Published online Jun 14, 2025. doi: 10.3748/wjg.v31.i22.106575
Revised: April 9, 2025
Accepted: May 26, 2025
Published online: June 14, 2025
Processing time: 103 Days and 6.2 Hours
Epidemiological evidence suggests that there is a direct relationship between the degree of obesity and acute pancreatitis severity. Intake of different fatty acids leads to different types of hyperlipidemias. Adipose degradation by pancreatic lipase generates different free fatty acids, which can exacerbate pancreatitis. Saturated fatty acids (SFAs) play an inflammatory role in human metabolic syndrome and obesity, whereas unsaturated fatty acids (UFAs) are “good fats” that are thought to enhance overall health status. However, it appears that serum UFAs correlate with severe acute pancreatitis. Additionally, the “obesity paradox” suggests that UFAs potentially minimize direct harm to the organ. This review provides an in-depth overview of the role of SFAs and UFAs in acute pancreatitis of hyperlipidemia and discusses potential prevention targets for severe acute pancreatitis.
Core Tip: This review explores the potential causes of hyperlipidemic pancreatitis. The increased intake of saturated fatty acids (SFAs) initially elevates pancreatic lipase activity, accelerating lipolysis and fat necrosis, which in turn increases free fatty acids levels and creates a permissive inflammatory environment in severe acute pancreatitis. This process accelerates immune response of M1 macrophage polarization and subsequent acinar damage. Over time, unsaturated fatty acids (UFAs) exacerbate acinar cell damage and impair β-cell function in the pancreas, leading to diabetes. This cumulative effect is a consistent and damaging process. Therefore, the ratio of UFAs to SFAs is crucial in the pathogenesis of severe acute pancreatitis, and the progression is complex. It is essential to clearly define the primary roles of SFAs and UFAs in the development of severe acute pancreatitis.
- Citation: Wang YX, Ge P, Chen HL. Induction of hyperlipidemic pancreatitis by different fatty acids: A narrative review. World J Gastroenterol 2025; 31(22): 106575
- URL: https://www.wjgnet.com/1007-9327/full/v31/i22/106575.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i22.106575
Acute pancreatitis (AP) is a rapid-onset inflammatory disease marked by the self-digestion of pancreatic tissue, which can cause both local pancreatic damage and a systemic inflammatory response[1]. Although the biological mechanisms underlying AP are not fully understood, gallstones and chronic alcohol consumption are recognized as predominant etiologies of AP, with hypertriglyceridemia being the third most common cause. Although hypertriglyceridemia-induced pancreatitis (HTGP) accounts for approximately 5% of all AP cases, its reported prevalence can be as high as 22% and it may be responsible for up to 56% of cases during pregnancy[2]. HTGP pathophysiology is associated with the accumulation of free fatty acids (FFAs) and activation of the inflammatory response[3]. Researchers have suggested that FFA release is related to the onset and severity of AP[4]. Circulating FFAs differ in their saturation level [saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), or polyunsaturated fatty acids (PUFAs)], as well as in their carbon chain length, (short-, medium-, and long-chain). These FFAs have been implicated in AP pathogenesis by contributing to systemic inflammatory response syndrome (SIRS), multiple organ dysfunction, and circulatory shock[4]. Specifically, FFAs can trigger the activation of trypsinogen to initiate AP[5]. In addition, FFAs exert cytotoxic and proinflammatory effects that contribute to adipose tissue (AT) injury and necrosis. Notably, AT necrosis has been frequently observed in cases of severe AP (SAP)[6].
In this review, we provide a detailed overview of the role of different FFAs in AP, particularly in the context of coexisting obesity and hyperlipidemia. Furthermore, we summarize inflammatory states, levels of AT necrosis, and alterations in carbohydrate and lipid metabolism in AP induced by high fat diets. Finally, we seek to elucidate the key roles of saturated and unsaturated fatty acids (UFAs) in modulating AP progression and severity, with the overall aim of providing recommendations for lifestyle modifications that effectively reduce the risk of AP onset.
AP is an acute inflammatory disease of the digestive system. Elevated concentrations of FFAs in plasma following high-fat dietary intake is a common feature of inflammatory diseases, including AP[7]. Although AP pathogenesis is not fully understood, oxidative stress is widely considered a key mechanism in its early development. Oxidative stress results from a disruption to the balance between pro-oxidant and antioxidant systems, leading to inflammatory cell recruitment and activation, which in turn amplify both oxidative stress and inflammation through a positive feedback loop[8]. Gulhane et al[9] demonstrated that a high-fat diet (HFD) induced the expression of genes associated with endoplasmic reticulum (ER) stress, including key markers of the unfolded protein response (UPR) pathway, such as spliced X-box binding protein 1, the ER chaperone glucose-regulated protein 78, and the ER-associated degradation component Edem1. Oxidative stress impairs ER functionality, leading to the initiation of the UPR, a tightly regulated signaling network that aims to re-establish proteostasis and prevent intracellular accumulation of aberrant polypeptides. This adaptive response involves the induction of ER-resident chaperones, transient suppression of protein synthesis to alleviate translocation burden, and promotion of retrotranslocation mechanisms that export defective proteins to the cytoplasm for ubiquitin tagging and subsequent degradation via lysosomal pathways[10]. ER dysfunction has been associated with activation of inflammatory signaling modules, including the IκB kinase (IKKβ) and c-Jun N-terminal kinase (JNK) axes, upregulation of the transcription factor CREB-H, and increased reactive oxygen species (ROS) generation[11]. During inflammation, factors such as dysregulated adipokine secretion, AT expansion, and elevated SFAs further exacerbate ER stress, perpetuating a self-amplifying cycle of inflammation[12-15].
Regardless of etiology, injury to pancreatic acinar cells leads to the leakage of digestive enzymes, such as lipases and phospholipase A2, into the pancreatic interstitium and systemic circulation[16]. Lipolytic activity on peripancreatic AT results in the release of large quantities of FFAs. This process is closely associated with multisystem organ failure in SAP, particularly in the context of obesity[17]. In experimental settings, both total lipid extracts and individual FA fractions derived from necrotic AT enhance the expression of proinflammatory mediators in pancreatic acinar cells. In rat models of AP, peripancreatic fat necrosis is characterized by a predominance of SFAs, particularly palmitic acid (PA, C16:0) and stearic acid (SA, C18:0), while oleic acid (OA, C18:1) and linoleic acid (LA, C18:2) constitute the major UFAs present in affected tissue[18]. However, the specific contributions of individual FAs to the initiation and propagation of inflammatory responses in acinar cells remain unclear. The harmful effects of FAs have been attributed to their cytotoxic properties, particularly their ability to disrupt cellular membranes, as well as their role in promoting inflammatory signaling pathways[19,20]. Excessive dietary fat intake stimulates mitochondrial β-oxidation of FFAs, leading to increased ROS production, which in turn contributes to the initiation of proinflammatory responses[21,22]. Interestingly, while clear proinflammatory effects have been associated with SFAs[23], both pro- and anti-inflammatory effects have been reported for UFAs[24-26]. These conflicting findings may be attributed to cell-type-specific responses and the distinct biological effects of individual fatty acids, which vary according to their chemical structure. To mechanistically explain the effects of UFAs and SFAs, Mateu et al[27] investigated key intracellular signaling pathways involved in the inflammatory response of pancreatic acinar cells. Their findings demonstrated that c-JNK, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinases (p38-MAPKs), and Janus kinase (JAK) are critical upstream regulators of C-C motif chemokine ligand 2 (CCL2; also known as monocyte chemoattractant protein-1, MCP-1) expression induced by the UFAs OA and LA. These results highlight the necessity of evaluating the distinct effects of individual UFAs and SFAs on a uniform cell type, as well as elucidating the molecular mechanisms that mediate their inflammatory actions.
Triglycerides are the primary chemical form in which fat is stored in the body. Structurally, they consist of three fatty acids chains attached to a single glycerol molecule. Patients with HTG-AP typically present 10-15 years earlier than those with AP of other etiologies. Approximately two-thirds of HTG-AP cases occur in males, and patients with HTG-AP also tend to have higher body mass indexes[28]. Notably, triglycerides themselves are not directly responsible for initiating AP. Rather, it is their hydrolysis by lipase into FFAs, particularly when concentrations of UFAs are sufficiently elevated, that leads to acinar cell injury. This injury is mediated through trypsin activation and a dose-dependent increase in cytosolic calcium levels, a process influenced by the ratio of UFAs to SFAs, ultimately contributing to pancreatitis.
Ingested triglycerides cannot be directly absorbed by the small intestine without prior digestion. Within the jejunum, pancreatic lipase (PNLIP) catalyzes the breakdown of triglycerides into FFAs and glycerol, which are subsequently taken up by intestinal epithelial cells. Following translocation across the intestinal barrier, FFAs are re-esterified with glycerol to reform triglycerides. These resynthesized lipids are incorporated into chylomicron-lipoprotein complexes composed of triglycerides, cholesterol, phospholipids, and apolipoproteins (APOs). Chylomicrons are secreted into the lymphatic system via the thoracic duct and eventually enter the systemic circulation. Once in the bloodstream, they serve as transport vehicles, delivering lipid constituents to peripheral tissues such as skeletal muscle, cardiac tissue, and the liver for further metabolism. Lipoprotein lipase (LPL), a key hydrolytic enzyme localized to the endothelial surface of capillaries in adipose and muscle tissue, is primarily synthesized as a precursor protein by adipocytes and myocytes. Lipase maturation factor 1 facilitates proper LPL folding and maturation, which is subsequently secreted into the interstitial space, transported across the endothelium, and anchored to the luminal surface by glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. LPL activity is tightly regulated by a variety of apolipoproteins (APOs) and angiopoietin-like proteins, with apolipoprotein C-II (APOC2), APOC3, APOA5, apolipoprotein E (APOE), ANGPTL3, ANGPTL4, and ANGPTL8 playing particularly critical roles. APOC2 is an essential cofactor required for LPL activation, and APOE is a key ligand in the hepatic clearance of triglyceride-rich lipoproteins[29]. Conversely, ANGPTL proteins predominantly function by inhibiting LPL activity through paracrine or endocrine mechanisms, and their expression is influenced by systemic nutritional, metabolic, and hormonal signals.
Enzymatic defects in lipid metabolism can lead to significantly elevated blood lipid levels, a condition known as primary hypertriglyceridemia. Under normal physiological conditions, FFAs hydrolyzed from triglycerides bind to albumin, rendering them nontoxic. However, when serum triglyceride levels become excessively high, the binding capacity of albumin is exceeded, resulting in the accumulation of unbound FFAs[30]. These unbound FFAs are cytotoxic and contribute to AP pathogenesis in severe HTG. In particular, FFAs exert proinflammatory effects that damage pancreatic acinar cells and vascular endothelium. In an acidic microenvironment, FFAs can activate trypsinogen, initiating premature enzyme activation within the pancreas and triggering local inflammation[31]. This sets off a self-amplifying cascade involving the release of inflammatory mediators and ROS, which can lead to tissue edema, necrosis, and widespread pancreatic injury if unresolved[32]. In advanced stages, necrotizing pancreatitis can trigger SIRS, frequently leading to multiorgan dysfunction, protracted clinical courses, or mortality[33]. Although the exact pathophysiological mechanisms linking elevated serum triglycerides to the onset of AP remain incompletely elucidated, the prevailing theory, first articulated by Havel in 1969, proposes that in the context of pronounced HTG, excessive accumulation of large lipoprotein particles such as chylomicrons compromises capillary microcirculation, thereby exacerbating pancreatic injury[34]. When such events are localized to the pancreas, reduced perfusion leads to ischemia and creates an increasingly acidic microenvironment in the acinar tissue. Under these conditions, PNLIP leaks from damaged acinar cells and hydrolyzes surrounding triglycerides, resulting in FFA accumulation, which exacerbates pancreatic injury and inflammation. Notably, experimental models of HTG and AP have demonstrated that FFAs also enhance Protein Kinase C (PKC) activity, further contributing to the pathophysiological cascade[35]. High UFA concentrations can mimic the effects of CCK at supraphysiological levels by activating specific PKC isoforms, including PKC-α, -δ, and -ζ, suggesting that hyperlipidemic pancreatitis (HLP) may be partially mediated through UFA-induced PKC activation[5]. Cui et al[36] found that FFAs promote ER stress in pancreatic β cells by enhancing calcium influx, leading to β cell dysfunction and apoptosis. Similarly, in an arginine-induced experimental model of AP, ER stress signaling pathways were activated early in pancreatic acinar cells[37]. Moreover, hypertriglyceridemia exacerbates ER stress[38]. These findings highlight the need for continued research into the impact of elevated UFA concentrations on critical cellular pathways implicated in AP pathogenesis. Key processes warranting further investigation include calcium mobilization from acidic organelles, mitochondrial dysfunction, ER stress, dysregulated autophagy, impaired intracellular trafficking, and aberrant lysosomal and secretory responses. Notably, Navina et al[23] demonstrated that UFAs elevate intracellular calcium concentrations [ic (Ca2+)], inhibit mitochondrial complexes I and V, and induce necrosis in pancreatic acinar cells, contributing to tissue inflammation. In contrast, SFAs did not elicit these deleterious effects. UFAs have also been shown to directly influence pancreatic ductal cell function and ion channel protein expression. Although SFAs are less acutely cytotoxic, long-term exposure can affect pancreatic ductal cell plasticity, promoting their transdifferentiation into adipocytes or insulin-producing β-cells[39]. Prolonged lipotoxic stress from high concentrations of FAs can also stimulate insulin production and leptin release from pancreatic ductal cells, as observed in palmitate-treated rat ductal cell lines. Furthermore, HFD elevates serum triglyceride and FFA levels in experimental animals and alters pancreatic ductal function. Consistent with this notion, Huang et al[40] demonstrated that HFD can promote pancreatic ductal epithelial cell transdifferentiation into insulin-producing phenotypes.
Lipases constitute a family of enzymes responsible for the hydrolysis of triglycerides into FFAs and glycerol. These lipases are differentially expressed across various tissues: hepatic lipase in the liver, hormone-sensitive lipase in adipocytes, LPL on the vascular endothelium, and PNLIP in the small intestine[41]. PNLIP plays a critical role in dietary fat digestion and the absorption of fat-soluble vitamins[42]. During AP, PNLIP is released in an uncontrolled manner into the circulation and surrounding visceral AT. This results in FFA generation from both circulating triglycerides and damaged adipocytes due to the aberrant activity of PNLIP following acinar cell injury[43,44]. Excessive levels of FFAs, particularly those not bound to albumin, can exert direct lipotoxic effects by integrating into cellular membranes and interacting with other biomolecules, thereby promoting cell death and cytokine release[23,45]. Among FFAs, UFAs have been more strongly associated with organ failure in AP animal models compared to their saturated counterparts[44]. The higher reactivity of UFAs due to their double bonds may contribute to their greater cytotoxic potential. While some UFAs (e.g., ω-3 and ω-6) exhibit anti-inflammatory properties under physiological conditions, others have been implicated in exacerbating AP pathogenesis[46,47]. Clinical studies support this distinction. For instance, one investigation found that serum saturated:unsaturated FFA ratio was significantly lower in AP patients compared to healthy controls[17]. A separate study demonstrated that necrotic pancreatic collections contained elevated levels of both UFAs and SFAs. Notably, UFAs, particularly oleic and LA, induced acinar cell necrosis at concentrations less than half of those detected in the necrotic material, whereas SFAs failed to elicit necrosis even at twice those levels[44]. Furthermore, LA exhibited greater cytotoxicity than PA, as evidenced by increased lactate dehydrogenase release and impaired mitochondrial function in acinar cells[23]. Collectively, these findings indicate that while not all UFAs exert harmful effects, specific subtypes may play a pivotal role in FFA-mediated pancreatic injury during AP.
Obesity is an independent risk factor for increased severity in AP[48] Although the association between obesity and increased severity of AP is well recognized, the underlying mechanisms remain incompletely understood. Fat necrosis can arise through both enzymatic and non-enzymatic pathways[49]. While non-enzymatic fat necrosis is commonly associated with traumatic events such as direct physical injury, enzymatic fat necrosis is typically observed in AP following aberrant PNLIP activity[49]. Obesity leads to AT accumulation, especially visceral fat. Similarly, increased fat accumulation within the pancreas, referred to as fatty pancreas or pancreatic steatosis, has been frequently observed in individuals with obesity. However, our current understanding of the clinical implications and pathophysiological consequences of pancreatic fat deposition remains limited and largely speculative[50]. During AP, saponification of peripancreatic fat is a common pathological event. This process occurs because of adipocyte injury, leading to uncon
High SFA has been linked to enhanced lipid accumulation in both hepatic and visceral compartments, whereas PUFA-rich diets are linked to greater lean body mass accrual[55]. These findings underscore the significant impact of dietary fat composition on metabolic health. Nevertheless, the molecular mechanisms by which overfeeding and varying fatty acid profiles influence lipid accumulation and metabolism remain incompletely understood. Emerging evidence from both animal and human studies suggests that excessive fatty acid intake can modulate the epigenetic landscape of AT[56,57]. However, data from randomized, food-based intervention trials are scarce. To date, only a few studies have reported epigenetic changes in response to lipid supplementation in human blood samples, with limited insight into adipose-specific effects[58-61]. Despite inducing comparable weight gain, SFA overfeeding led to a greater increase in liver fat and visceral adiposity than PUFA overfeeding. These divergent metabolic outcomes may, in part, be explained by epigenetic modifications. Indeed, recent data indicate that both SFA and PUFA overfeeding can alter the epigenome of human AT, potentially contributing to the distinct metabolic phenotypes observed during in vivo dietary interventions. Interestingly, only SFA overfeeding has been reported to significantly alter gene expression in AT, including that of acyl-CoA oxidase 1[62].
AT harbors a significant immune cell population, which actively contribute to systemic inflammation by secreting cytokines and other inflammatory mediators into circulation. Among immune cell populations, two principal macrophage subsets serve as key inflammation modulators: classically activated M1 and alternatively activated M2 macrophages[63]. M1 polarization is typically driven by Th1-type cytokines, including interferon-γ and granulocyte-macrophage colony-stimulating factor, or by the recognition of pathogen-associated molecular patterns (PAMPs) like lipopolysaccharide (LPS). These proinflammatory macrophages secrete cytokines including tumor necrosis factor α (TNF-α), IL-1β, and IL-6[64]. In contrast, M2 macrophages are induced by Th2-type mediators, primarily IL-4, IL-10, and IL-13. They are associated with anti-inflammatory and tissue-repair functions. Their roles include facilitating efferocytosis, promoting regulatory T cell (Treg) differentiation, and secreting immunomodulatory cytokines such as IL-10 and transforming growth factor-β (TGF-β). An imbalance in M1/M2 polarization has been implicated in the development and persistence of various chronic inflammatory diseases. To explore the role of obesity in SAP, Han et al[65] established an HFD-induced obesity model in rats. Their study demonstrated a significant increase in serum non-esterified fatty acid levels and a marked shift toward M1 macrophage polarization within AT. These findings suggest that obesity exacerbates inflammatory responses in SAP by enhancing lipotoxicity and altering immune cell phenotypes.
Early recruitment of inflammatory monocytes into pancreatic tissue during AP is critically dependent on CCL2. Experimental studies in rodent models have shown that pharmacological inhibition of CCL2 or its receptors, including CCR2, ACKR1, and ACKR2, attenuates disease severity[66]. Upon infiltration, these monocytes differentiate into classically activated M1 macrophages, amplifying the inflammatory cascade. In contrast, pancreatic tissues from patients with chronic pancreatitis (CP) exhibit increased infiltration of alternatively activated M2 macrophages[67], which are implicated in fibrogenesis via pancreatic stellate cell activation. These findings highlight distinct macrophage polarization patterns in AP vs CP. Macrophages are central mediators of AT inflammation, and shifts in their polarization states are strongly associated with metabolic dysregulation, obesity, and insulin resistance. In both humans with obesity and HFD-induced obese mouse models, macrophage accumulation in AT is characterized by a shift from the anti-inflammatory M2 phenotype toward the pro-inflammatory M1 phenotype[68-70]. This phenotypic switch correlates with heightened AT inflammation and systemic insulin resistance[71]. Recent studies have identified interferon regulatory factor 3 (IRF3) as a pivotal transcriptional regulator in HFD-induced inflammation. Kiran et al[72] demonstrated that IRF3, together with TGF-β1 and STAT3 signaling pathways, downregulates peroxisome proliferator-activated receptor gamma (PPARγ) expression in AT, thereby sustaining chronic low-grade inflammation. Additionally, enhanced infiltration of Th17 cells and dendritic cells within AT contributes to M1 macrophage polarization and disrupts the Th17/Treg equilibrium, further propagating metabolic inflammation. Decreased PPARγ expression in obesity may exacerbate this immune imbalance by impairing Th17/Treg homeostasis[73]. PPARγ, a ligand-activated nuclear receptor, plays an essential role in regulating lipid metabolism and inflammatory responses[74]. It is highly expressed in both pancreatic acinar cell lines
Toll-like receptors (TLRs) are type I transmembrane proteins that play a pivotal role in innate immune response initiation and propagation during inflammation[81]. Among pattern recognition receptors, TLR4 detects a broad spectrum of exogenous ligands and transduces extracellular danger signals into intracellular proinflammatory responses[82]. It is widely expressed in immune cells, including monocytes, macrophages, and Kupffer cells, as well as in non-immune cells such as intestinal epithelial and endothelial cells, adipocytes, hepatocytes, and pancreatic acinar cells[83]. One of the most well-characterized ligands of TLR-4 is LPS, which interacts with the LPS-binding protein and its co-receptor, cluster of differentiation 14, to activate TLR4. This ligand-receptor complex triggers a downstream signaling cascade involving focal adhesion kinase activation, facilitating the recruitment of myeloid differentiation primary response gene 88 and interleukin (IL)-1 receptor-associated kinase 4. These events culminate in the NF-κB and MAPK pathway activation[84-88], leading to proinflammatory gene upregulation, including TNF-α, IL-6, iNOS, and MCP-1[89]. A secondary activation signal promotes the assembly of inflammasome complexes (e.g., NLRP3), enabling the cleavage of pro-cytokines into their mature, active forms. This dual-signal mechanism underscores the central role of innate immune activation in amplifying pancreatic inflammation.
In individuals with genetic susceptibility, recurrent pancreatic injury promotes the release of damage-associated molecular patterns (DAMPs), including fibronectin extra domain A (fibronectin-EDA) and tenascin-C, which further amplify innate immune activation. These endogenous molecules act as danger signals and are sensed by TLR4 expressed on resident immune and stromal cells. TLR4 activation not only amplifies fibrogenic gene expression and promotes myofibroblast differentiation but also enhances fibroblast sensitivity to transforming TGF-β, thereby contributing to tissue remodeling and fibrosis. Moreover, TLRs function as key sensors of both DAMPs and PAMPs within pancreatic cells. Upon activation, TLRs initiate canonical inflammatory signaling pathways, most notably the nuclear NF-κB cascade, culminating in the transcriptional induction of proinflammatory cytokine precursors, including pro-IL1β and pro-IL18[90]. Interestingly, in the context of AP, TLR4 is highly expressed in pancreatic tissue. Experimental models have shown that genetic deletion or pharmacological inhibition of TLR4 reduces acinar cell necrosis and attenuates disease severity[91,92]. It is also implicated in regulating chemokine production, neutrophil infiltration, and tissue injury in SAP[93]. Although AP may be initially triggered by intra-acinar events, such as premature trypsinogen activation, its progression and severity are predominantly influenced by subsequent inflammatory and immunometabolism responses. These processes are driven by innate immune pathways, including TLR-mediated signaling and inflammasome activation, underscoring their potential as key therapeutic targets.
In addition to its proinflammatory role, TLR4 has been increasingly recognized as a trigger of necroptosis, a regulated form of necrotic cell death characterized by the activation of receptor-interacting protein kinase 1 (RIP1), RIP3, and mixed lineage kinase domain-like pseudokinase[94]. Unlike apoptosis, necroptosis elicits a robust inflammatory response due to intracellular DAMP release. In models of severe experimental pancreatitis, necroptosis is the dominant mode of acinar cell death[95]. Notably, an inverse correlation between RIP1 and RIP3 expression levels have been reported in AP mouse models[96]. Emerging evidence further implicates TLR4-mediated necroptosis in the pathogenesis of inflammatory diseases, including AP[97,98].
Recent research suggests that SFAs can act as endogenous TLR4 ligands, thereby promoting inflammatory responses. Lipid A, a component of LPS, contains saturated acyl chains that are essential for TLR4 activation, supporting the notion that saturated lipid moieties can engage this receptor[99,100]. Besides LPS, several endogenous molecules-such as heat shock proteins (Hsp60, Hsp70), the type III repeat extra domain A of fibronectin, hyaluronic acid fragments, heparan sulfate polysaccharides, and fibrinogen-have been identified as TLR4 ligands[101]. In 2001, Lee et al[102] demonstrated that SFAs directly induced inflammatory gene expression via TLR4 signaling in vitro. Notably, fibrinogen, an acute-phase reactant elevated during obesity, can activate TLR4, thereby amplifying systemic inflammation[103]. Consistent with these findings, Kim et al[104] showed that TLR4-deficient mice failed to mount a proinflammatory cytokine response upon HFD exposure, highlighting the centrality of TLR4 in mediating HFD-induced inflammation. Furthermore, a proposed feedback loop suggests that RIP3-dependent necroptosis augments TLR4-driven inflammation through the induction of cytokines such as TNF-α via NF-κB signaling.
Among SFAs, lauric acid (C12:0) has the highest potential to activate COX-2 expression through NF-κB in macrophage cell lines, followed by PA (C16:0) and SA (C18:0)[102]. Palmitate activation of TLR4 was associated with increased JNK and IKK-β phosphorylation and elevated proinflammatory cytokine secretion[105]. In contrast, neither monounsaturated nor PUFAs activated TLR4. Interestingly, pretreatment with ω-3 PUFA docosahexaenoic acid (DHA; C22:6, ω-3) or OA (ω-9) significantly attenuated the lauric acid-induced inflammatory response in vitro[102]. PUFAs, particularly eicosapentaenoic acid (EPA; C20:5, ω-3) and DHA, modulate inflammation by acting as precursors for anti-inflammatory eicosanoids and by directly suppressing inflammatory gene expression in macrophages, including COX-2, iNOS, and IL-1β[102,106]. These ω-3 PUFAs also inhibit the TLR4-induced NF-κB pathway[102,107,108]. Mechanistically, EPA and DHA bind to PPARs, notably PPAR-α, PPAR-γ, and PPAR-β/δ, which heterodimerize with retinoid X receptor and interact with peroxisome proliferator response elements in the promoter regions of lipid metabolism and anti-inflammatory genes[109]. Moreover, EPA and DHA inhibit NADPH oxidase activity, thereby reducing ROS production, which is essential for TLR4 recruitment into lipid rafts and subsequent dimerization and activation[110]. Incorporation of DHA into the plasma membrane can further disrupt lipid raft formation, reducing TLR4 surface localization and downstream NF-κB activation[110-112]. These findings underscore the differential immunomodulatory roles of SFAs and PUFAs, with implications for dietary interventions in inflammatory diseases such as obesity-related AP.
HFD-induced dysbiosis is linked to a range of molecular abnormalities, with disruption of gut barrier function being particularly critical. Like the airways and genitourinary tract, the gastrointestinal tract serves as a semipermeable barrier, facilitating nutrient absorption while preventing the translocation of harmful antigens and microorganisms from the gut lumen. Intestinal barrier integrity is preserved by a complex system involving the gut microbiota, a protective mucus layer, the epithelium, and immune cells within the lamina propria and submucosa[113]. The process of intestinal fatty acid absorption involves two distinct steps: (1) Intraluminal fatty acid entry into intestinal absorptive epithelial cells (uptake); and (2) The transfer of absorbed fatty acids into the lymphatic circulation, specifically the thoracic duct (absorption)[114]. In experimental models, intraduodenal infusion of emulsified fatty acids into rats, consisting of 14C-labeled PA or LA mixed with monoolein and taurocholate, demonstrated significant differences in absorption efficiency. PA consistently required a longer intestinal length for complete absorption than LA[115]. Furthermore, although mucosal uptake of both fatty acids was similar in short-term settings, palmitate esterification was significantly less efficient than that of linoleate. These findings suggest that saturated fats exert localized effects on the intestinal mucosa before being systemically metabolized by peripheral organs such as AT, liver, or skeletal muscle. Therefore, early exposure of the gut barrier to SFAs may initiate a cascade of barrier dysfunction, immune activation, and dysbiosis, contributing to systemic inflammation and metabolic impairment.
Excessive SFA consumption is associated with adverse health effects[116]. Even short-term overconsumption of SFAs can trigger low-grade inflammation and initiate intracellular damage, laying the groundwork for obesity and its associated comorbidities[117-119]. Emerging evidence indicates that disruption of the gut-pancreas axis plays a central role in SAP pathogenesis. For instance, Li et al[120] demonstrated that HFD induces dysbiosis characterized by the overgrowth of pathogenic genera such as Escherichia-Shigella, Enterococcus, and Klebsiella, which significantly exacerbates pancreatic injury. In contrast, certain UFAs have anti-inflammatory potential through the activation of specific G-protein-coupled receptors. GPR120 (also known as the ω-3 fatty acid receptor) and GPR40 are selectively activated by long-chain fatty acids, predominantly ω-3 and ω-9 species like DHA and OA, respectively[121]. Both receptors signal through the Gq protein family, leading to increased concentrations of intracellular calcium. GPR40 is predominantly expressed in pancreatic β-cells, where its activation by long-chain fatty acids enhances glucose-stimulated insulin secretion[122]. Meanwhile, GPR120 is expressed in AT and, when activated by ω-3 fatty acids, suppresses inflammation in macrophages and promotes adipocyte differentiation. GPR120 dysfunction has been associated with obesity, hepatic steatosis, and insulin resistance. The early detrimental effects of a HFD on intestinal permeability may act as initial obesogenic triggers. Diets enriched with ω-3 fatty acids have shown modest local protective effects on the intestinal barrier. Supporting this, Rodrigues et al[123] suggest that reducing SFA intake or substituting them with ω-3 fatty acids may be effective in preserving intestinal barrier function and mitigating inflammatory responses.
Diabetes of the exocrine pancreas (DEP), also referred to as type 3c diabetes mellitus (T3cDM), is a secondary form of diabetes that arises from underlying pancreatic disorders, including acute and CP. Despite its clinical distinctiveness, DEP is frequently underdiagnosed and commonly misclassified as type 2 diabetes mellitus (T2DM)[124]. Notably, HFD-induced AP contributes to the development of metabolic syndrome, a constellation of metabolic disturbances including insulin resistance, dyslipidemia, hyperglycemia, hepatic steatosis, and hypertension. This syndrome increases T2DM risk, cardiovascular disease, nonalcoholic fatty liver disease, and certain malignancies. Environmental and genetic factors, such as overnutrition, enhanced lipolysis, and adipocyte hypertrophy, drive metabolic syndrome progression, with insulin resistance as the central pathological feature. Following HFD-induced AP, patients may first develop HFD-driven diabetes, evolving into DEP. Diabetes mellitus itself exacerbates HTG and gallstone formation, both of which are risk factors for AP. Li et al[125] identified diabetes mellitus as an independent risk factor for HTG-AP, highlighting the bidirectional relationship between metabolic dysregulation and pancreatic inflammation. However, the specific subtype of diabetes following AP is often unclear, and DEP warrants specific attention in this context. In T2DM, hyperglycemia and elevated plasma FFAs, particularly SFAs such as PA, are key contributors to β-cell lipotoxicity and insulin resistance[126-128]. SFAs impair insulin secretion and promote β-cell apoptosis through oxidative stress, ER stress, and proinflammatory signaling pathways. In contrast, PUFAs, particularly ω-3 PUFAs, exhibit protective effects on pancreatic β-cells. In transgenic mfat-1 mice, which endogenously convert ω-6 to ω-3 PUFAs, increased ω-3 PUFA levels were associated with improved insulin secretion, enhanced expression of key β-cell genes (e.g., PDX-1, glucokinase, insulin-1), reduced PGE2 levels, and suppression of proinflammatory pathways such as NF-κB and ERK1/2[129]. ω-3 PUFAs also improve β-cell survival by modulating membrane lipid rafts and binding to nuclear receptors such as PPARs, GPR40 and GPR120. However, the effects of different types of UFAs are nuanced. While ω-3 PUFAs generally confer anti-inflammatory and antioxidant effects, some studies suggest that high ω-6 PUFA levels, particularly those with cis-configured double bonds, can induce significant lipid peroxidation and ferroptosis in β-cells[130]. On the other hand, long-chain SFAs primarily trigger apoptosis but can also induce ferroptosis when combined with high PUFA levels. Interestingly, MUFAs such as OA appear to mitigate oxidative stress and prevent ferroptotic β-cell death. Therefore, the dietary balance of fatty acid subtypes plays a pivotal role in β-cell health and diabetes pathogenesis. Avoiding high dietary intake of ω-6 PUFAs and excessive SFAs, while favoring MUFAs and ω-3 PUFAs, may reduce the risk of β-cell dysfunction and progression to T3cDM in individuals with AP or metabolic syndrome. Nonetheless, due to inconsistent findings in human studies, further large-scale, prospective investigations are necessary to delineate the specific mechanisms and therapeutic potential of ω-3 PUFA supplementation in diabetes prevention and management.
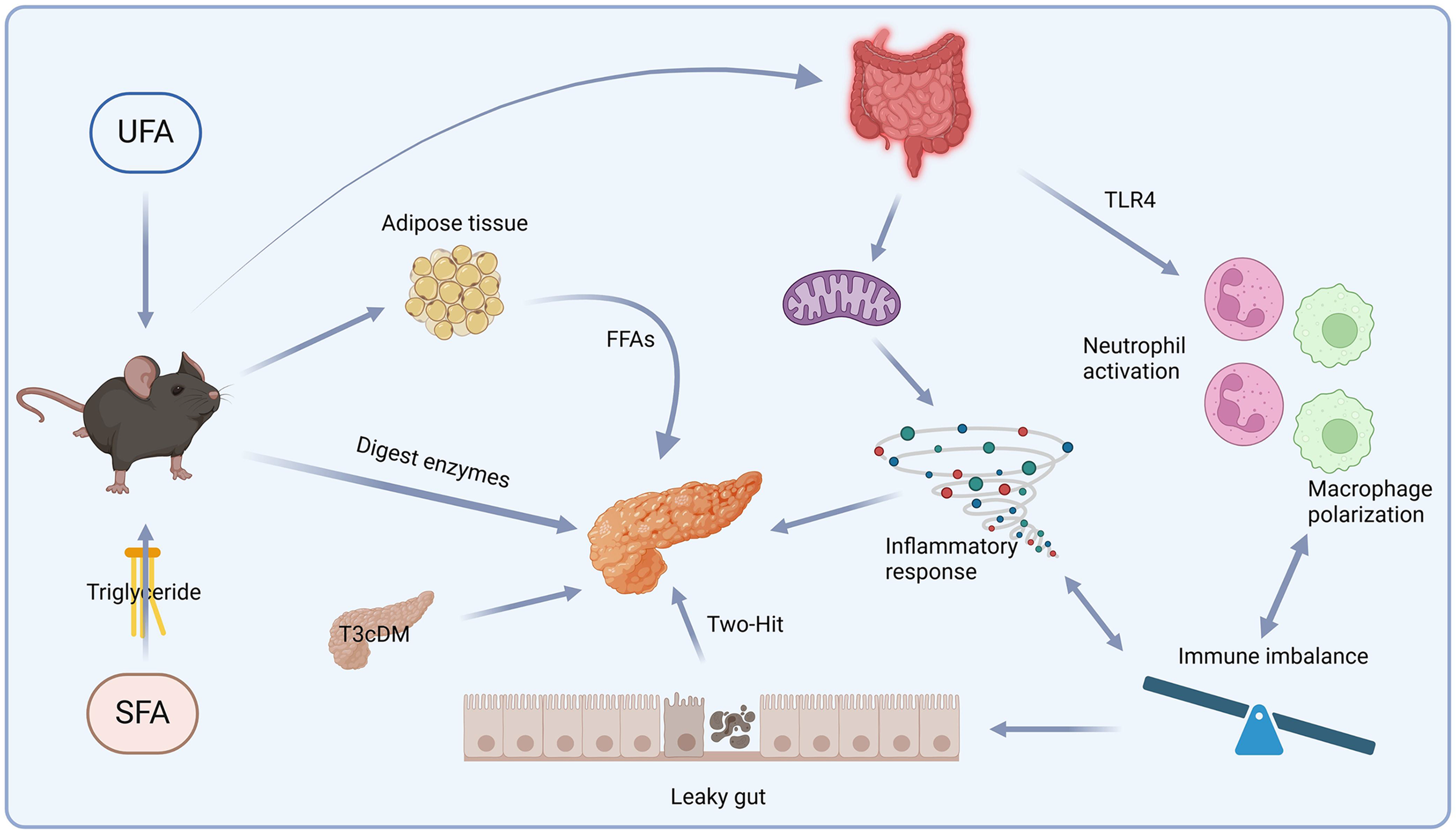
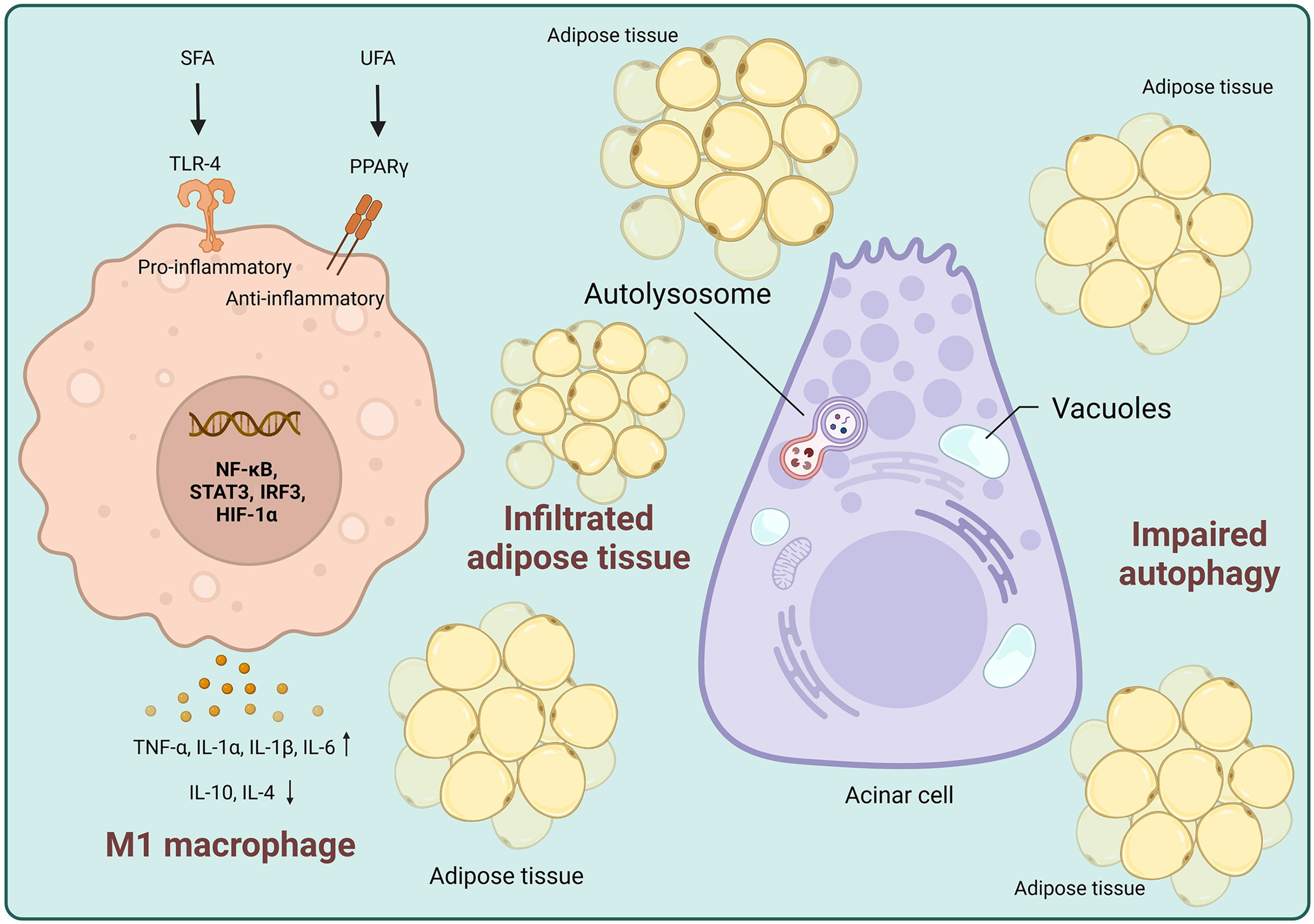
AP is a complex disease varying in severity and course. The different effects of HFD-induced fatty acids on AP depend on the components and substrates involved. Each stage of SAP has its own distinct mechanism. In the case of HTGP, the first step involves hypertriglyceridemia, where a high SFA diet accelerates PNLIP production, and subsequently degrades AT into triglycerides and FFAs. These FFAs directly contribute to acinar cell damage. Additionally, the acceleration of chylomicron emulsion increases pressure on the pancreatic ducts, while degradation of AT leads to necrosis and inflammation (Figure 1). The second step involves autophagy, which acts as a protective mechanism to eliminate cytokines and chemokines causing damage. However, excessive or selective blocking of autophagy promotes pro-inflammatory cytokine release. SFAs directly interact with TLR4 to stimulate an immune response, which leads to mitochondrial dysfunction, ER stress, and ROS generation, all of which promote M1 macrophage polarization (Figure 2). The third step involves increased gut permeability, which leads to elevated levels of LPS and UFAs in the bloodstream and further potentiates cytokine release. HFD-induced damage to the liver and pancreas impairs gluconeogenesis and leads to insulin resistance, contributing to acinar cell damage in the pancreas. UFAs play a critical role in AP, potentially linking obesity to poorer AP outcomes. This concept is further supported by findings from Noel et al[44], who reported elevated UFA levels in necrotic fluid collections from obese patients with AP. UFAs induce acinar cell necrosis and multisystem organ failure through pathways independent of pancreatic necrosis. These observations underscore the importance of eluci
We thank Justin Clark, PhD for helping with revising the manuscript.
| 1. | Hong YP, Chen C, Guo WY, Zhao L, Mei FC, Xiang MW, Wang WX. Effects of Castanospermine on Inflammatory Response in a Rat Model of Experimental Severe Acute Pancreatitis. Arch Med Res. 2016;47:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 4. | Phillips AE, Wilson AS, Greer PJ, Hinton A, Culp S, Paragomi P, Pothoulakis I, Singh V, Lee PJ, Lahooti I, Whitcomb DC, Papachristou GI. Relationship of circulating levels of long-chain fatty acids to persistent organ failure in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2023;325:G279-G285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 5. | Chang YT, Chang MC, Tung CC, Wei SC, Wong JM. Distinctive roles of unsaturated and saturated fatty acids in hyperlipidemic pancreatitis. World J Gastroenterol. 2015;21:9534-9543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 6. | Pereda J, Pérez S, Escobar J, Arduini A, Asensi M, Serviddio G, Sabater L, Aparisi L, Sastre J. Obese rats exhibit high levels of fat necrosis and isoprostanes in taurocholate-induced acute pancreatitis. PLoS One. 2012;7:e44383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Henderson GC. Plasma Free Fatty Acid Concentration as a Modifiable Risk Factor for Metabolic Disease. Nutrients. 2021;13:2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Dong Z, Shang H, Chen YQ, Pan LL, Bhatia M, Sun J. Sulforaphane Protects Pancreatic Acinar Cell Injury by Modulating Nrf2-Mediated Oxidative Stress and NLRP3 Inflammatory Pathway. Oxid Med Cell Longev. 2016;2016:7864150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, Denman S, Begun J, Florin TH, Perkins A, Cuív PÓ, McGuckin MA, Hasnain SZ. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci Rep. 2016;6:28990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1537] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 11. | Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2323] [Article Influence: 92.9] [Reference Citation Analysis (1)] |
| 13. | Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 14. | Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 428] [Article Influence: 23.8] [Reference Citation Analysis (2)] |
| 15. | Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917-33925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 331] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 16. | Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 17. | Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 18. | Mateu A, Ramudo L, Manso MA, Closa D, De Dios I. Acinar inflammatory response to lipid derivatives generated in necrotic fat during acute pancreatitis. Biochim Biophys Acta. 2014;1842:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Morita Y, Yoshikawa T, Takeda S, Matsuyama K, Takahashi S, Yoshida N, Clemens MG, Kondo M. Involvement of lipid peroxidation in free fatty acid-induced isolated rat pancreatic acinar cell injury. Pancreas. 1998;17:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 20. | Volpe CM, Nogueira-Machado JA. The dual role of free fatty acid signaling in inflammation and therapeutics. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 21. | Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Kobayasi R, Akamine EH, Davel AP, Rodrigues MA, Carvalho CR, Rossoni LV. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J Hypertens. 2010;28:2111-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (2)] |
| 24. | Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S-1519S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1674] [Cited by in RCA: 1645] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 25. | Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Fang IM, Yang CH, Yang CM, Chen MS. Comparative effects of fatty acids on proinflammatory gene cyclooxygenase 2 and inducible nitric oxide synthase expression in retinal pigment epithelial cells. Mol Nutr Food Res. 2009;53:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Mateu A, De Dios I, Manso MA, Ramudo L. Unsaturated but not saturated fatty acids induce transcriptional regulation of CCL2 in pancreatic acini. A potential role in acute pancreatitis. Biochim Biophys Acta. 2015;1852:2671-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Pothoulakis I, Paragomi P, Archibugi L, Tuft M, Talukdar R, Kochhar R, Goenka MK, Gulla A, Singh VK, Gonzalez JA, Ferreira M, Barbu ST, Stevens T, Nawaz H, Gutierrez SC, Zarnescu NO, Easler J, Triantafyllou K, Pelaez-Luna M, Thakkar S, Ocampo C, de-Madaria E, Wu BU, Cote GA, Tang G, Papachristou GI, Capurso G. Clinical features of hypertriglyceridemia-induced acute pancreatitis in an international, multicenter, prospective cohort (APPRENTICE consortium). Pancreatology. 2020;20:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 29. | Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis. 2017;267:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Penn AH, Schmid-Schönbein GW. The intestine as source of cytotoxic mediators in shock: free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294:H1779-H1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Qiu M, Zhou X, Zippi M, Goyal H, Basharat Z, Jagielski M, Hong W. Comprehensive review on the pathogenesis of hypertriglyceridaemia-associated acute pancreatitis. Ann Med. 2023;55:2265939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 32. | Gan SI, Edwards AL, Symonds CJ, Beck PL. Hypertriglyceridemia-induced pancreatitis: A case-based review. World J Gastroenterol. 2006;12:7197-7202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Lams E. Pancreatitis and hypertriglyceridaemia. Eur J Anaesthesiol. 2006;23:1067-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 34. | Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (2)] |
| 35. | Wang YJ, Sun JB, Li F, Zhang SW. Hyperlipidemia intensifies cerulein-induced acute pancreatitis associated with activation of protein kinase C in rats. World J Gastroenterol. 2006;12:2908-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Cui W, Ma J, Wang X, Yang W, Zhang J, Ji Q. Free fatty acid induces endoplasmic reticulum stress and apoptosis of β-cells by Ca2+/calpain-2 pathways. PLoS One. 2013;8:e59921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238-G245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Zeng Y, Wang X, Zhang W, Wu K, Ma J. Hypertriglyceridemia aggravates ER stress and pathogenesis of acute pancreatitis. Hepatogastroenterology. 2012;59:2318-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 39. | Gezginci-Oktayoglu S, Sancar S, Karatug-Kacar A, Bolkent S. miR-375 induces adipogenesis through targeting Erk1 in pancreatic duct cells under the influence of sodium palmitate. J Cell Physiol. 2021;236:3881-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 40. | Huang W, Su L, Zhang X, Xu X, Li R. Endocrinological characterization of pancreatic ducts in HFD and HGD fed mice. J Cell Biochem. 2019;120:16153-16159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Pirahanchi Y, Sharma S. Biochemistry, Lipase. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 42. | Holmes RS, Cox LA, VandeBerg JL. Comparative studies of mammalian acid lipases: Evidence for a new gene family in mouse and rat (Lipo). Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:217-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 43. | de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, Patel KS, Guerra AA, Martinez MN, Trivedi S, McCullough A, Lam-Himlin DM, Navina S, Faigel DO, Fukami N, Pannala R, Phillips AE, Papachristou GI, Kershaw EE, Lowe ME, Singh VP. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest. 2020;130:1931-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Noel P, Patel K, Durgampudi C, Trivedi RN, de Oliveira C, Crowell MD, Pannala R, Lee K, Brand R, Chennat J, Slivka A, Papachristou GI, Khalid A, Whitcomb DC, DeLany JP, Cline RA, Acharya C, Jaligama D, Murad FM, Yadav D, Navina S, Singh VP. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016;65:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Durgampudi C, Noel P, Patel K, Cline R, Trivedi RN, DeLany JP, Yadav D, Papachristou GI, Lee K, Acharya C, Jaligama D, Navina S, Murad F, Singh VP. Acute lipotoxicity regulates severity of biliary acute pancreatitis without affecting its initiation. Am J Pathol. 2014;184:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Shahidi F, Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol. 2018;9:345-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 721] [Article Influence: 120.2] [Reference Citation Analysis (1)] |
| 47. | D'Angelo S, Motti ML, Meccariello R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients. 2020;12:2751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 48. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 49. | Russo AL, Taghian AG. Fat necrosis of the breast in the accelerated partial breast irradiation era: the need for a universal grading system. Breast Cancer Res Treat. 2013;140:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Majumder S, Philip NA, Takahashi N, Levy MJ, Singh VP, Chari ST. Fatty Pancreas: Should We Be Concerned? Pancreas. 2017;46:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Aloysius TA, Carvajal AK, Slizyte R, Skorve J, Berge RK, Bjørndal B. Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines (Basel). 2018;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, Petersen BT, Baron TH, Farnell MB, Sarr MG. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include "moderately severe acute pancreatitis". Am J Gastroenterol. 2009;104:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond). 2010;34:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 54. | Matsuda A, Makino N, Tozawa T, Shirahata N, Honda T, Ikeda Y, Sato H, Ito M, Kakizaki Y, Akamatsu M, Ueno Y, Kawata S. Pancreatic fat accumulation, fibrosis, and acinar cell injury in the Zucker diabetic fatty rat fed a chronic high-fat diet. Pancreas. 2014;43:735-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, Risérus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 322] [Article Influence: 29.3] [Reference Citation Analysis (3)] |
| 56. | Burdge GC, Lillycrop KA. Fatty acids and epigenetics. Curr Opin Clin Nutr Metab Care. 2014;17:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Gillberg L, Perfilyev A, Brøns C, Thomasen M, Grunnet LG, Volkov P, Rosqvist F, Iggman D, Dahlman I, Risérus U, Rönn T, Nilsson E, Vaag A, Ling C. Adipose tissue transcriptomics and epigenomics in low birthweight men and controls: role of high-fat overfeeding. Diabetologia. 2016;59:799-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 58. | Ma Y, Smith CE, Lai CQ, Irvin MR, Parnell LD, Lee YC, Pham LD, Aslibekyan S, Claas SA, Tsai MY, Borecki IB, Kabagambe EK, Ordovás JM, Absher DM, Arnett DK. The effects of omega-3 polyunsaturated fatty acids and genetic variants on methylation levels of the interleukin-6 gene promoter. Mol Nutr Food Res. 2016;60:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Hoile SP, Clarke-Harris R, Huang RC, Calder PC, Mori TA, Beilin LJ, Lillycrop KA, Burdge GC. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS One. 2014;9:e109896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Voisin S, Almén MS, Moschonis G, Chrousos GP, Manios Y, Schiöth HB. Dietary fat quality impacts genome-wide DNA methylation patterns in a cross-sectional study of Greek preadolescents. Eur J Hum Genet. 2015;23:654-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Hermsdorff HH, Mansego ML, Campión J, Milagro FI, Zulet MA, Martínez JA. TNF-alpha promoter methylation in peripheral white blood cells: relationship with circulating TNFα, truncal fat and n-6 PUFA intake in young women. Cytokine. 2013;64:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Perfilyev A, Dahlman I, Gillberg L, Rosqvist F, Iggman D, Volkov P, Nilsson E, Risérus U, Ling C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am J Clin Nutr. 2017;105:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 63. | Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, Striz I. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). 2023;137:1067-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 224] [Reference Citation Analysis (0)] |
| 64. | Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol. 2015;6:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (1)] |
| 65. | Han H, Zhang L, Fu Q, Zhang B, Chen J. Plasma Exosomes Aggravate Acute Pancreatitis by Promoting M1 Polarization of Adipose Tissue Macrophages in Obesity-Related Severe Acute Pancreatitis. Dig Dis Sci. 2023;68:3660-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 66. | Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology. 2017;153:1212-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 67. | Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2202] [Cited by in RCA: 2088] [Article Influence: 130.5] [Reference Citation Analysis (1)] |
| 68. | Liang W, Qi Y, Yi H, Mao C, Meng Q, Wang H, Zheng C. The Roles of Adipose Tissue Macrophages in Human Disease. Front Immunol. 2022;13:908749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Crook KR, Liu P. Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol. 2014;4:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 70. | Kontaki E, Boumpas DT, Tzardi M, Mouzas IA, Papadakis KA, Verginis P. Aberrant function of myeloid-derived suppressor cells (MDSCs) in experimental colitis and in inflammatory bowel disease (IBD) immune responses. Autoimmunity. 2017;50:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 71. | Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591-23599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 72. | Kiran S, Rakib A, Kodidela S, Kumar S, Singh UP. High-Fat Diet-Induced Dysregulation of Immune Cells Correlates with Macrophage Phenotypes and Chronic Inflammation in Adipose Tissue. Cells. 2022;11:1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 73. | Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 74. | Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 75. | Masamune A, Satoh K, Sakai Y, Yoshida M, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma induce apoptosis in AR42J cells. Pancreas. 2002;24:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Xu P, Lou XL, Chen C, Yang ZW. Effects of peroxisome proliferator-activated receptor-γ activation on apoptosis in rats with acute pancreatitis. Dig Dis Sci. 2013;58:3516-3523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 78. | Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Britti D, Patel NS, Di Paola R, Genovese T, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute pancreatitis induced by cerulein. Intensive Care Med. 2004;30:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Xu P, Xu K, Wang J, Jiang JP, Chen LQ. Pioglitazone: a promising therapeutic tool in sodium taurocholate-induced severe acute pancreatitis. Dig Dis Sci. 2011;56:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | He X, Jia H, Jing Z, Liu D. Recognition of pathogen-associated nucleic acids by endosomal nucleic acid-sensing toll-like receptors. Acta Biochim Biophys Sin (Shanghai). 2013;45:241-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol. 2022;13:812774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 453] [Article Influence: 151.0] [Reference Citation Analysis (1)] |
| 83. | Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78:1233-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 840] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 84. | Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 508] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 85. | Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol. 2015;195:4999-5010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 86. | Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 87. | Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 88. | Elena G, Giovanna D, Brunella P, De Anna F, Alessandro M, Antonietta TM. Proinflammatory signal transduction pathway induced by Shigella flexneri porins in caco-2 cells. Braz J Microbiol. 2009;40:701-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 89. | Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 90. | Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4683] [Article Influence: 203.6] [Reference Citation Analysis (0)] |
| 91. | Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Wu J, Ma X, Chen W, Yang N, Gao L, Mao W, Yang J, Yang Q, Dong J, Tong Z, Li B, Lu G, Li W. Protective effects of HTD4010, a Reg3α/PAP-derived peptide, in mouse model of acute pancreatitis via toll-like receptor 4 pathway. Biochem Biophys Res Commun. 2019;512:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Awla D, Abdulla A, Regnér S, Thorlacius H. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflamm Res. 2011;60:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4:e128834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 95. | Louhimo J, Steer ML, Perides G. Necroptosis Is an Important Severity Determinant and Potential Therapeutic Target in Experimental Severe Pancreatitis. Cell Mol Gastroenterol Hepatol. 2016;2:519-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 96. | Wu J, Mulatibieke T, Ni J, Han X, Li B, Zeng Y, Wan R, Wang X, Hu G. Dichotomy between Receptor-Interacting Protein 1- and Receptor-Interacting Protein 3-Mediated Necroptosis in Experimental Pancreatitis. Am J Pathol. 2017;187:1035-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Lim J, Park H, Heisler J, Maculins T, Roose-Girma M, Xu M, Mckenzie B, van Lookeren Campagne M, Newton K, Murthy A. Autophagy regulates inflammatory programmed cell death via turnover of RHIM-domain proteins. Elife. 2019;8:e44452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 98. | Cao M, Chen F, Xie N, Cao MY, Chen P, Lou Q, Zhao Y, He C, Zhang S, Song X, Sun Y, Zhu W, Mou L, Luan S, Gao H. c-Jun N-terminal kinases differentially regulate TNF- and TLRs-mediated necroptosis through their kinase-dependent and -independent activities. Cell Death Dis. 2018;9:1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 99. | Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 447] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 100. | Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (1)] |
| 101. | Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 493] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 102. | Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683-16689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 951] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 103. | Al-ofi E, Coffelt SB, Anumba DO. Fibrinogen, an endogenous ligand of Toll-like receptor 4, activates monocytes in pre-eclamptic patients. J Reprod Immunol. 2014;103:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 833] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 105. | Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 426] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 106. | Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 415] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 107. | Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev. 2004;62:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 108. | Stryjecki C, Mutch DM. Fatty acid-gene interactions, adipokines and obesity. Eur J Clin Nutr. 2011;65:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Li AC, Glass CK. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 2004;45:2161-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 110. | Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. 2016;785:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 111. | Meital LT, Sandow SL, Calder PC, Russell FD. Abdominal aortic aneurysm and omega-3 polyunsaturated fatty acids: Mechanisms, animal models, and potential treatment. Prostaglandins Leukot Essent Fatty Acids. 2017;118:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Puglisi MJ, Hasty AH, Saraswathi V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J Nutr Biochem. 2011;22:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Di Sabatino A, Santacroce G, Rossi CM, Broglio G, Lenti MV. Role of mucosal immunity and epithelial-vascular barrier in modulating gut homeostasis. Intern Emerg Med. 2023;18:1635-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 114. | Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013;43:1203-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 115. | Ockner RK, Pittman JP, Yager JL. Differences in the intestinal absorption of saturated and unsaturated long chain fatty acids. Gastroenterology. 1972;62:981-992. [PubMed] |
| 116. | Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr Atheroscler Rep. 2010;12:384-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 117. | Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1413] [Article Influence: 100.9] [Reference Citation Analysis (1)] |
| 118. | Da Silva AS, Pauli JR, Ropelle ER, Oliveira AG, Cintra DE, De Souza CT, Velloso LA, Carvalheira JB, Saad MJ. Exercise intensity, inflammatory signaling, and insulin resistance in obese rats. Med Sci Sports Exerc. 2010;42:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 119. | Nakandakari SCBR, Muñoz VR, Kuga GK, Gaspar RC, Sant'Ana MR, Pavan ICB, da Silva LGS, Morelli AP, Simabuco FM, da Silva ASR, de Moura LP, Ropelle ER, Cintra DE, Pauli JR. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav Immun. 2019;79:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 120. | Li X, Zheng P, Zou Y, Guan L, Li N, Liu J, Lu N, Zhu Y, He C. Dietary inulin ameliorates obesity-induced severe acute pancreatitis via gut-pancreas axis. Gut Microbes. 2024;16:2436949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 121. | Oliveira V, Marinho R, Vitorino D, Santos GA, Moraes JC, Dragano N, Sartori-Cintra A, Pereira L, Catharino RR, da Silva AS, Ropelle ER, Pauli JR, De Souza CT, Velloso LA, Cintra DE. Diets Containing α-Linolenic (ω3) or Oleic (ω9) Fatty Acids Rescues Obese Mice From Insulin Resistance. Endocrinology. 2015;156:4033-4046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008;57:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 123. | Rodrigues PB, Dátilo MN, Sant'Ana MR, Nogueira GADS, Marin RM, Nakandakari SCBR, de Moura LP, da Silva ASR, Ropelle ER, Pauli JR, Cintra DE. The early impact of diets enriched with saturated and unsaturated fatty acids on intestinal inflammation and tight junctions. J Nutr Biochem. 2023;119:109410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 125. | Li Q, Hou C, Peng Y, Zhu X, Shi C, Zhang K, Tu M, Guo F, Huang D, Miao Y. Diabetes and Younger Age Are Vital and Independent Risk Factors for Acute Pancreatitis in Patients with Severe Hypertriglyceridemia. Biomed Res Int. 2019;2019:2620750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 126. | I S Sobczak A, A Blindauer C, J Stewart A. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients. 2019;11:2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 127. | Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front Endocrinol (Lausanne). 2018;9:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 128. | Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 129. | Wei D, Li J, Shen M, Jia W, Chen N, Chen T, Su D, Tian H, Zheng S, Dai Y, Zhao A. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes. 2010;59:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 130. | Lobato TB, Santos ESS, Iser-Bem PN, Falcão HS, Gimenes GM, Pauferro JRB, Rodrigues GT, Correa IS, Pereira ACG, Passos MEP, Borges JCO, Alves ACDA, Santos CSD, Araújo MJL, Diniz VLS, Levada-Pires AC, Pithon-Curi TC, Masi LN, Curi R, Hirabara SM, Gorjão R. Omega-3 Fatty Acids Weaken Lymphocyte Inflammatory Features and Improve Glycemic Control in Nonobese Diabetic Goto-Kakizaki Rats. Nutrients. 2024;16:4106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |