Published online Jan 14, 2025. doi: 10.3748/wjg.v31.i2.100234
Revised: September 28, 2024
Accepted: October 25, 2024
Published online: January 14, 2025
Processing time: 129 Days and 17.6 Hours
Rebleeding after recovery from esophagogastric variceal bleeding (EGVB) is a severe complication that is associated with high rates of both incidence and mortality. Despite its clinical importance, recognized prognostic models that can effectively predict esophagogastric variceal rebleeding in patients with liver cirrhosis are lacking.
To construct and externally validate a reliable prognostic model for predicting the occurrence of esophagogastric variceal rebleeding.
This study included 477 EGVB patients across 2 cohorts: The derivation cohort (n = 322) and the validation cohort
Six predictors, including albumin and aspartate aminotransferase concentrations, white blood cell count, and the presence of ascites, portal vein thrombosis, and bleeding signs, were selected for the rebleeding event prediction following endoscopic treatment (REPET) model. In predicting rebleeding within 1 year, the REPET model ex
We constructed and validated a new prognostic model for variceal rebleeding with excellent predictive per
Core Tip: Rebleeding is a serious complication in liver cirrhosis patients following esophagogastric variceal bleeding, and there is no widely recognized prognostic model to reliably predict this risk. To address this gap, we developed and externally validated the rebleeding event prediction following endoscopic treatment model, which incorporates readily accessible clinical variables from multiple domains. The rebleeding event prediction following endoscopic treatment model enables effective risk stratification, facilitating improved patient management and the tailoring of follow-up and treatment strategies.
- Citation: Zhan JY, Chen J, Yu JZ, Xu FP, Xing FF, Wang DX, Yang MY, Xing F, Wang J, Mu YP. Prognostic model for esophagogastric variceal rebleeding after endoscopic treatment in liver cirrhosis: A Chinese multicenter study. World J Gastroenterol 2025; 31(2): 100234
- URL: https://www.wjgnet.com/1007-9327/full/v31/i2/100234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i2.100234
Esophagogastric variceal bleeding (EGVB) is a major consequence of portal hypertension in patients with liver cirrhosis and is associated with considerable mortality[1]. Rebleeding is a serious complication following the recovery from EGVB. In patients who do not receive secondary prophylaxis, the incidence of rebleeding can reach up to 60%, accompanied by a higher risk of mortality[2,3]. The Baveno VII workshop guidelines recommend the use of a combination of endoscopic variceal ligation and nonselective beta-blockers (NSBB) as a first-line therapy to prevent variceal rebleeding[1], and this method has been widely adopted. However, a recent meta-analysis revealed that the rebleeding rate following standard secondary prophylaxis still ranged from 22% to 33%[4]. Given the varied risk of rebleeding among patients, there is a clear need to tailor therapeutic interventions and follow-up regimens on the basis of each individual patient’s anticipated risk.
There is a lack of widely recognized prognostic models that effectively predict esophagogastric variceal rebleeding in patients with liver cirrhosis[1]. Several models for predicting rebleeding have been reported[5-9], but these models are limited in their selection of predictive factors, focusing primarily on laboratory indicators while overlooking a wide range of clinical information, such as cirrhosis complications, endoscopic features, and treatment regimens. As a result, the predictive performance of these models is constrained. Furthermore, the lack of external validation reduces their generalizability. Therefore, there is an urgent need for the development of a prognostic model for variceal rebleeding using long-term follow-up cohorts, a multidimensional collection of clinical data and reliable validation.
In the present study, we screened EGVB patients who underwent endoscopy combined with NSBB secondary prophylaxis, with the aim of constructing and externally validating a reliable prognostic model for variceal rebleeding. The developed model not only facilitates active monitoring and treatment of high-risk patients but also helps spare low-risk patients from unnecessary treatment[10], thereby optimizing the allocation of medical resources.
The study was performed in patients with cirrhosis and EGVB who were derived from two retrospective cohorts. The derivation cohort included patients with cirrhosis and EGVB who underwent endoscopy combined with NSBB secondary prophylaxis at Shuguang Hospital Affiliated with Shanghai University of Traditional Chinese Medicine between December 2015 and April 2023. External validation was performed on patients who were treated with endoscopy combined with NSBB for EGVB between April 2022 and April 2023 at Zhongshan Hospital Affiliated with Fudan University. The clinical data were collected retrospectively, with each collection independently gathered and verified by two data managers. All patients were followed up by telephone, outpatient visits, and inpatient records until rebleeding, or up to 1 year. This study was performed in accordance with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis guidelines (Supplementary Table 1)[11]. The study was approved by the local ethics committees and complied with the Declaration of Helsinki. The raw data did not contain any personal identifying information that could be linked to particular individuals and were anonymized before use. Given that all the clinical data were obtained retrospectively, the need for informed consent was waived.
The inclusion criteria for the derivation and validation cohorts were as follows: (1) Had a diagnosis of cirrhosis (on the basis of clinical characteristics and laboratory and imaging tests or liver biopsy); (2) Were admitted due to EGVB confirmed by endoscopy; (3) ≥ 18 years of age; and (4) Underwent endoscopy combined with NSBB secondary pro
The exclusion criteria were as follows: (1) Previous endoscopic primary or secondary prophylaxis; (2) Previous trans
Patients were managed according to the Baveno consensus[1,12] and the American Association for the Study of Liver Diseases guidelines[13,14] at the time of treatment. The patients were initially treated with vasoactive drugs and prophylactic antibiotics within 12 hours, and endoscopic emergency hemostasis was performed when necessary. After control of the index bleed, secondary prophylaxis with endoscopy combined with NSBB was instituted to prevent rebleeding. Decisions to institute therapeutic modifications were made according to individual center policy and the clinical assessment of the patient by the attending physician.
Each center has two senior endoscopists with more than five years of therapeutic experience who are in charge of endoscopic examinations and treatments. Esophageal varices were treated with endoscopic variceal ligation, with ligation from the cardia to the oral side using a commercial multiband device. When combined with gastric varices, intravenous injections of lauroylmorpholine and tissue adhesive were performed using a therapeutic endoscope and a transparent Teflon syringe.
The primary outcome was defined as rebleeding events within 1 year. The secondary outcomes were rebleeding events within 6 weeks and 2 years. Rebleeding was defined as the reoccurrence of a clinically significant active bleeding event after bleeding control (hematemesis, dark stools, or melena; decrease in systolic blood pressure > 20 mmHg or increase in heart rate > 20 beats/minute; decrease in hemoglobin > 30 g/L in the absence of transfusion)[15]. The assessment of rebleeding events was performed by senior endoscopists at each center.
The candidate predictors were restricted to variables collected at admission on the first visit. The candidate predictors are listed in Table 1. Information on esophageal varices and gastric varices was recorded according to the classification of the Japanese Research Society for Portal Hypertension[16]. Bleeding signs, as observed through endoscopy, were defined as gushing bleeding, spurting bleeding, oozing bleeding, red plug, or white plug[16,17]. Bacterial infections were defined as spontaneous peritonitis, pulmonary infections or urinary tract infections, depending on the collected clinical data.
| Characteristic | Derivation cohort | Missing data in derivation cohort (%) | Validation cohort | Missing data in validation cohort (%) | P value |
| Demographics | |||||
| Age (year) | 58.38 ± 11.86 | 0 | 57.7 ± 13.37 | 0 | 0.57 |
| Sex, n (%) | 0 | 0 | 0.36 | ||
| Female | 132 (40.99) | 56 (36.13) | |||
| Male | 190 (59.01) | 99 (63.87) | |||
| Smoking history, n (%) | 52 (16.25) | 0.6 | 26 (16.77) | 0 | 0.99 |
| Drinking history, n (%) | 74 (23.12) | 0.6 | 30 (19.35) | 0 | 0.42 |
| Surroundings, n (%) | 0 | 0 | 0.67 | ||
| Rural | 93 (28.88) | 50 (32.26) | |||
| Suburban | 58 (18.01) | 24 (15.48) | |||
| Urban | 171 (53.11) | 81 (52.26) | |||
| Symptom, n (%) | 0 | 0 | 0.44 | ||
| Melena or hematochezia | 115 (35.71) | 49 (31.61) | |||
| Hematemesis | 207 (64.29) | 106 (68.39) | |||
| Etiology, n (%) | 0 | 0 | 0.8 | ||
| Chronic HBV infection | 156 (48.45) | 69 (44.52) | |||
| Autoimmune liver disease | 55 (17.08) | 31 (20.00) | |||
| Alcohol-related | 50 (15.53) | 23 (14.84) | |||
| Other | 61 (18.94) | 32 (20.65) | |||
| Course of cirrhosis (year) | 2 (0.5-6) | 3.7 | 2 (0.2-6) | 0 | 0.46 |
| Family history of cirrhosis, n (%) | 52 (16.20) | 0.3 | 7 (4.52) | 0 | < 0.01 |
| Endoscopic treatment and presentation | |||||
| Received emergency endoscopic hemostasis, n (%) | 28 (8.70) | 0 | 10 (6.45) | 0 | 0.5 |
| Types of varices, n (%) | 0 | 0 | < 0.01 | ||
| EV | 177 (54.97) | 28 (18.06) | |||
| EV combined with GV | 145 (45.03) | 127 (81.94) | |||
| Red color sign, n (%) | 302 (93.79) | 0 | 150 (96.77) | 0 | 0.25 |
| Bleeding signs, n (%) | 49 (15.22) | 0 | 29 (18.71) | 0 | 0.4 |
| Form of varices, n (%) | 0 | 0 | 0.57 | ||
| F1 | 56 (17.39) | 23 (14.84) | |||
| F2-F3 | 266 (82.61) | 132 (85.16) | |||
| Location of varices, n (%) | 0.3 | 0 | < 0.01 | ||
| Low to middle | 252 (78.50) | 146 (94.19) | |||
| High | 69 (21.50) | 9 (5.81) | |||
| Numbers of varices | 4 (4-4) | 4 (4-4) | 0.73 | ||
| Diameter of varices (cm) | 0.9 (0.8-1) | 0.6 | 1 (0.8-1) | 0 | 0.53 |
| Number of EVL (times) | 2 (1-3) | 0 | 2 (1.5-3) | 0 | 0.05 |
| Received sclerotherapy injections, n (%) | 139 (43.17) | 0 | 74 (47.74) | 0 | 0.4 |
| Received tissue glue injections, n (%) | 125 (38.82) | 0 | 65 (41.94) | 0 | 0.58 |
| Comorbidities and complications | |||||
| Ascites, n (%) | 220 (68.32) | 0 | 95 (61.29) | 0 | 0.16 |
| Bacterial infection, n (%) | 48 (14.91) | 0 | 12 (7.74) | 0 | 0.04 |
| Encephalopathy, n (%) | 13 (4.04) | 0 | 3 (1.94) | 0 | 0.36 |
| Diabetes, n (%) | 67 (20.87) | 0.3 | 31 (20.00) | 0 | 0.92 |
| Hypertension, n (%) | 78 (24.30) | 0.3 | 20 (12.90) | 0 | 0.01 |
| Hemorrhagic shock, n (%) | 10 (3.12) | 0.3 | 2 (1.29) | 0 | 0.38 |
| Heart disease, n (%) | 16 (4.98) | 0.3 | 7 (4.52) | 0 | 1.00 |
| Laboratory tests | |||||
| Total bilirubin (μmol/L) | 21.3 (15-32.02) | 3.1 | 15.8 (10.85-20.85) | 0 | < 0.01 |
| Alanine aminotransferase (U/L) | 25 (17-36) | 4.3 | 21 (15-30) | 0 | 0.01 |
| Aspartate aminotransferase (U/L) | 35 (26-47) | 4.3 | 28 (21-38.5) | 0 | < 0.01 |
| Alkaline phosphatase (U/L) | 87 (68-130) | 5 | 83 (63.25-107) | 0.6 | 0.05 |
| Total protein (g/L) | 62.45 ± 8.92 | 3.8 | 62.9 ± 9.16 | 0 | 0.62 |
| Albumin (g/L) | 31.89 ± 6.07 | 3.1 | 35.3 ± 5.94 | 0 | < 0.01 |
| Globulin (g/L) | 29.55 (25.9-34.42) | 5.6 | 27 (23-32) | 0 | < 0.01 |
| A/G | 1.05 (0.87-1.3) | 5.6 | 1.33 (1.08-1.59) | 0 | < 0.01 |
| Blood urea nitrogen (mmol/L) | 5.72 (4.2-7.72) | 6.8 | 5.2 (4.1-6.18) | 0.6 | < 0.01 |
| Creatinine (μmoI/L) | 65.4 (55-79) | 4.7 | 69 (57.25-80.5) | 0.6 | 0.14 |
| Uric acid (μmoI/L) | 298 (238-372) | 4.7 | 288 (239-357) | 0 | 0.26 |
| Total cholesterol (mmol/L) | 3.17 (2.61-3.94) | 7.5 | 3.17 (2.6-3.94) | 25.8 | 0.94 |
| White blood cell (× 109/L) | 3.1 (2.09-4.75) | 5.6 | 2.66 (1.92-4.47) | 0 | 0.07 |
| Neutrophil (× 109/L) | 1.96 (1.25-3.52) | 5.3 | 1.6 (1.1-2.77) | 0.6 | < 0.01 |
| Lymphocyte (× 109/L) | 0.7 (0.5-1.03) | 5.9 | 0.7 (0.5-1) | 1.9 | 0.69 |
| Red blood cell (× 109/L) | 3.05 ± 0.74 | 3.4 | 3.19 ± 0.77 | 0 | 0.08 |
| Hemoglobin (g/L) | 88.37 ± 24.42 | 1.9 | 89.1 ± 22.33 | 0 | 0.76 |
| Platelet count (× 109/L) | 68 (45.75-91.25) | 3.1 | 68 (45.5-103) | 0 | 0.68 |
| Prothrombin time (second) | 14.8 (13.6-16.25) | 3.4 | 14.4 (13.7-15.6) | 0 | 0.07 |
| INR (unit) | 1.27 (1.15-1.4) | 4 | 1.26 (1.17-1.37) | 0 | 0.48 |
| D-dipolymer (μg/mL) | 1.45 (0.56-3.34) | 7.1 | 1.1 (0.45-2.37) | 1.9 | 0.12 |
| Thrombin time (second) | 17.8 (16.9-19.1) | 6.5 | 17.8 (16.67-18.72) | 1.9 | 0.34 |
| Fibrinogen (g/L) | 200.26 ± 80.04 | 5.6 | 185.67 ± 69.84 | 1.9 | 0.06 |
| CTP (points) | 7 (6-8) | 0 | 6 (6-7) | 0 | < 0.01 |
| CTP class, n (%) | 0 | 0 | < 0.01 | ||
| A (5-6) | 101 (31.37) | 83 (53.55) | |||
| B (7-9) | 190 (59.01) | 69 (44.52) | |||
| C (10-13) | 31 (9.63) | 3 (1.94) | |||
| MELD (points) | 11 (9-13) | 0 | 9 (8-11) | 0 | < 0.01 |
| Radiological features | |||||
| Portal vein thrombosis, n (%) | 68 (21.12) | 1.2 | 49 (31.61) | 0 | 0.02 |
| Splenic vein diameter (mm) | 9.6 ± 3.45 | 6.8 | 9.08 ± 3.94 | 4.5 | 0.16 |
| Inner diameter of portal vein trunk (mm) | 13.17 ± 2.34 | 6.5 | 12.7 ± 2.97 | 4.5 | 0.07 |
| Use of medication | |||||
| Use of NSBB, n (%) | 314 (65.83) | 0 | 204 (63.35) | 0 | 0.12 |
| Use of PPI, n (%) | 450 (94.34) | 0 | 306 (95.03) | 0 | 0.47 |
| Use of antibiotics, n (%) | 335 (70.23) | 0 | 219 (68.01) | 0 | 0.16 |
| Outcomes | |||||
| Rebleeding within 6 weeks, n (%) | 26 (8.07) | 0 | 7 (4.52) | 0 | 0.21 |
| Rebleeding within 6 months, n (%) | 60 (18.63) | 0 | 22 (14.19) | 0 | 0.28 |
| Rebleeding within 1 year, n (%) | 83 (25.78) | 0 | 36 (23.23) | 0 | 0.62 |
| Rebleeding within 2 years, n (%) | 111 (34.47) | 0 | 51 (32.90) | 0 | 0.81 |
| Follow-up time (day) | 408.5 (322-885) | 0 | 447 (366-1108) | 0 | 0.06 |
The derivation cohort sample size was calculated using the methodology proposed by Riley et al[18]. The calculation was based on the assumption of 6 predictive parameters in the model, an adjusted R-square of 0.2, a shrinkage of 10%, and a 22% incidence of the primary outcome (rebleeding within 1 year). Given this, the minimum sample size required for model development was 239 patients with 53 outcome events. The external validation sample size was calculated with reference to a statistical article published by Riley et al[19]. The calculation was based on the assumption of an observed/expected statistic of 1, a target confidence interval width of 0.7 for observed/expected statistic, and a 22% incidence of rebleeding within 1 year. Thus, at least 77 participants (approximately 17 events) were required to satisfy these criteria.
Statistical analyses were conducted using R 4.3.2 (https://www.R-project.org). Descriptive results are presented as n (%) or mean ± SD, as appropriate. Missing data were imputed with multiple imputations. Variables for which more than 10% of the values were missing were excluded (the excluded data are shown in Supplementary Table 2). Group comparisons of continuous variables were made using Student’s t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Group comparisons of categorical variables were made using Pearson’s χ2 test or Fisher’s exact test. A 2-sided P < 0.05 was considered to indicate statistical significance.
Least absolute shrinkage and selection operator (LASSO) was applied to screen independent variables of the training set for selection of candidate predictors. This approach performs simultaneous feature selection by applying a regularization penalty term to the model that causes some coefficients to shrink to zero. The Cox proportional hazards model was constructed using the features that had nonzero coefficients in the LASSO regression model. Proportionate risk assumptions were tested via the Global Schoenfeld Test. Multicollinearity was assessed using the variance inflation factor (VIF), and a VIF < 5 indicates the absence of multicollinearity. We also developed and used a nomogram to calculate the predicted probability of rebleeding at 6 weeks, 1 year, and 2 years.
Model performance was assessed using the concordance index (C-index), area under the receiver operating curve (AUC), calibration plots, Brier scores and decision curve analysis (DCA). The predictive performance of the prognostic model was compared with that of the Child-Turcotte-Pugh (CTP) score, the model for end-stage liver disease (MELD) score, albumin-bilirubin grade and fibrosis 4 (FIB-4) score[20,21] (the calculations are shown in Supplementary Table 3). Internal validation was performed via the bootstrap resampling method with 1000 repetitions. For external validation, prognostic scores were calculated for each individual using the formula developed for the derivation cohort, and the performance of the prognostic scores was subsequently evaluated. We used X-tile software[22] to determine the optimal threshold for prognostic scores and plotted Kaplan-Meier survival curves.
Given that the incidence of rebleeding after emergency endoscopic hemostasis is significantly higher[23,24], we performed a sensitivity analysis to exclude patients who underwent emergency endoscopic hemostasis. To assess whether there was heterogeneity in the predictive value of the prognostic model, we assessed the prognostic scores of the subgroups individually according to the etiology of cirrhosis (hepatitis B virus, autoimmune hepatitis, alcoholic hepatitis, and other etiologies), variceal type (esophageal varices, combined with gastric varices), sex (male or female), age (< 60, ≥ 60), and use of NSBB (non-NSBB group, NSBB group).
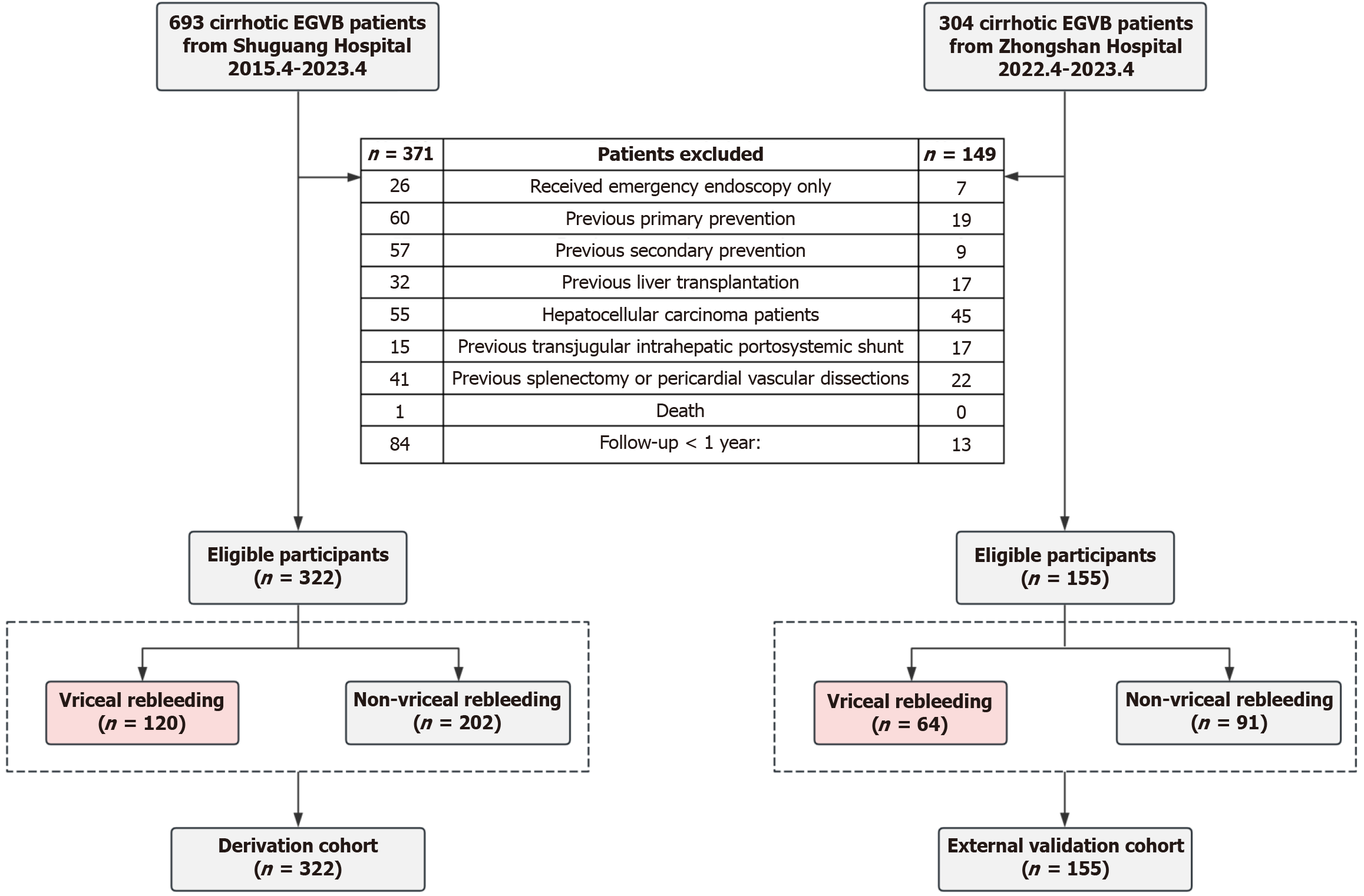
A total of 997 patients with liver cirrhosis and EGVB were enrolled during the study period. Of these, 520 were excluded for the reasons illustrated in Figure 1. The remaining 477 patients were included in subsequent model development and validation, with the derivation cohort comprising 322 patients and the external validation cohort comprising 155 patients. The baseline characteristics of the patients are presented in Table 1, with missing values noted. A total of 56 variables were included in the statistical analyses after excluding variables with more than 10% missing values. There was no significant difference in the distribution of cirrhosis etiology between the two cohorts (P = 0.8). Despite notable differences in liver function tests and variceal types between the derivation and validation cohorts, the rebleeding rates within 6 weeks (8.07% vs 4.52%, P = 0.21), 1 year (25.78% vs 23.23%, P = 0.62), and 2 years (34.47% vs 32.90%, P = 0.81) did not significantly differ.
All patients were followed up until rebleeding occurred or for up to 1 year. The median follow-up times for the two cohorts were 408.5 days and 447 days, respectively. During the follow-up period, 25 patients developed HCC. Two patients underwent TIPS and 1 patient underwent splenectomy before rebleeding occurred. A total of 27 patients died, with 26 deaths attributed to hemorrhagic shock or multi-organ failure related to EGVB, and 1 death resulting from HCC.
A total of 56 independent variables from the training set were screened using LASSO regression to identify candidate predictors (Supplementary Figure 1A and B). To facilitate the model’s practical clinical application, we selected the most concise scheme (the lambda.1se scheme) for the final modeling variables[25], which included albumin (ALB) and aspartate aminotransferase (AST) concentrations, white blood cell (WBC) count, and the presence of ascites, portal vein thrombosis (PVT), bacterial infection, and recent bleeding manifestations. Owing to their closely related clinical significance, bacterial infection and WBC count were considered collinear variables. After careful discussion between two senior physicians, it was decided to exclude bacterial infection from the model to enhance the model’s practical clinical application. Therefore, six predictors were ultimately selected for the rebleeding event prediction following endoscopic treatment (REPET) model, including ALB, AST, and WBC, and the presence of ascites, PVT, and bleeding signs.
We then performed variable analysis using Cox multivariable regression, constructed a forest plot of risk factors for rebleeding (Supplementary Figure 1C), and constructed a Cox proportional risk prognostic model. The global Schoenfeld test yielded a P value > 0.5, indicating that the prognostic model complies with the proportional risk assumption (Supplementary Figure 2). Multicollinearity analysis revealed no covariance (VIF < 5) (Supplementary Figure 3).
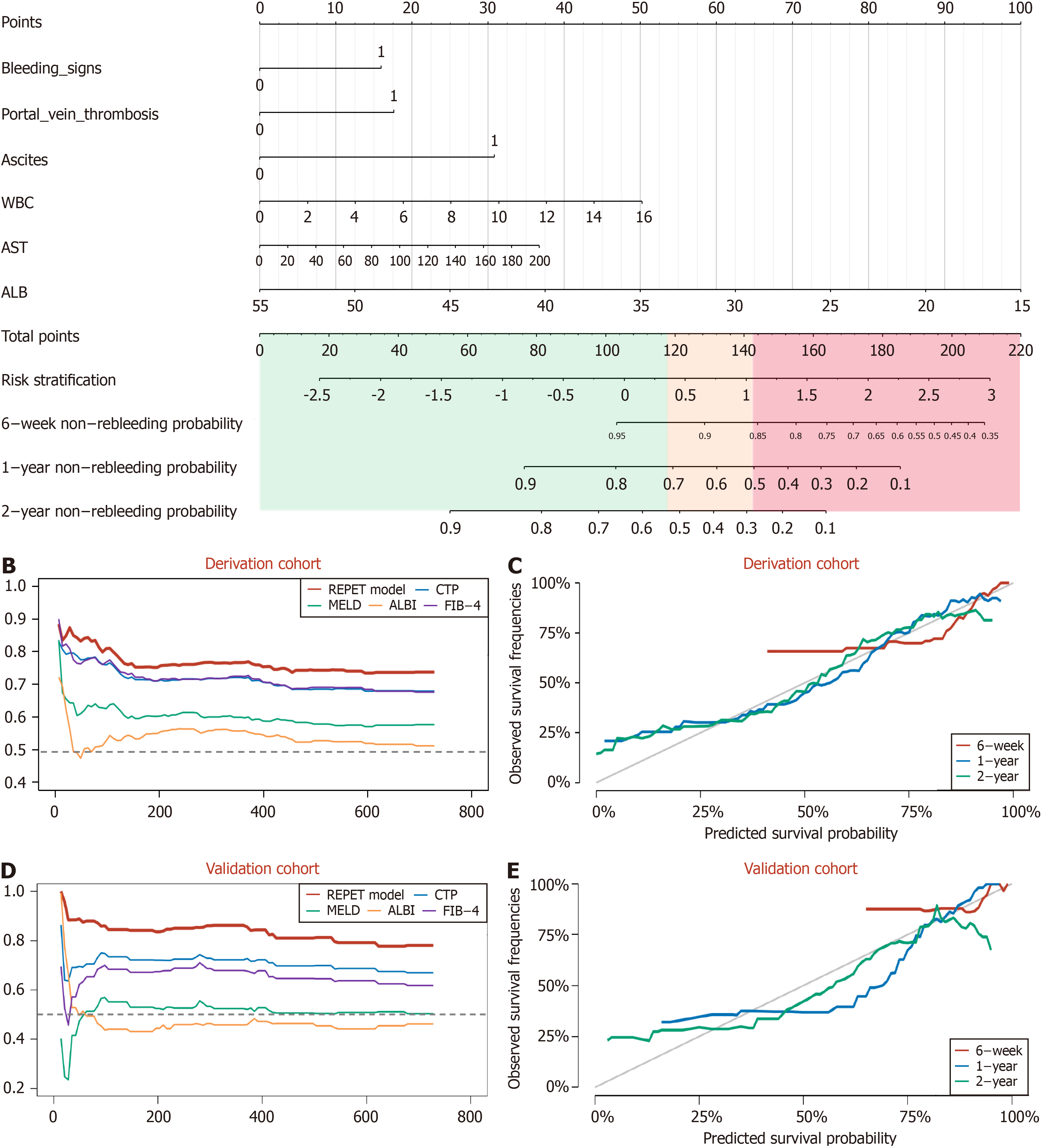
A nomogram based on the REPET model was developed (Figure 2A). Using easily accessible clinical characteristics, clinicians can calculate a risk score according to the following formula: REPET score = 137.5 - 2.5 × ALB (g/L) + 30.799 × Ascites (1, 0) + 17.609 × PVT (1, 0) + 15.927 × bleeding signs (1, 0) + 0.184 × AST (U/L) + 3.138 × WBC (109/L). The incidences of rebleeding at 6 weeks, 1 year and 2 years can be obtained by combining the REPET score with the no
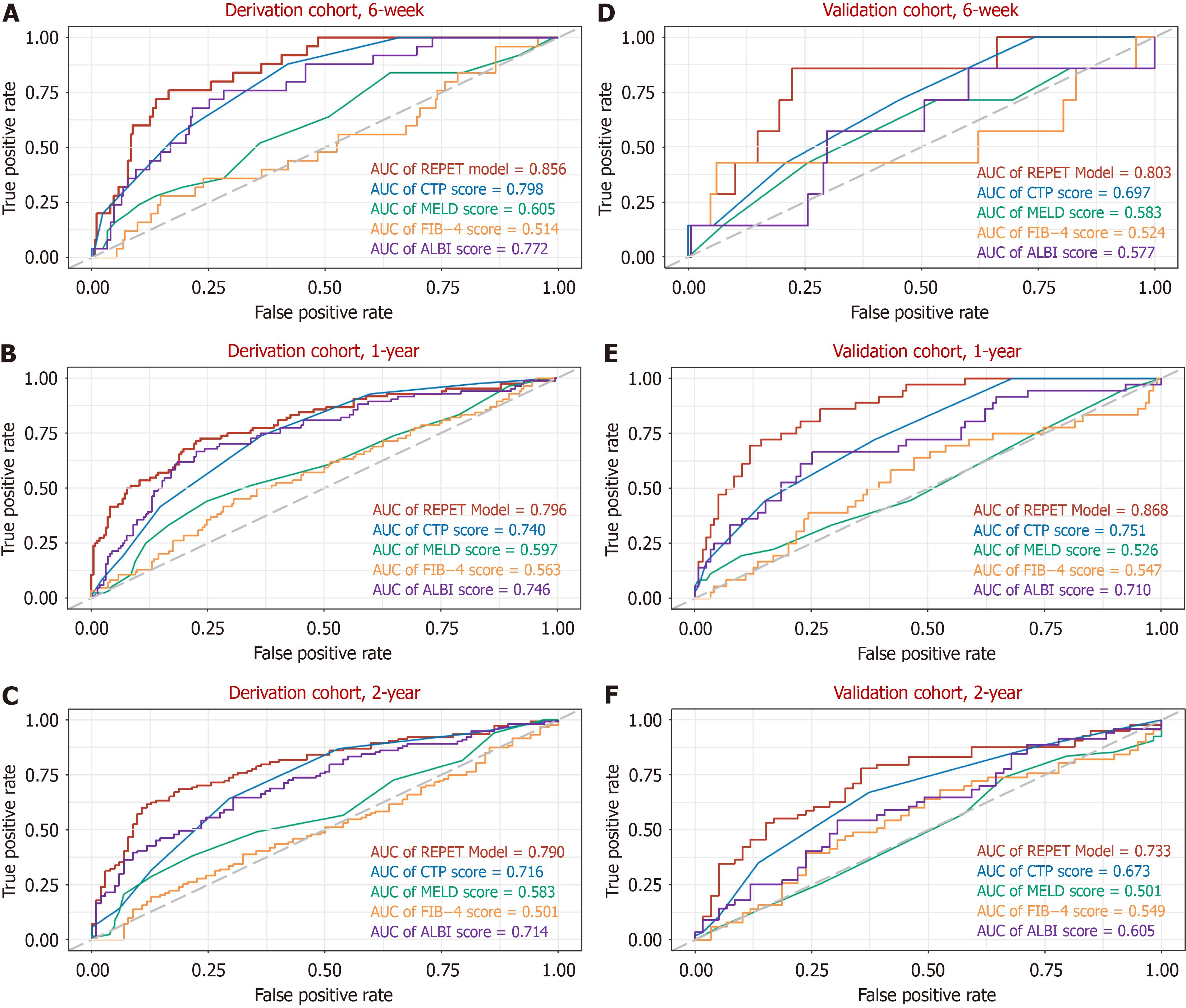
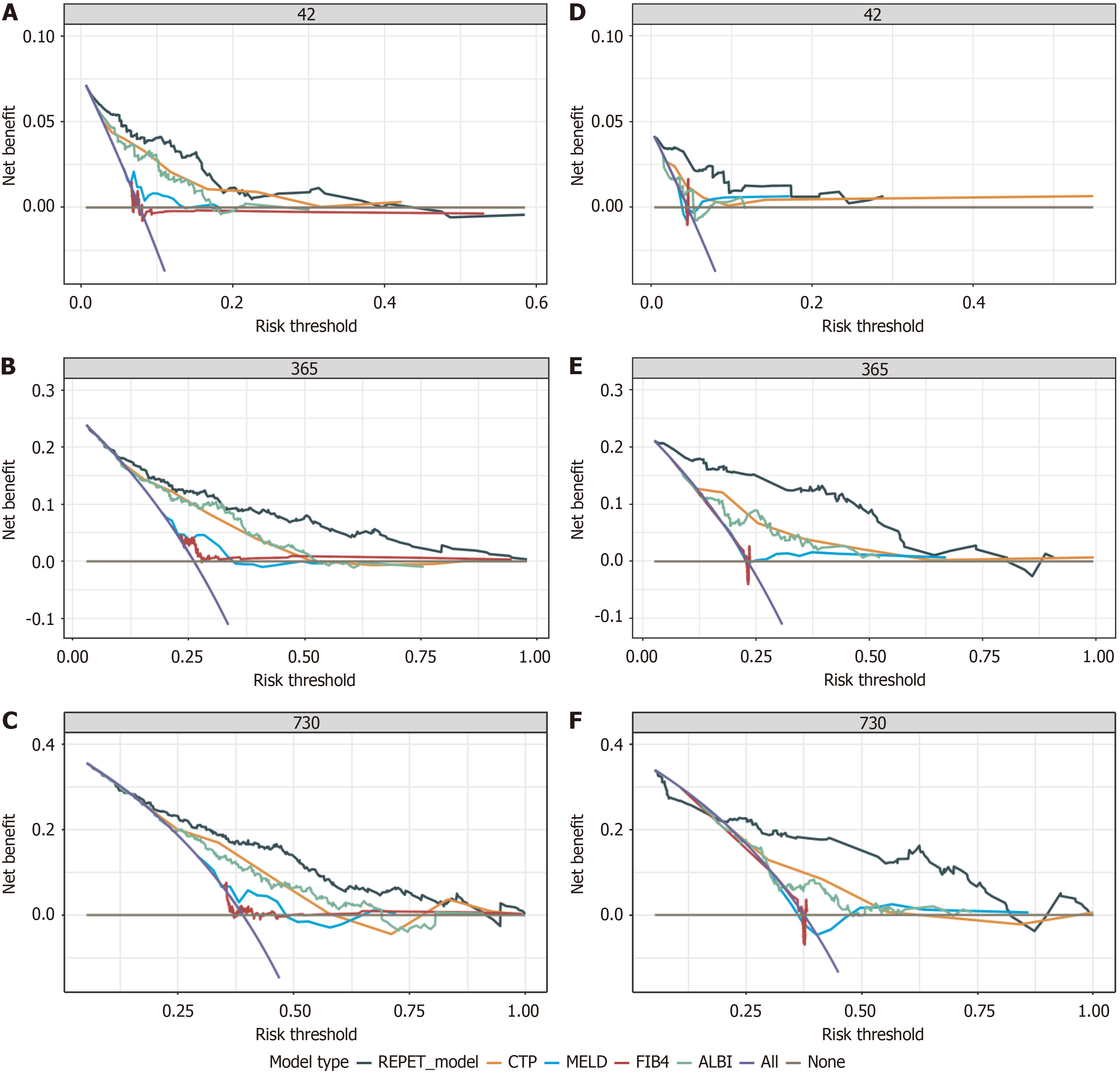
The C-index values of the REPET for predicting 6-week, 1-year and 2-year rebleeding in the derivation cohort were 0.857, 0.775, and 0.741, respectively. As shown in Figure 2B, the C-index values of the REPET consistently exceeded those of the other scores across all time points. The AUC values for the ability of the REPET to predict rebleeding were 0.856, 0.796, and 0.790 at 6 weeks, 1 year, and 2 years, respectively, which also surpassed those of the comparator scores (Figure 3A-C). Additionally, compared with the other scores, the DCA of the REPET model demonstrated a greater net benefit (Figure 4A-C). The calibration plot revealed that the REPET model had acceptable calibration at 6 weeks and good calibration at 1 year and 2 years (Figure 2C). The Brier scores for the REPET model at various time points were con
| C-index | AUC (95%CI) | Brier (95%CI) | |
| The derivation cohort | |||
| 6 weeks | 85.7 | 0.856 (0.794-0.918) | 0.062 (0.042-0.083) |
| 1 year | 77.5 | 0.796 (0.737-0.855) | 0.143 (0.121-0.165) |
| 2 years | 74.1 | 0.790 (0.727-0.853) | 0.177 (0.149-0.205) |
| Internal validation | |||
| 6 weeks | 84.2 | 0.841 (0.755-0.925) | 0.066 (0.036-0.102) |
| 1 year | 75.9 | 0.783 (0.712-0.842) | 0.153 (0.127-0.185) |
| 2 years | 73.5 | 0.779 (0.685-0.867) | 0.184 (0.146-0.233) |
| External validation | |||
| 6 weeks | 88.5 | 0.803 (0.647-0.959) | 0.04 (0.014-0.066) |
| 1 year | 86.2 | 0.868 (0.808-0.928) | 0.127 (0.096-0.159) |
| 2 years | 76.8 | 0.733 (0.634-0.832) | 0.194 (0.151-0.237) |
The REPET model was internally validated using 1000 bootstrap resampling data points. Our prognostic model showed good discriminative ability, with C-index values of 0.842, 0.759, and 0.735 at 6 weeks, 1 year, and 2 years, respectively (Supplementary Figure 4), and AUC values of 0.841, 0.783, and 0.779, respectively (Supplementary Figure 5). DCA for internal validation revealed favorable clinical net benefit (Supplementary Figure 6). The internal validation data showed good calibration at 1 year and 2 years but had limited calibration at 6 weeks (Supplementary Figure 7). Moreover, the internal validation Brier scores confirmed the excellent accuracy of the model (Table 2).
In the external validation set, the REPET model maintained impressive discriminative ability, with C-index values of 0.885, 0.862, and 0.768 at 6 weeks, 1 year, and 2 years, respectively, outperforming the compared models (Figure 2D). The AUC values for predicting rebleeding were 0.803, 0.868, and 0.733 at 6 weeks, 1 year, and 2 years, respectively, further demonstrating the solid predictive performance of the REPET model (Figure 3D-F). Similarly, DCA of the external validation cohort demonstrated the greatest clinical net benefit among all the models tested (Figure 4D-F). The REPET model displayed good calibration at 1 and 2 years but limited calibration at 6 weeks (Figure 2E). The REPET model also exhibited exceptional accuracy, with Brier scores of 0.04, 0.127, and 0.194 at 6 weeks, 1 year, and 2 years, respectively.
In the sensitivity analysis, we excluded patients who received emergency endoscopic hemostasis, and surprisingly, the REPET model maintained similarly excellent discriminatory ability (C-index: 6 weeks/1 year/2 years: 0.847/0.78/0.724; AUC: 6 weeks/1 year/2 years: 0.847/0.8/0.75) and accuracy (Brier scores: 6 weeks/1 year/2 years: 0.053/0.136/0.188) (Table 3). In the subgroup analyses, the REPET model showed good to excellent performance in predicting rebleeding at 6 weeks, 1 year, and 2 years across subgroups according to the etiology of cirrhosis, types of varices, sex, age and use of NSBB (Table 3).
| C-index | AUC (95%CI) | Brier score (95%CI) | |
| Sensitivity analysis | |||
| Exclude patients underwent emergency endoscopy | |||
| 6 weeks | 0.847 | 0.847 (0.781-0.913) | 0.053 (0.036-0.07) |
| 1 year | 0.78 | 0.8 (0.749-0.85) | 0.136 (0.117-0.156) |
| 2 years | 0.724 | 0.75 (0.691-0.81) | 0.188 (0.163-0.213) |
| Subgroup analysis | |||
| Etiology of cirrhosis | |||
| Chronic HBV infection | |||
| 6 weeks | 0.9 | 0.915 (0.858-0.973) | 0.04 (0.021-0.059) |
| 1 year | 0.779 | 0.793 (0.718-0.867) | 0.137 (0.11-0.164) |
| 2 years | 0.717 | 0.731 (0.646-0.816) | 0.195 (0.158-0.232) |
| Autoimmune liver disease | |||
| 6 weeks | 0.913 | 0.884 (0.761-1) | 0.048 (0.011-0.085) |
| 1 year | 0.84 | 0.892 (0.814-0.97) | 0.122 (0.083-0.162) |
| 2 years | 0.798 | 0.821 (0.69-0.953) | 0.154 (0.103-0.206) |
| Alcohol-related | |||
| 6 weeks | 0.803 | 0.817 (0.705-0.929) | 0.093 (0.04-0.147) |
| 1 year | 0.786 | 0.828 (0.723-0.934) | 0.138 (0.094-0.183) |
| 2 years | 0.757 | 0.783 (0.66-0.907) | 0.174 (0.126-0.222) |
| Other | |||
| 6 weeks | 0.745 | 0.718 (0.532-0.903) | 0.067 (0.025-0.109) |
| 1 year | 0.812 | 0.793 (0.695-0.892) | 0.153 (0.11-0.196) |
| 2 years | 0.743 | 0.777 (0.658-0.897) | 0.2 (0.141-0.259) |
| Types of varices | |||
| EV | |||
| 6 weeks | 0.862 | 0.868 (0.796-0.94) | 0.061 (0.036-0.087) |
| 1 year | 0.836 | 0.854 (0.799-0.909) | 0.129 (0.103-0.155) |
| 2 years | 0.776 | 0.822 (0.747-0.896) | 0.164 (0.131-0.197) |
| EV combined with GV | |||
| 6 weeks | 0.845 | 0.823 (0.727-0.919) | 0.05 (0.029-0.071) |
| 1 year | 0.768 | 0.791 (0.726-0.857) | 0.144 (0.119-0.17) |
| 2 years | 0.715 | 0.726 (0.65-0.802) | 0.203 (0.169-0.237) |
| Sex | |||
| Male | |||
| 6 weeks | 0.872 | 0.894 (0.845-0.943) | 0.062 (0.04-0.084) |
| 1 year | 0.796 | 0.809 (0.75-0.869) | 0.139 (0.115-0.163) |
| 2 years | 0.719 | 0.724 (0.651-0.798) | 0.2 (0.167-0.234) |
| Female | |||
| 6 weeks | 0.703 | 0.71 (0.539-0.882) | 0.044 (0.02-0.067) |
| 1 year | 0.781 | 0.831 (0.764-0.899) | 0.136 (0.108-0.164) |
| 2 years | 0.772 | 0.831 (0.756-0.907) | 0.164 (0.13-0.197) |
| Age | |||
| < 60 | |||
| 6 weeks | 0.889 | 0.862 (0.77-0.954) | 0.046 (0.025-0.067) |
| 1 year | 0.771 | 0.783 (0.711-0.854) | 0.134 (0.107-0.161) |
| 2 years | 0.706 | 0.731 (0.649-0.812) | 0.191 (0.155-0.227) |
| ≥ 60 | |||
| 6 weeks | 0.814 | 0.826 (0.742-0.909) | 0.064 (0.039-0.089) |
| 1 year | 0.801 | 0.848 (0.792-0.904) | 0.142 (0.117-0.166) |
| 2 years | 0.761 | 0.796 (0.723-0.87) | 0.181 (0.150-0.213) |
| Use of NSBB | |||
| Non-NSBB group | |||
| 6 weeks | 0.846 | 0.837 (0.753-0.921) | 0.079 (0.046-0.113) |
| 1 year | 0.802 | 0.835 (0.762-0.908) | 0.146 (0.114-0.178) |
| 2 years | 0.794 | 0.871 (0.805-0.937) | 0.141 (0.11-0.171) |
| NSBB group | |||
| 6 weeks | 0.85 | 0.846 (0.754-0.939) | 0.042 (0.025-0.06) |
| 1 year | 0.79 | 0.806 (0.75-0.862) | 0.134 (0.112-0.156) |
| 2 years | 0.708 | 0.705 (0.631-0.78) | 0.21 (0.177-0.242) |
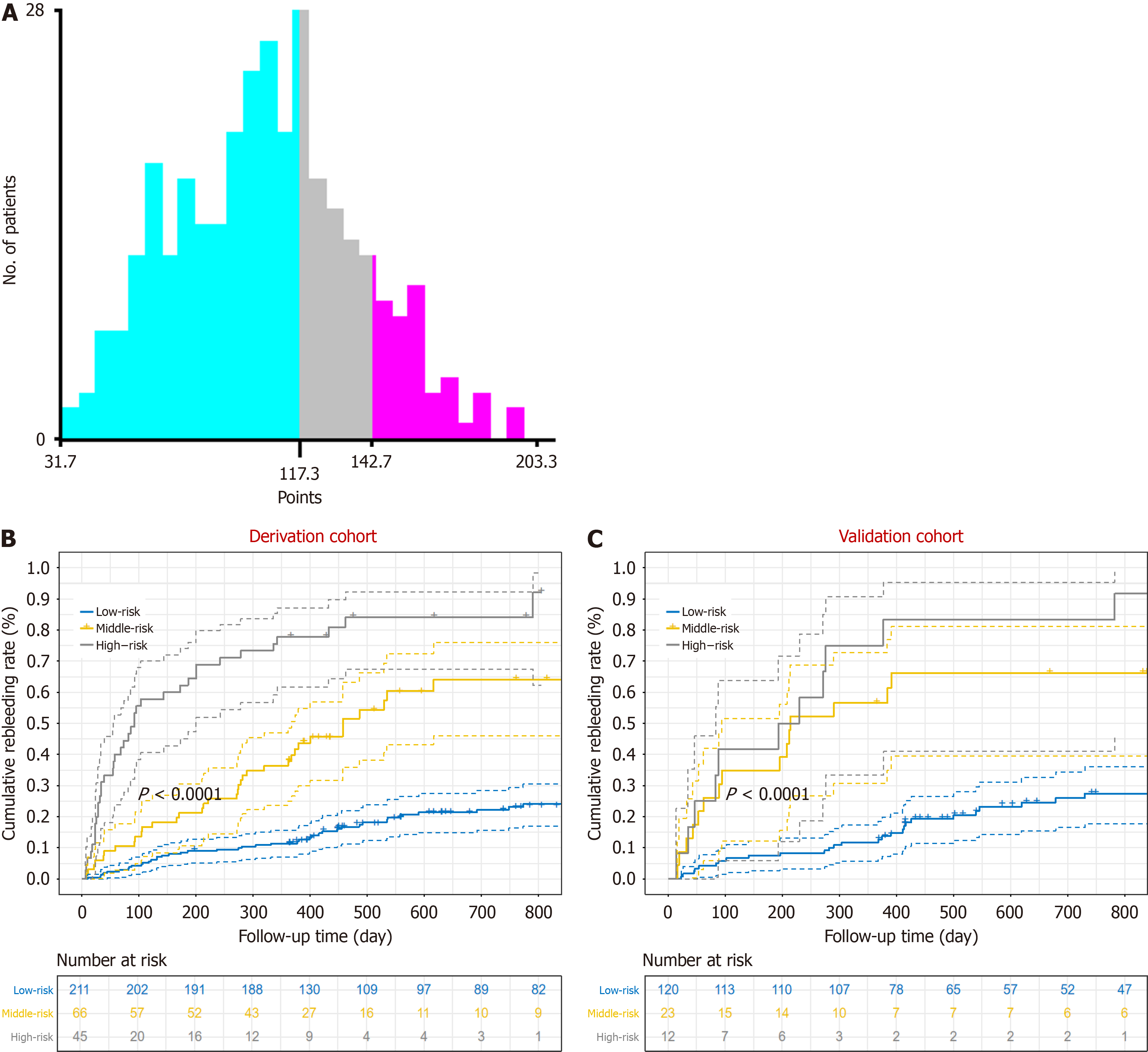
In the derivation cohort, X-tile identified 2 optimal thresholds (117.3 and 142.7) that classified patients into three distinct risk groups with significantly different probabilities of rebleeding: Low-risk (score < 117.3, n = 211), intermediate-risk (score between 117.3 and 142.7, n = 66), and high-risk (score > 142.7, n = 45) (Figure 5A). The cumulative probabilities of rebleeding for the low-risk, intermediate-risk and high-risk groups were 1.9%, 9.09%, and 33.33%, respectively, at 6 weeks, 11.85%, 37.88%, and 77.78%, respectively, at 1 year, and 30.46%, 77.78%, and 92.5%, respectively, at 2 years (Figure 5B). In the external cohort, the cumulative probabilities of rebleeding for the low-risk, intermediate-risk and high-risk groups were 1.67%, 13.04%, and 16.67%, respectively, at 6 weeks, 11.67%, 56.52%, and 75%, respectively, at 1 year, and 33.33%, 69.57%, and 83.33%, respectively, at 2 years (Figure 5C).
Variceal rebleeding is a concerning complication following EGVB in patients with liver cirrhosis, yet a recognized prognostic model that effectively predicts rebleeding is lacking[1-3]. In this longitudinal study, we investigated 56 clinical characteristics to construct and validate an easy-to-apply model comprising 6 items (ALB and AST concentrations, the WBC count, and the presence of ascites, PVT, and bleeding signs) to assist clinicians in risk stratification of rebleeding in cirrhotic EGVB patients who undergo endoscopic therapy combined with NSBB secondary prophylaxis. The model exhibited good discrimination and accuracy in predicting short-term (6 weeks) to long-term (1 and 2 years) variceal rebleeding risk, outperforming existing liver function assessment tools (CTP, MELD, albumin-bilirubin) and noninvasive fibrosis markers (FIB-4)[20,21]. There are several strengths of our study. First, the selection of predictive factors incorporated variables from multiple clinical domains. This study pioneers the integration of endoscopic features and the presence of significant complications into the model, thereby fully utilizing clinically available information to increase the model’s predictive accuracy. Second, all patients were followed up until rebleeding or for up to 1 year, with 67.92% of the cohort being followed for up to 2 years or until rebleeding occurred. This comprehensive follow-up allows the REPET model to be used for predicting long-term rebleeding risk. Third, the study proposes a risk stratification scheme for rebleeding, which can assist clinicians in tailoring treatment plans according to patient risk levels. Finally, the external validation of the model with an independent cohort enhances the generalizability of our findings.
To date, several alternative models/scores have been proposed to predict variceal rebleeding risk, but the results have been mixed. Wang et al[7] attempted to repurpose commonly used cirrhosis scoring systems for predicting rebleeding, concluding that the MELD-Na and MELD were effective predictors, with AUC values of 0.85 and 0.80, respectively, whereas the CTP demonstrated lower predictive accuracy, with an AUC of 0.65. In 2023, Liu et al[5] developed a prognostic model for esophageal variceal rebleeding in hepatitis B-associated cirrhosis patients that was independent of those risk scores, comprising the body mass index, liver stiffness measurement, NSBB usage, platelet count, and hemoglobin concentration, and achieved good predictive results (C-index: 0.772). However, the model’s predictive accuracy may be weakened by two key issues: The inaccuracy of liver stiffness measurement in patients with ascites and its limited use in EGVB patients, along with the assumption of uniform NSBB effectiveness without considering va
The most commonly reported variables influencing rebleeding can be categorized as follows: Liver dysfunction, severity of portal hypertension, infection indicators, and variceal features[1,17,27-32]. The present study revealed that higher serum ALB concentrations protect against EGVB, whereas elevated AST levels and WBC counts are risk factors. A decrease in ALB levels typically indicates impaired liver synthetic function, whereas elevated AST reflects hepatocellular injury, and both of these changes are manifestations of liver dysfunction. An elevated WBC indicates the presence of an infection or an inflammatory response in the body. Consistent with our findings, bacterial infection has been identified as a significant risk factor for rebleeding, underscoring the critical importance of prophylactic antibiotic use in managing EGVB[1]. In addition to routine biochemical indicators, our model also assessed comorbidities and complications at admission. Ascites detected via ultrasonography reflected, in part, liver dysfunction and the severity of portal hy
By integrating clinical characteristics from several domains, we developed a novel model (the REPET model) to predict the risk of experiencing rebleeding in patients with cirrhosis and EGVB. The REPET model achieved great prognostic performance, as confirmed in an independent cohort. In the external cohort, 77.42% of patients were classified as low risk, and their rebleeding rates at 6 weeks and 1 year were 1.67% and 11.67%, respectively. However, the risk of rebleeding increased sharply to 33.33% at 2 years. These findings suggest that follow-up at approximately 1 year is critical for low-risk patients, as it may help identify and prevent rebleeding events effectively. The remaining 22.58% of patients were categorized as intermediate- to high-risk, corresponding to a greater risk of variceal rebleeding. Thus, more frequent follow-up and more aggressive prophylaxis may be needed for this population. In summary, the REPET score can distinguish between low-risk patients and intermediate- to high-risk patients well, which may be useful for guiding follow-up and treatment regimen adjustment.
We subsequently performed further sensitivity analyses and subgroup analyses based on patient characteristics. Notably, the REPET model maintained excellent predictive performance in all of the sensitivity analyses and subgroup analyses. This demonstrates the robustness and generalizability of our risk stratification system to “real-world” clinical practice, where standardization may be lacking[35]. Our research has certain limitations. First, in this study, we utilized retrospective data to construct and validate a predictive model, which has inherent information and recall bias, requiring further validation via prospective data. Accurate recording of patients’ conditions and regular follow-up at each medical center may help to reduce these biases. Second, the model cannot predict rebleeding in patients who are undergoing other treatment regimens, such as TIPS and balloon-occluded retrograde transvenous obliteration. Prediction of rebleeding risk in these patients require the development of additional specialized models that consider factors such as preoperative imaging assessments, the hepatic venous pressure gradient, the portal pressure gradient, and other intraoperative parameters. Additionally, given the increased rebleeding risk and distinct metabolic, tumor, and treatment profiles in HCC patients, we excluded them from this study. Therefore, further validation of the REPET model is needed for the HCC population. Third, although the REPET model can be used to effectively distinguish low-risk patients from intermediate- to high-risk patients, its ability to discriminating between intermediate- and high-risk patients in external validation cohort is lower than expected. One possible reason for this may be sampling bias and reduced statistical power due to the small sample size in the high-risk group. Thus, these findings need further validation in a large independent cohort. The current REPET model can be used for clinical risk stratification to some extent, as up to 69.39% of patients were classified as low risk and were spared from aggressive treatment. Fourth, there were statistically significant differences between the two cohorts in certain serological markers. These differences may be attributed to variations in sample size and time span, reflecting the complexity of real-world data. Nevertheless, the REPET model consistently maintained robust predictive performance across both the training and external validation sets, further demonstrating the model’s strong generalizability. Finally, although a comprehensive investigation of the available clinical characteristics was performed in this study, certain gaps in the data persist, and potential risk factors for rebleeding may have been missed. Future prospective studies should implement standardized protocols for data evaluation and collection and should take into account potential rebleeding risk factors such as liver stiffness, spleen stiffness, and relevant blood electrolyte concentrations.
In conclusion, we developed and externally validated a new REPET model for predicting rebleeding in patients with cirrhosis and EGVB comprising a set of broadly available clinical variables obtained from multiple perspectives. By revealing the expected probability of rebleeding for individuals at different time points, the REPET model allows for rational risk stratification of patients, which helps optimize follow-up and treatment regimens. In addition, the model presented here should be further evaluated in prospective cohorts and in independent cohorts at other centers.
We extend our appreciation to Dr. Kang-Li Yin from Shanghai Hospital of Integrated Traditional Chinese and Western Medicine for his invaluable contribution to the statistical analysis of our study. This study was not commercially funded.
| 1. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1477] [Article Influence: 492.3] [Reference Citation Analysis (2)] |
| 2. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1438] [Article Influence: 179.8] [Reference Citation Analysis (3)] |
| 3. | Jakab SS, Garcia-Tsao G. Evaluation and Management of Esophageal and Gastric Varices in Patients with Cirrhosis. Clin Liver Dis. 2020;24:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Yang J, Ge K, Chen L, Yang JL. The efficacy comparison of carvedilol plus endoscopic variceal ligation and traditional, nonselective β-blockers plus endoscopic variceal ligation in cirrhosis patients for the prevention of variceal rebleeding: a meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:1518-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Liu L, Nie Y, Liu Q, Zhu X. A Practical Model for Predicting Esophageal Variceal Rebleeding in Patients with Hepatitis B-Associated Cirrhosis. Int J Clin Pract. 2023;2023:9701841. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Li F, Wang T, Liang J, Qian B, Tang F, Gao Y, Lv J. Albuminbilirubin grade and INR for the prediction of esophagogastric variceal rebleeding after endoscopic treatment in cirrhosis. Exp Ther Med. 2023;26:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Wang J, Wang AJ, Li BM, Liu ZJ, Chen L, Wang H, Shi F, Zhu X. MELD-Na: effective in predicting rebleeding in cirrhosis after cessation of esophageal variceal hemorrhage by endoscopic therapy. J Clin Gastroenterol. 2014;48:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Wang X, Han H, Yang J, Cheng Y, Yin X, Gu L, Xiao J, Wang Y, Zou X, Wang L, Zhang M, Zhuge Y, Zhang F. Liver stiffness-spleen diameter to platelet ratio score (LSPS model) predicts variceal rebleeding for cirrhotic patients. Eur J Gastroenterol Hepatol. 2023;35:488-496. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Yokoyama S, Honda T, Ishizu Y, Imai N, Ito T, Yamamoto K, Mizuno K, Nakamura M, Kawashima H. Predicting early rebleeding and mortality after endoscopic hemostasis of esophagogastric varices: Diagnostic performance of aspartate aminotransferase-to-platelet ratio index and model for end-stage liver disease-Na score. J Hepatobiliary Pancreat Sci. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Seo YS. Prevention and management of gastroesophageal varices. Clin Mol Hepatol. 2018;24:20-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 2292] [Article Influence: 229.2] [Reference Citation Analysis (0)] |
| 12. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2290] [Article Influence: 229.0] [Reference Citation Analysis (3)] |
| 13. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 14. | Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, Bosch J. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (1)] |
| 15. | Chinese Society of Hepatology; Chinese Society of Gastroenterology; and Chinese Society of Digestive Endoscopology of Chinese Medical Association. [Guidelines on the management of esophagogastric variceal bleeding in cirrhotic portal hypertension]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:1029-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, Tajiri H, Tsukada K, Nonami T, Hashizume M, Hirota S, Murashima N, Moriyasu F, Saigenji K, Makuuchi H, Oho K, Yoshida T, Suzuki H, Hasumi A, Okita K, Futagawa S, Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Sarin SK, Kumar A, Angus PW, Baijal SS, Baik SK, Bayraktar Y, Chawla YK, Choudhuri G, Chung JW, de Franchis R, de Silva HJ, Garg H, Garg PK, Helmy A, Hou MC, Jafri W, Jia JD, Lau GK, Li CZ, Lui HF, Maruyama H, Pandey CM, Puri AS, Rerknimitr R, Sahni P, Saraya A, Sharma BC, Sharma P, Shiha G, Sollano JD, Wu J, Xu RY, Yachha SK, Zhang C; Asian Pacific Association for the Study of the Liver (APASL) Working Party on Portal Hypertension. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011;5:607-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Riley RD, Ensor J, Snell KIE, Harrell FE Jr, Martin GP, Reitsma JB, Moons KGM, Collins G, van Smeden M. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1129] [Article Influence: 225.8] [Reference Citation Analysis (1)] |
| 19. | Riley RD, Debray TPA, Collins GS, Archer L, Ensor J, van Smeden M, Snell KIE. Minimum sample size for external validation of a clinical prediction model with a binary outcome. Stat Med. 2021;40:4230-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 20. | Cifci S, Ekmen N. Evaluation of Non-invasive Fibrosis Markers in Predicting Esophageal Variceal Bleeding. Clin Endosc. 2021;54:857-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Mancini M, Strazzullo P, Ferrara LA, Barba G. [Treatment of hypertension associated with metabolic anomalies]. Cardiologia. 1988;33:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2928] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 23. | Diaz-Soto MP, Garcia-Tsao G. Management of varices and variceal hemorrhage in liver cirrhosis: a recent update. Therap Adv Gastroenterol. 2022;15:17562848221101712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Primignani M, Dell'Era A. Endoscopy in acute variceal bleeding: Not always the sooner, the better? Dig Liver Dis. 2019;51:999-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Wu B, Niu Z, Hu F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab J. 2021;45:526-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Balcar L, Mandorfer M, Hernández-Gea V, Procopet B, Meyer EL, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Samaniego LI, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Herrera EL, Laleman W, Palazón Azorín JM, Alonso JC, Gluud LL, Ferreira CN, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Grønbæk H, Hernandez Guerra MN, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Catalina MV, Albillos A, Rudler M, Tapias EA, Guardascione MA, Tantau M, Schwarzer R, Reiberger T, Laursen SB, Lopez-Gomez M, Cachero A, Ferrarese A, Ripoll C, La Mura V, Bosch J, García-Pagán JC; International Variceal Bleeding Observational Study Group by the Baveno Cooperation: an EASL consortium. Predicting survival in patients with 'non-high-risk' acute variceal bleeding receiving β-blockers+ligation to prevent re-bleeding. J Hepatol. 2024;80:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Giri S, Sundaram S, Jearth V, Bhrugumalla S. Predictors of early bleeding after endoscopic variceal ligation for esophageal varices: a systematic review and meta-analysis. Clin Exp Hepatol. 2022;8:267-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Gao Z, Zhao J, Liu X, Li S, Wang M, Gao Y. Portal vein thrombosis associated with high 14-day and 6-week rebleeding in patients after oesophageal variceal band ligation: a retrospective, multicentre, nested case-control study. Hepatol Int. 2021;15:1183-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Chen PH, Chen WC, Hou MC, Liu TT, Chang CJ, Liao WC, Su CW, Wang HM, Lin HC, Lee FY, Lee SD. Delayed endoscopy increases re-bleeding and mortality in patients with hematemesis and active esophageal variceal bleeding: a cohort study. J Hepatol. 2012;57:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Wu L, Fang QQ, Huang XQ, Xue CY, Rao CY, Luo JJ, Xu PJ, Chen Y, Chen S, Li F. Risk factors associated with failure of endoscopic combined treatment to prevent varices rebleeding in patients with liver cirrhosis. Expert Rev Gastroenterol Hepatol. 2023;17:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Jung JH, Jo JH, Kim SE, Bang CS, Seo SI, Park CH, Park SW. Minimal and Maximal Extent of Band Ligation for Acute Variceal Bleeding during the First Endoscopic Session. Gut Liver. 2022;16:101-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Wang AJ, Wang J, Zheng XL, Liao WD, Yu HQ, Gong Y, Gan N, You Y, Guo GH, Xie BS, Zhong JW, Hong JB, Liu L, Shu X, Zhu Y, Li BM, Zhu X. Second-look endoscopy-guided therapy under sedation prevents early rebleeding after variceal ligation for acute variceal bleeding. J Dig Dis. 2020;21:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Tonon M, Piano S. Cirrhosis and Portal Hypertension: How Do We Deal with Ascites and Its Consequences. Med Clin North Am. 2023;107:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, Development, and Treatment of Portal Vein Thrombosis in Patients With and Without Cirrhosis. Gastroenterology. 2019;156:1582-1599.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 35. | Lv Y, Bai W, Zhu X, Xue H, Zhao J, Zhuge Y, Sun J, Zhang C, Ding P, Jiang Z, Zhu X, Ren W, Li Y, Zhang K, Zhang W, Li K, Wang Z, Luo B, Li X, Yang Z, Guo W, Xia D, Xie H, Pan Y, Yin Z, Fan D, Han G. Development and validation of a prognostic score to identify the optimal candidate for preemptive TIPS in patients with cirrhosis and acute variceal bleeding. Hepatology. 2024;79:118-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |