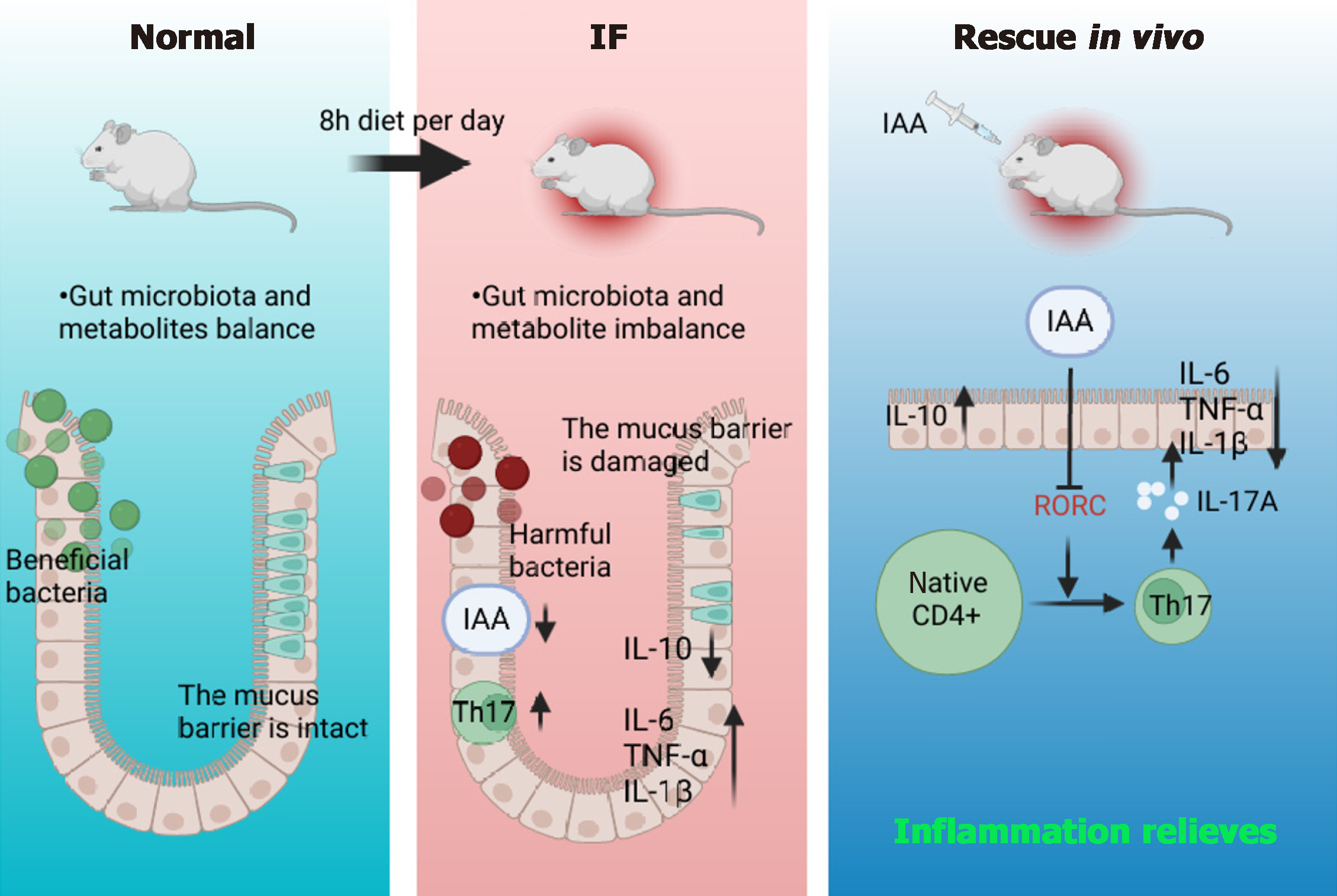
This study demonstrates that long-term intermittent diet significantly reduces mouse body weight, causes intestinal damage, and alters intestinal metabolism and microbiota, ultimately triggering intestinal inflammation. Transcriptome sequencing further reveals that intermittent diet influences the “Th17 cell differentiation” pathway, accelerating Th17 cell differentiation and exacerbating intestinal inflammation. The experimental results highlight the pivotal role of IAA content in Th17 cell differentiation, with IAA serving as a critical metabolic product that alleviates colon inflammation by inhibiting Th17 cell differentiation (Figure 7).
We thank Li WY for his technical supports. We would like to thank Geng QL and Li HY for their guidance on my experimental techniques. Thank you for Guo YQ’s support and companionship. We further thank all volunteers who participated in this study.
| 1. | Son DS, Done KA, Son J, Izban MG, Virgous C, Lee ES, Adunyah SE. Intermittent Fasting Attenuates Obesity-Induced Triple-Negative Breast Cancer Progression by Disrupting Cell Cycle, Epithelial-Mesenchymal Transition, Immune Contexture, and Proinflammatory Signature. Nutrients. 2024;16:2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 2. | Strilbytska O, Klishch S, Storey KB, Koliada A, Lushchak O. Intermittent fasting and longevity: From animal models to implication for humans. Ageing Res Rev. 2024;96:102274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Cardiometabolic Benefits of Intermittent Fasting. Annu Rev Nutr. 2021;41:333-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Matta L, Weber P, Erener S, Walth-Hummel A, Hass D, Bühler LK, Klepac K, Szendroedi J, Guerra J, Rohm M, Sterr M, Lickert H, Bartelt A, Herzig S. Chronic intermittent fasting impairs β cell maturation and function in adolescent mice. Cell Rep. 2025;44:115225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Yin W, Sun L, Liang Y, Luo C, Feng T, Zhang Y, Zhang W, Yin Y. Maternal intermittent fasting deteriorates offspring metabolism via suppression of hepatic mTORC1 signaling. FASEB J. 2023;37:e22831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Dje Kouadio DK, Wieringa F, Greffeuille V, Humblot C. Bacteria from the gut influence the host micronutrient status. Crit Rev Food Sci Nutr. 2024;64:10714-10729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18:309-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 180] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 8. | Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, Hong D, Tian S, Sun C. Effect of Intermittent Fasting Diet on Glucose and Lipid Metabolism and Insulin Resistance in Patients with Impaired Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. Int J Endocrinol. 2022;2022:6999907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Mendonça MLM, Carvalho MR, Romanenghi RB, Santos DSD, Filiú WFO, Pagan LU, Okoshi K, Okoshi MP, Oliveira RJ, Oliveira-Junior SA, Martinez PF. Impact of combined intermittent fasting and high-intensity interval training on apoptosis and atrophy signaling in rat fast- and slow-twitch muscles. Physiol Rep. 2024;12:e16181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Li C, Zhang H, Wu H, Li R, Wen D, Tang Y, Gao Z, Xu R, Lu S, Wei Q, Zhao X, Pan M, Ma B. Intermittent fasting reverses the declining quality of aged oocytes. Free Radic Biol Med. 2023;195:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 11. | Antarianto RD, Kadharusman MM, Wijaya S, Hardiny NS. The Impact of Prolonged and Intermittent Fasting on PGC-1α, Oct-4, and CK-19 Liver Gene Expression. Curr Aging Sci. 2023;16:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Gallage S, Ali A, Barragan Avila JE, Seymen N, Ramadori P, Joerke V, Zizmare L, Aicher D, Gopalsamy IK, Fong W, Kosla J, Focaccia E, Li X, Yousuf S, Sijmonsma T, Rahbari M, Kommoss KS, Billeter A, Prokosch S, Rothermel U, Mueller F, Hetzer J, Heide D, Schinkel B, Machauer T, Pichler B, Malek NP, Longerich T, Roth S, Rose AJ, Schwenck J, Trautwein C, Karimi MM, Heikenwalder M. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. 2024;36:1371-1393.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (1)] |
| 13. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 339] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 14. | Liu A, Liang X, Wang W, Wang C, Song J, Guo J, Sun D, Wang D, Song M, Qian J, Zhang X. Human umbilical cord mesenchymal stem cells ameliorate colon inflammation via modulation of gut microbiota-SCFAs-immune axis. Stem Cell Res Ther. 2023;14:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Palenca I, Seguella L, Del Re A, Franzin SB, Corpetti C, Pesce M, Rurgo S, Steardo L, Sarnelli G, Esposito G. N-Palmitoyl-D-Glucosamine Inhibits TLR-4/NLRP3 and Improves DNBS-Induced Colon Inflammation through a PPAR-α-Dependent Mechanism. Biomolecules. 2022;12:1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Cai J, Liu J, Fan P, Dong X, Zhu K, Liu X, Zhang N, Cao Y. Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int Immunopharmacol. 2021;99:108015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Aldars-García L, Chaparro M, Gisbert JP. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms. 2021;9:977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 18. | Martín-Hernández D, Gutiérrez IL, González-Prieto M, MacDowell KS, Robledo-Montaña J, Tendilla-Beltrán H, Calleja-Rodríguez N, Bris ÁG, Ulecia-Morón C, Moreno B, Caso JR, García-Bueno B, Rodrigues-Mascarenhas S, Marín-Jiménez I, Leza JC, Menchén L. Sphk2 deletion is involved in structural abnormalities and Th17 response but does not aggravate colon inflammation induced by sub-chronic stress. Sci Rep. 2022;12:4073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 19. | Pan H, Chen X, Wang P, Peng J, Li J, Ding K. Effects of Nemacystus decipiens polysaccharide on mice with antibiotic associated diarrhea and colon inflammation. Food Funct. 2023;14:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Regmi SC, Kim JA, Cho MH, Yun H, Lee CS, Lee J. Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect Immun. 2011;79:4819-4827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Shen J, Yang L, You K, Chen T, Su Z, Cui Z, Wang M, Zhang W, Liu B, Zhou K, Lu H. Indole-3-Acetic Acid Alters Intestinal Microbiota and Alleviates Ankylosing Spondylitis in Mice. Front Immunol. 2022;13:762580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 22. | Kang J, Ratamess NA, Faigenbaum AD, Bush JA, Beller N, Vargas A, Fardman B, Andriopoulos T. Effect of Time-Restricted Feeding on Anthropometric, Metabolic, and Fitness Parameters: A Systematic Review. J Am Nutr Assoc. 2022;41:810-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Endo S, Uto A, Miyashita K, Sato M, Inoue H, Fujii K, Hagiwara A, Ryuzaki M, Oshida T, Kinouchi K, Itoh H. Intermittent Fasting Sustainably Improves Glucose Tolerance in Normal Weight Male Mice Through Histone Hyperacetylation. J Endocr Soc. 2023;7:bvad082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Song S, Chen L, Bai M, Wang S, Ye X, Lin Y, Luo X, Li Z, Zhang L, Zhu X, Wang Z, Chen Y. Time-restricted feeding ameliorates dextran sulfate sodium-induced colitis via reducing intestinal inflammation. Front Nutr. 2022;9:1043783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Song S, Bai M, Ling Z, Lin Y, Wang S, Chen Y. Intermittent administration of a fasting-mimicking diet reduces intestinal inflammation and promotes repair to ameliorate inflammatory bowel disease in mice. J Nutr Biochem. 2021;96:108785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Dai L, Liu Y, Cheng L, Wang H, Lin Y, Shi G, Dong Z, Li J, Fan P, Wang Q, Su X, Zhang S, Yang Y, Hu X, Huang W, Zhou Z, Yu D, Probert C, Wei Y, Deng H. SARI attenuates colon inflammation by promoting STAT1 degradation in intestinal epithelial cells. Mucosal Immunol. 2019;12:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Zheng T, Zhang B, Chen C, Ma J, Meng D, Huang J, Hu R, Liu X, Otsu K, Liu AC, Li H, Yin Z, Huang G. Protein kinase p38α signaling in dendritic cells regulates colon inflammation and tumorigenesis. Proc Natl Acad Sci U S A. 2018;115:E12313-E12322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Yang M, Tang L, Wang F, Huang S, Liu S, Lei Y, Wang S, Xie Z, Wang W, Zhao X, Tang B, Yang S. TLR4 regulates RORγt(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome. 2022;10:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 29. | Lian Q, Yan S, Yin Q, Yan C, Zheng W, Gu W, Zhao X, Fan W, Li X, Ma L, Ling Z, Zhang Y, Liu J, Li J, Sun B. TRIM34 attenuates colon inflammation and tumorigenesis by sustaining barrier integrity. Cell Mol Immunol. 2021;18:350-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Li M, Han X, Sun L, Liu X, Zhang W, Hao J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes. 2024;16:2329147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 31. | Grosheva I, Zheng D, Levy M, Polansky O, Lichtenstein A, Golani O, Dori-Bachash M, Moresi C, Shapiro H, Del Mare-Roumani S, Valdes-Mas R, He Y, Karbi H, Chen M, Harmelin A, Straussman R, Yissachar N, Elinav E, Geiger B. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology. 2020;159:1807-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 32. | Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol Gastroenterol Hepatol. 2021;11:1463-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 33. | Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 429] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 34. | He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, Zhang S, Zhu L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci. 2020;21:6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 548] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 35. | Shin Y, Han S, Kwon J, Ju S, Choi TG, Kang I, Kim SS. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients. 2023;15:4466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 36. | Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV, Turnbaugh PJ. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe. 2022;30:17-30.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 37. | Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, Huang SJ, Yang M, Wu LY, Wang W, Liu S, Yang SM, Zhao XY. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10:5225-5241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 38. | Xue HH, Li JJ, Li SF, Guo J, Yan RP, Chen TG, Shi XH, Wang JD, Zhang LW. Phillygenin Attenuated Colon Inflammation and Improved Intestinal Mucosal Barrier in DSS-induced Colitis Mice via TLR4/Src Mediated MAPK and NF-κB Signaling Pathways. Int J Mol Sci. 2023;24:2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |