Published online May 7, 2025. doi: 10.3748/wjg.v31.i17.105438
Revised: March 31, 2025
Accepted: April 21, 2025
Published online: May 7, 2025
Processing time: 89 Days and 1.6 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as a prominent and pervasive global health challenge. Bicuculline (BIC), which is a key active component of the anti-MASLD prescription "Eight Zhes Decoction", has been preliminarily shown by our research team to have significant potential in treating MASLD.
To determine BIC's efficacy in treating MASLD by regulating lipid metabolism and suppressing hepatic inflammation via nuclear factor-kappa B (NF-κB) path
This study explored the potential of BIC in preventing and treating MASLD using zebrafish, cellular (HepG2 and AML12), and mouse models.
Our results indicate that BIC significantly reduces lipid accumulation and inflammation both in vivo and in vitro. Transcriptomic analysis suggested that the anti-MASLD effects of BIC are linked to the inhibition of the NF-κB pathway, which plays a critical role in mitigating inflammation and lipid deposition.
This study is the first to demonstrate that BIC specifically alleviates lipid accumulation and hepatic steatosis in MASLD models via the NF-κB signaling pathway. Overall, BIC has emerged as a promising candidate for treating MASLD.
Core Tip: This study identifies bicuculline (BIC) as a novel agent for treating metabolic dysfunction-associated steatotic liver disease (MASLD), demonstrating for the first time the dual efficacy of BIC in reducing hepatic lipid accumulation and inflammation via nuclear factor-kappa B (NF-κB) pathway inhibition. Experiments involving zebrafish, mouse, and cellular (HepG2 and AML12) models confirmed that, mechanistically, BIC disrupts the NF-κB signaling pathway, which is a central hub that links metabolic dysfunction and inflammation in MASLD. Transcriptomic insights confirm pathway-specific targeting, distinguishing BIC from broad-spectrum therapies. These findings not only reveal a new axis that can be targeted in the treatment of MASLD but also position BIC as a precision therapeutic candidate with translational promise. The multimodel validation highlights the robustness of BIC, advancing this agent toward clinical exploration.
- Citation: Wang XM, Dai Z, Lu DJ, Bao CQ, Yang NB, Zhou YP. Bicuculline ameliorates metabolic dysfunction-associated steatotic liver disease by inhibiting the nuclear factor-kappa B pathway and reducing lipid accumulation. World J Gastroenterol 2025; 31(17): 105438
- URL: https://www.wjgnet.com/1007-9327/full/v31/i17/105438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i17.105438
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease worldwide, and it poses significant public health challenges. Owing to excessive hepatic lipid accumulation (hepatic steatosis), MASLD can progress to inflammation (steatohepatitis) and fibrosis[1]. Dysregulation of lipid metabolism, including increased free fatty acid (FFA) uptake and lipogenesis, impaired FFA oxidation, and reduced lipid export, contributes to the dysfunction of liver lipid metabolism[2]. In 2023, three major multinational liver societies reached a consensus, agreeing to replace the term “nonalcoholic fatty liver disease” with “metabolic dysfunction-associated steatotic liver disease”[3], highlighting the close association of this condition with metabolic syndrome and emphasizing the need to investigate the role of fatty acid metabolism in MASLD development[4]. Currently, no medical treatments have been approved for MASLD management, and lifestyle modifications, such as weight loss and exercise, remain the primary treatment approach[5]. Investigating pharmacological interventions that inhibit fatty acid synthesis and regulate lipid metabolism pathways may provide promising therapeutic strategies for reducing hepatic fat accumulation and treating MASLD.
Traditional Chinese medicines (TCMs) and certain natural products have attracted significant attention because of their potential for use in treating MASLD and its related complications due to their multicomponent, multitarget, and multipathway characteristics[6]. In our previous study, we developed a new TCM prescription called "Eight Zhes Decoction" (EZD), which has shown promising clinical efficacy in the treatment of MASLD. To identify the active components of EZD that function in the treatment of MASLD and to elucidate the underlying mechanisms, we employed a comprehensive approach integrating lipidomics, network pharmacology, and pharmacokinetics[7]. Bicuculline (BIC), which might be the main active substance of EZD that promotes the lowering of lipid levels, was identified as one of the most important active ingredients of BIC in the treatment of MASLD. BIC is a highly active isoquinoline alkaloid that is found in the natural product Corydalis decumbens (C. decumbens)[8]. In China, C. decumbens is listed in the Chinese Pharmacopoeia as a TCM that is used to treat paralytic stroke, headaches, rheumatoid arthritis, and sciatica[8]. Recently, it was discovered that C. decumbens can also be used as a remedy for eliminating blood stasis due to its antithrombotic effects, such as in the treatment of cardiovascular diseases[9]. At present, BIC is typically used as a GABAA receptor antagonist[10], and it has been shown to change the lipid composition of neuronal plasma membranes[11]. This study is the first report to identify BIC as a potential therapeutic agent for the management of MASLD, since BIC targets lipid metabolism and reduces liver inflammation. However, the precise mechanism by which BIC exerts these effects remains to be fully elucidated, warranting further investigation.
Although the pathogenesis of MASLD remains unclear, lipid metabolism disorders are commonly involved in the early and critical stages of its development[12]. In MASLD, abnormal lipid metabolism, such as whole-body lipolysis, increased hepatic FFA uptake, very-low-density lipoprotein synthesis, and decreased FFA oxidation and triglyceride (TG) output, can cause intrahepatic lipid accumulation[13]. The role of nuclear factor-kappa B (NF-κB) in regulating proinflammatory and anti-inflammatory processes, lipid metabolism, redox homeostasis, and systemic metabolic balance has been extensively discussed[14]. Under steady-state conditions, the NF-κB dimers p65 (RelA) and p50 are sequestered in the cytoplasm by IκB, which is an NF-κB inhibitor; sequestration by IκB prevents the translocation of p65 and p50 to the nucleus, thus preventing the transcription of target genes. However, under conditions of excessive fat accumulation, which disrupts homeostasis, the resulting fatty acids can induce the translocation of Bax to lysosomes, leading to NF-κB pathway activation[15,16]. These findings suggest that the NF-κB signaling pathway may underlie the mechanism by which BIC alleviates lipid metabolic disorders. Therefore, we hypothesize that BIC may ameliorate dyslipidemia and hepatic steatosis in mice with methionine/choline-deficient (MCD) diet-induced MASLD by inhibiting NF-κB activation, suggesting that BIC is a promising therapeutic candidate for clinical treatment.
To investigate the mechanism by which BIC promotes fatty acid catabolism, we established hepatocyte and zebrafish MASLD models to explore the therapeutic effects of BIC on MASLD. Through transcriptomic analysis, we identified the NF-κB signaling pathway as a potential target, and we subsequently validated this finding both in vivo and in vitro. To further confirm the anti-MASLD efficacy of BIC, an MCD diet-induced mouse model was used to assess the effects of BIC on lipid metabolism in vivo. Our findings demonstrate that BIC promotes fatty acid catabolism by inhibiting the NF-κB signaling pathway, thereby attenuating MASLD progression.
MCD feed was purchased from Synergy Biology (Batch No. XTM07-002, Jiangsu, China). AML12 specialized medium and DMEM basal medium were purchased from Pricella (Batch Nos. CM-0602 and PM150210B, Wuhan, China). Fetal bovine serum was purchased from Biological Industries (Batch No. 04-001-1ACS, Israel), and 100x penicillin-streptomycin solution (double antibody) solution and 0.25% trypsin solution were purchased from Pricella (Batch Nos. PB180120 and PB180224, Wuhan, China). The Cell Counting Kit-8 was purchased from New Cell and Molecular Biotech (Batch No. C6005, Suzhou, China). FFA solution with sodium oleate and sodium palmitate in solvent was purchased from Kunchuang Technology Development (Lot No. KC006, Xi'an, China). The total cholesterol (TCH/T-CHO) assay kit, TG assay kit, aspartate aminotransferase (AST/GOT) test kit, and alanine aminotransferase (ALT/GPT) test kit were purchased from Nanjing Jiancheng Bioengineering Institute (Batch Nos. A111-1-1, A110-1-1, C010-2-1, and C009-2-1, Nanjing, China). The oil red O staining kit for cells was purchased from Solarbio (Batch No. G1262, Beijing, China). Oil red O solution was purchased from Sigma-Aldrich (Batch No. O1516). Citrate sodium antigen retrieval solution, goat serum, and DAPI solution were purchased from Solarbio (Batch Nos. C1031, SL038, and C0065, Beijing, China). The TransScript All-in-One First-Strand cDNA Synthesis Kit for qPCR (with gDNA removed) was purchased from TransGen Biotech (Batch No. AT341, Beijing, China). RIPA lysis buffer was purchased from Beyotime (Batch No. P0013B, China). Rabbit anti-NF-κB/p65, rabbit anti-NF-κB/p50, rabbit anti-Lamin A/C, and mouse anti-GAPDH antibodies were purchased from Proteintech (Batch Nos. 10745-1-AP, 014220-1-AP, 10298-1-AP, and 60004-1-Ig, Wuhan, China). HRP-conjugated goat anti-rabbit IgG (H + L) and HRP-conjugated goat anti-mouse IgG (H + L) were purchased from Proteintech (Batch Nos. SA00001-2 and SA00001-1, Wuhan, China). The fluorescent secondary antibody Multi-rAb CoraLite® Plus 488-Goat Anti-Rabbit Recombinant Secondary Antibody (H + L) was purchased from Proteintech (Batch No. RGAR002, Wuhan, China).
Male C57BL/6 mice (20-22 g, 6 weeks old) were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. [License No. SCXK (Zhe) 2024-0001] with specific pathogen-free status. All the mice were raised in strict accordance with the guidelines for the care and use of laboratory animals at the Animal Experiment Center of Ningbo University. All the animals were bred and maintained in a controlled vivarium environment (temperature: 22 ± 1 °C; humidity: 55% ± 5%; 12-hour light/dark cycle) with ad libitum access to autoclaved water and standard chow. The bedding (corn cob) was replaced twice weekly under laminar airflow to minimize ammonia exposure.
The zebrafish parents were male and female zebrafish from the same batch of wild-type AB strains, and they were housed in the Zebrafish Model of Human Diseases and Drug Screening Laboratory at the School of Marine Sciences, Ningbo University. The zebrafish were maintained at 28 °C under a 14-hour/10-hour light-dark cycle. The water was maintained at a pH of 7.0-8.0 and a conductivity of 450-500 microsecond/cm. The zebrafish were fed freshly hatched brine shrimp twice daily, in the morning and evening.
HepG2 hepatocellular carcinoma cells and AML12 hepatocytes were both purchased from Procell. Both cell types were cultured in complete medium (89% DMEM + 10% fetal bovine serum + 1% penicillin-streptomycin solution) and AML12-specific medium and incubated at 37 °C in 5% CO2 in a cell culture incubator.
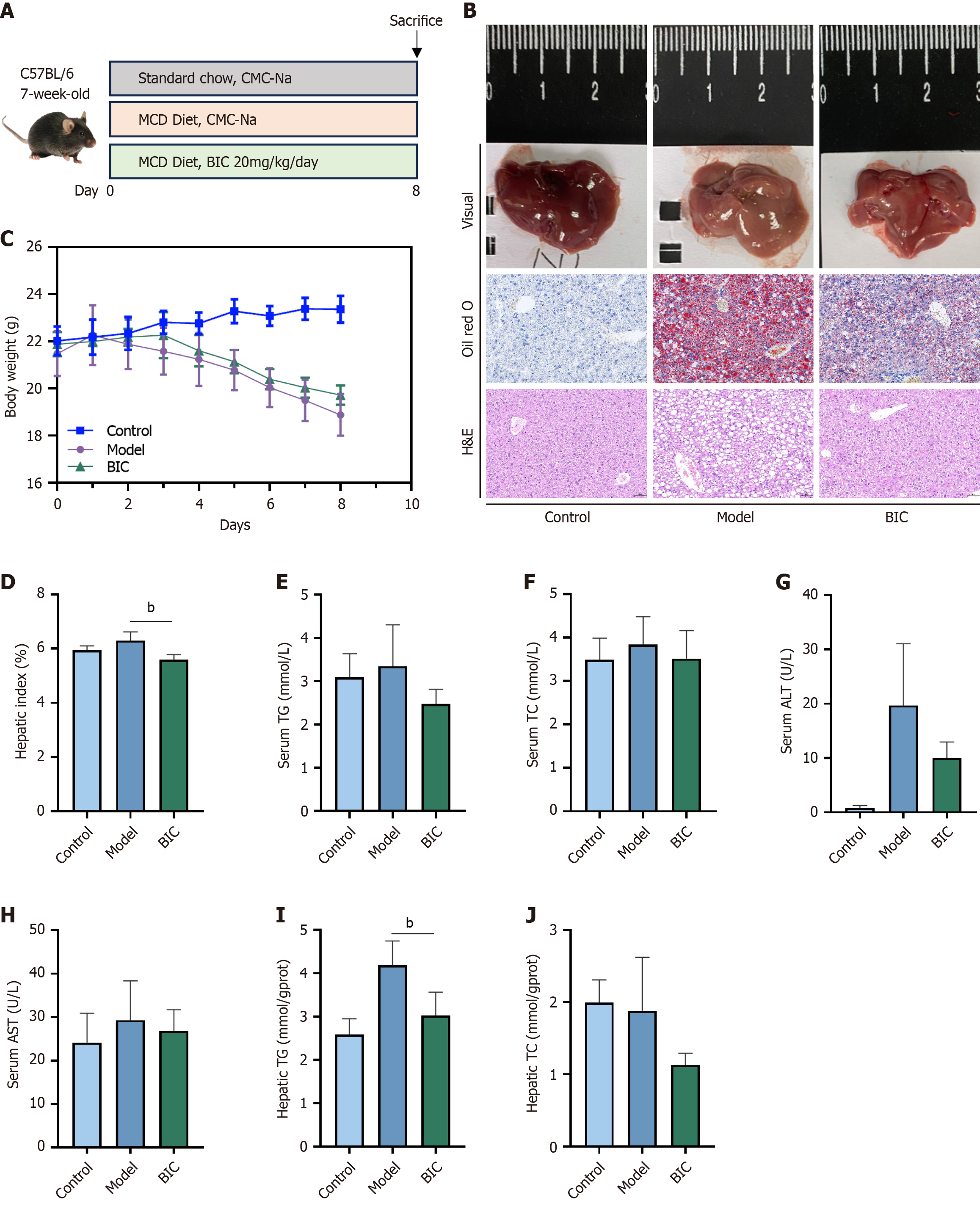
Wild-type male C57BL/6 mice aged 6 weeks and weighing 20-23 g were randomly divided into 3 groups (n = 5). After 1 week of acclimatization, the model group was fed an MCD diet (21% fat energy, methionine- and choline-deficient diet) for 8 days to establish a MASLD mouse model. The BIC group was fed the MCD diet and treated with BIC [dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na)] at a dosage of 20 mg/kg/day via oral administration. The control group was fed a normal diet and was administered the same volume of 0.5% CMC-Na as the BIC group. After 8 days, the mice were euthanized under anesthesia (sodium pentobarbital). Subsequent analyses were performed in a blinded manner.
Parental zebrafish were placed in tanks with mesh-bottom dividers at a female-to-male ratio of 2:3. After spawning, the eggs were collected and distributed into 24-well plates, and the eggs were randomly assigned to 14 groups with 40 eggs per group. Concentration groups of 25 μM, 50 μM, 100 μM, 200 μM, and 400 μM were established, and survival rates were recorded over 96 hours. The developmental status and heart rate of the zebrafish were observed at 96 hours posttreatment.
HepG2 cells were treated with a FFA solution containing 250 μM sodium palmitate and 500 μM sodium oleate for 24 hours, successfully establishing a high-fat cell model (nonalcoholic fatty liver cell model) in HepG2 cells. The cells were subsequently stained with oil red O according to the manufacturer's instructions. After staining, the cells were observed and photographed under an optical microscope. Semiquantitative analysis was performed using ImageJ software.
Slides of AML12 cells were prepared and treated with 25 μM BIC for 48 hours to establish the MASLD model. After intervention, the cells were washed twice with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, subjected to antigen retrieval with citrate sodium antigen retrieval solution, and blocked with goat serum. The cells were then incubated with an anti-NF-κB/p65 antibody (1:50 dilution) at 4 °C overnight. The next day, the slides were washed with PBST to remove unbound antibodies and incubated with the Multi-rAb CoraLite® Plus 488-goat anti-rabbit recombinant secondary antibody (H + L) in the dark for 2 hours. Then, the slides were washed three times with PBST and incubated with DAPI solution to stain the nuclei. The slides were mounted and observed using a confocal microscope (TCS SP8 X, Leica, Germany).
Plasma: Blood samples were collected from mice that had been fasted for 12 hours. Heparin was added to the blood samples as an anticoagulant. The blood samples were centrifuged at 3000 rpm for 8 minutes at 4 °C, and the plasma was collected for analysis. Liver: Liver tissue was accurately weighed, and anhydrous ethanol was added at a weight (g)-to-volume (mL) ratio of 1:9. The mixture was mechanically homogenized in an ice-water bath and centrifuged at 2500 rpm for 10 minutes. The supernatant was collected for analysis. Cells: The prepared cell suspension was centrifuged at 1000 rpm for 10 minutes, and the supernatant was discarded, leaving the cell pellet. The pellet was washed 1-2 times with PBS and centrifuged again at 1000 rpm for 10 minutes, after which the supernatant was discarded. The cell pellet was lysed with lysis buffer. The lysate was directly used for analysis without centrifugation. Finally, the TG, total cholesterol (TC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured with assay kits according to the manufacturers’ instructions.
Formaldehyde-fixed liver and zebrafish samples were embedded in paraffin, sectioned at a thickness of 4 μm, and subjected to hematoxylin & eosin (H&E) staining and immunohistochemistry. Frozen sections (7 μm thick) were stained with oil red O, counterstained with Mayer's hematoxylin, and observed under a light microscope to analyze hepatic fat accumulation. Immunohistochemistry was performed using an antip65 antibody (1:50). For each immunohistochemical staining process, PBS was used as a negative control. Digital images were captured using a Nikon DS-Ri1 camera equipped with NIS-Element AR 4.30.02 software. For each group, semiquantitative analysis was performed using images (final magnification: 400 ×) of 5 samples (Fiji ImageJ v1.2).
AML12 and HepG2 cells were seeded in 96-well plates and treated with different concentrations of BIC (12.5, 25, 50, or 100 μM) for 48 hours. At the end of the treatment period, serum-free medium supplemented with 10% CCK-8 was added, and the cells were incubated at 37 °C in 5% CO2 for 2 hours. The absorbance at 450 nm was measured using a Multiskan SkyHigh full-wavelength microplate reader (A51119500C, Thermo Scientific™, United States). Cell viability was calculated as [(As - Ab)/(Ac - Ab)] × 100%, where As is the absorbance of the drug-treated group, Ac is the absorbance of the control group, and Ab is the absorbance of the blank well.
Total RNA was isolated from tissues or cells via TRIzol reagent according to the manufacturer's protocol. The mRNA was reverse-transcribed into cDNA. qPCR analysis was performed using EvaGreen qPCR MasterMix on a MiniOpticon Real-Time PCR Detection System (Bio-Rad Laboratories, United States). The sequences of the primers that were used are listed in Tables 1 and 2.
| Primer | Primer sequence (5’-3’) | Primer sequence (5’-3’) |
| β-actin | ATGGATGAGGAAATCGCTGCC | CTCCCTGATGTCTGGGTCGTC |
| Srebp1 | CATCCACATGGCTCTGAGTG | CTCATCCACAAAGAAGCGGT |
| Fasn | GCACCGGTACTAAGGTTGGA | ACACAACCGACCATCTGTCA |
| Scd1 | ACGCTCCTCAGATACGCACT | AGTCGTAGGGAAACGTGTGG |
| Hmgcr | CCTGTTAGCCGTCAGTGGA | TCTTTGACCACTCGTGCCG |
| APOA1 | GCACTAAGCTGACCGAGCGT | GGAGGTCCTGGGTGTGTGGA |
| PPARγ | TGCTGGACTACCAGAACTGTGACA | TGCTGGCTGAGAACACTTCTGAG |
| IL-6 | CCTCAAACCTTCAGACCGCT | GAACAGGATCGAGTGGACCG |
| TNF-α | GCTTATGAGCCATGCAGTGA | TGCCCAGTCTGTCTCCTTCT |
| Primer | Primer sequence (5’-3’) | Primer sequence (5’-3’) |
| GAPDH | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT |
| Srebf1 | AGCCATCGACTACATCCG | TCCATAGACACATCTGTGCCTC |
| Fasn | TGGTGGTGTGGACATGGTCACAGA | CCGAAGCTGGGGGTCCATTGTGTG |
| Scd1 | ACACCATGGCGTTCCAGAAT | AGCTTCTCGGCTTTCAGGTC |
| Dgat1 | TCGTGGTATCCTGAATTGGTG | AGGTTCTCTAAAAATAACCTTGCATT |
| Dgat2 | GCTGGTGCCCTACTCCAAG | CCAGCTTGGGGACAGTGA |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| COX-2 | TTCCAATCCATGTCAAAACCGT | AGTCCGGGTACAGTCACACTT |
| TNF-α | AGGAGGAATTTGGCCAGGTG | GCTCACGAGGAGGCTAATCC |
To obtain total protein, tissues or cells were lysed with RIPA buffer supplemented with protease and phosphatase inhibitor cocktails. The lysates were centrifuged, and protein concentrations were measured using the BCA protein assay. The proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were then incubated overnight at 4 °C with primary antibodies, including rabbit anti-NF-κB/p65 (1:2000), rabbit anti-NF-κB/p50 (1:4000), rabbit anti-lamin A/C (1:10000), and mouse anti-GAPDH (1:10000) antibodies. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (1:10000) for 1 hour. The protein bands were visualized using an electrochemiluminescence system (Bio-Rad, United States) and quantified using ImageJ (v1.53) software. Protein expression levels were normalized to those of GAPDH (cytosolic fractions) or Lamin A/C (nuclear fractions). Statistical comparisons between groups were performed using one-way ANOVA followed by Tukey’s post hoc test.
Genome-wide gene expression analysis was performed on cells from the model and BIC groups. Total RNA was extracted using the TRIzol method, and cDNA samples were sequenced on the Illumina HiSeq 4000 platform (2 × 150 bp read length) by Shanghai Mailiao Biotechnology Co., Ltd. The reference mouse genome and gene information were downloaded from the National Center for Biotechnology Information database. Normalization and identification of differentially expressed genes (DEGs) were performed using DESeq2 (v1.34.0) in R. Genes with |log2 fold change| > 1 and adjusted P value (FDR) < 0.05 were considered to be significantly differentially expressed. All the DEGs were subjected to Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Enrichment analysis was conducted using the clusterProfiler (v4.2.2) R package. Terms with FDR-adjusted P values < 0.05 were retained. Pathway enrichment was performed via DAVID (v2021q3). The significance thresholds were set to P < 0.05 (Fisher’s exact test) with Benjamini-Hochberg correction.
All the experiments were performed with at least three biological replicates, and the data are expressed as the mean ± SD. Statistical analysis and the generation of graphs were performed using GraphPad Prism (10.2) software. For normally distributed data, one-way ANOVA with Tukey’s post hoc test was used for multiple group comparisons. Nonparametric data (e.g., lipid droplet area) were analyzed using the Kruskal-Wallis test followed by Dunn’s post hoc correction. P < 0.05 was considered to indicate statistically significant differences.
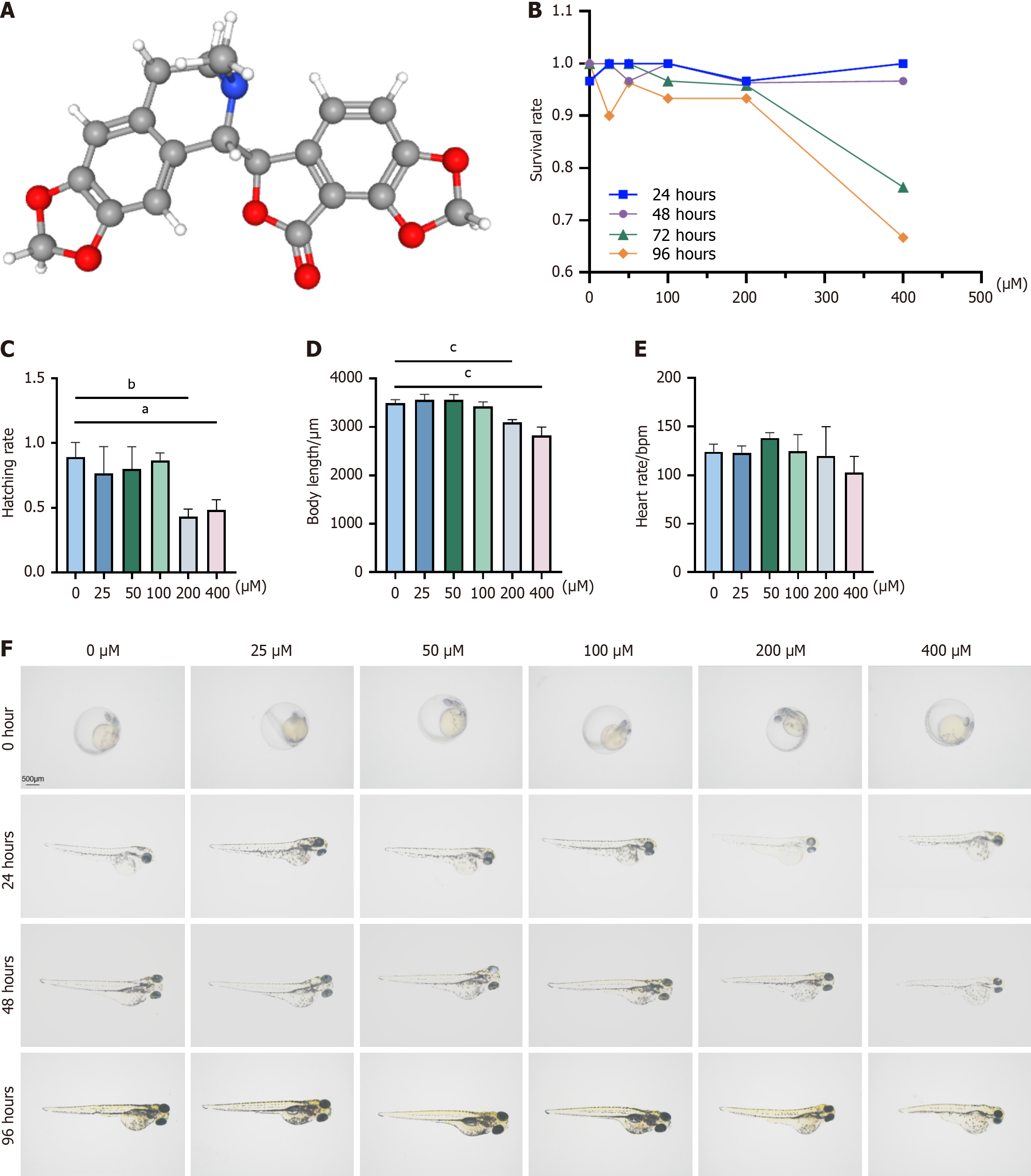
BIC is a highly active isoquinoline alkaloid that is found in traditional Chinese herbs, such as corydalis, and is included in the prescription EZD. The molecular weight of BIC is 367.4, and its three-dimensional structure is shown in Figure 1A. To determine the safe concentration range of BIC, zebrafish larvae were exposed to various concentrations (25 μM, 50 μM, 100 μM, 200 μM, or 400 μM), and survival, morphological changes, body length, and heart rate were monitored at 24, 48, 72, and 96 hours postfertilization (hpf). After treatment with BIC at concentrations of 200 μM and higher, a decrease in survival was observed at 72 and 96 hpf (Figure 1B), whereas lower concentrations (25-100 μM) had no adverse effects on development, as indicated by successful hatching at 48-72 hpf (Figure 1C). Lrvae that were exposed to 200 and 400 μM BIC presented shorter body lengths than those in the control group (Figure 1D), although no significant differences in heart rate were observed among the groups (Figure 1E). Additionally, morphological assessments revealed no significant changes in larvae that were exposed to 25-100 μM BIC, but dysplasia was observed in those that were treated with 200-400 μM BIC for 72-96 hpf (Figure 1F). The IC50 values of BIC in zebrafish embryos (24 hours IC50: 31.93 μM; 48 hours IC50: 164.0 μM) were used to observe the dose-response relationship. Given these findings, BIC concentrations of 25 μM, 50 μM, and 100 μM were selected for subsequent experiments, as these concentrations did not affect growth, development, or heart rate, with zero deaths and no teratogenic effects within 96 hours.
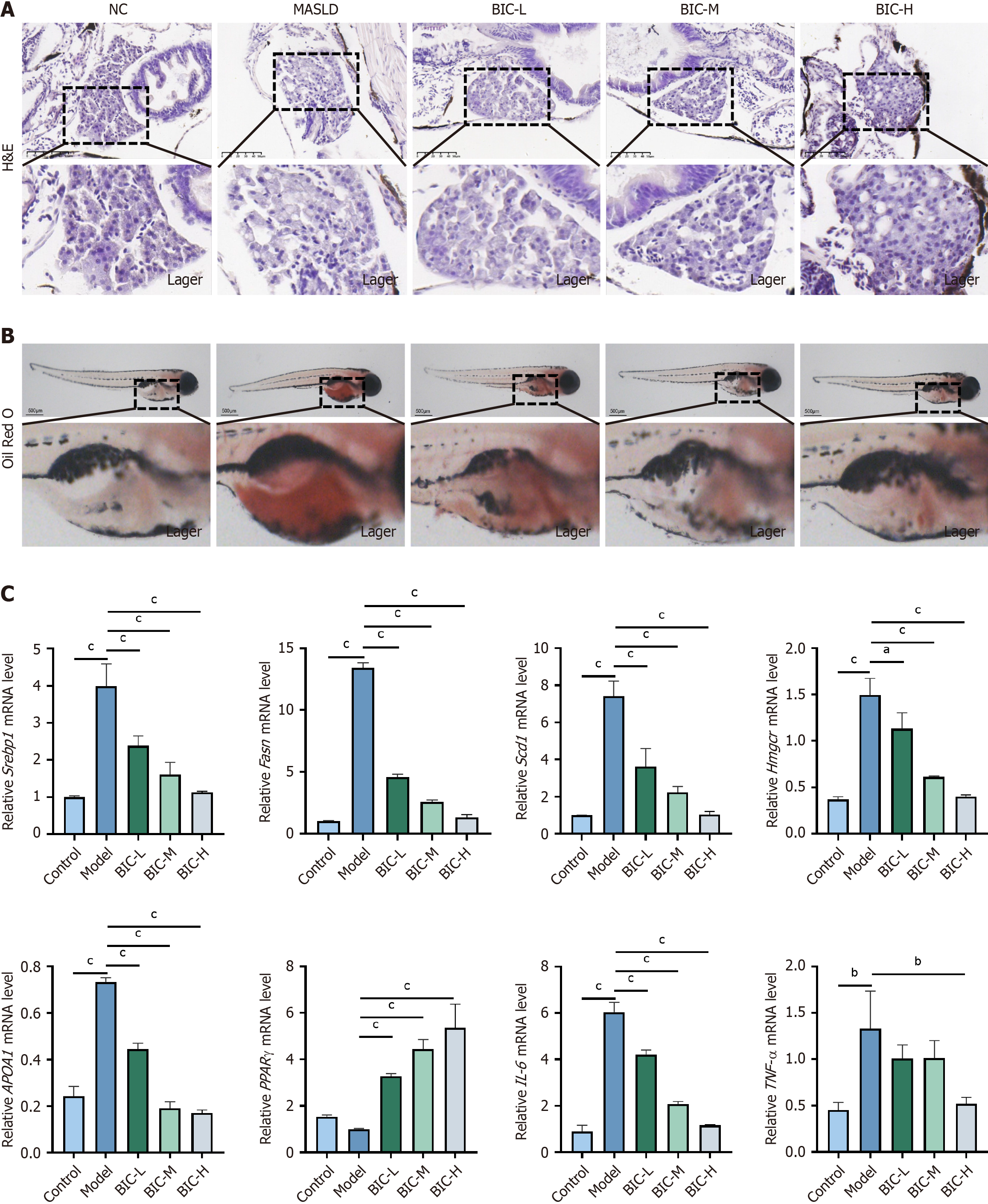
To investigate the effects of BIC on lipid deposition and inflammation in the liver, a thioacetamide (TAA)-induced zebrafish model was used. The livers of zebrafish that were treated with 0.4 mg/mL TAA exhibited loose intercellular contact and large vacuoles, which are indicative of liver damage and steatosis (Figure 2A). In contrast, treatment with low, medium, or high concentrations of BIC alleviated TAA-induced lipid deposition, with high BIC concentrations having the most pronounced effect (Figure 2B). Consistent with these histological findings, whole-fish oil red O staining confirmed the reduction in lipid accumulation (Figure 2B). Gene expression analysis via RT-PCR revealed that TAA treatment significantly upregulated the expression of genes that are involved in lipid metabolism, including Srebp1, Fasn, Scd1, Hmgcr, and APOA1 (P < 0.05), and downregulated PPARγ expression. Additionally, TAA-induced inflammation was evident by the increased expression of IL-6 and TNF-α (P < 0.05). Notably, BIC treatment mitigated these metabolic and inflammatory changes in a dose-dependent manner (Figure 2C). These findings suggest that BIC exerts a protective effect against TAA-induced MASLD by modulating lipid metabolism and reducing inflammation, highlighting its poten
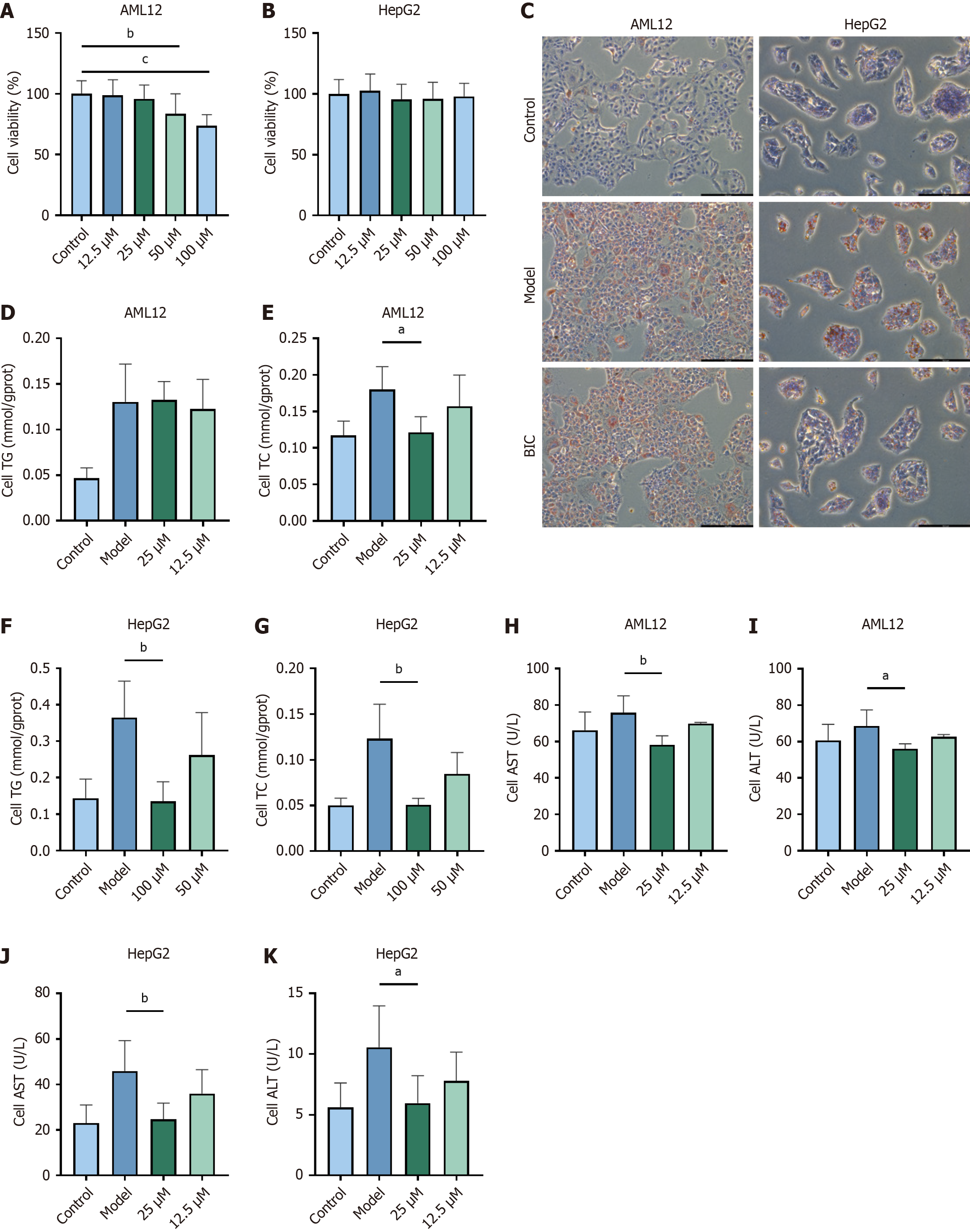
To evaluate the potential of BIC to treat MASLD, HepG2 and AML12 cell models were established by exposing these cells to a FFA solution. Then, cells that were treated with the FFA solution were exposed to BIC at concentrations of 12.5, 25, 50, or 100 μmol/L for 48 hours, followed by an assessment of cell viability (Figure 3A and B). When added at concentrations 100 μM, BIC exerted no cytotoxic effects on HepG2 cells, whereas moderate toxicity was observed in AML12 cells (IC50: 113.7 μM). In zebrafish, BIC concentrations ≤ 100 μM caused no mortality or developmental abnormalities, which was consistent with its therapeutic safety window. Nontoxic concentrations were subsequently selected for further pharmacological interventions. Oil red O staining revealed a significant reduction in lipid accumulation in FFA-treated cells after BIC treatment (Figure 3C). Furthermore, measurements of TG and TC levels demonstrated the lipid-lowering effect of BIC, and a notable reduction in TC levels were observed after treatment with higher doses of BIC (Figure 3D-G). Additionally, analysis of the levels of ALT and AST, which are key indicators of liver function, indicated that BIC significantly alleviated hepatic impairment in the cell model of MASLD, thereby exerting hepatoprotective effects (Figure 3H-K). Collectively, these findings suggest that BIC effectively reduces lipid accumulation and protects liver function in cell models of MASLD.
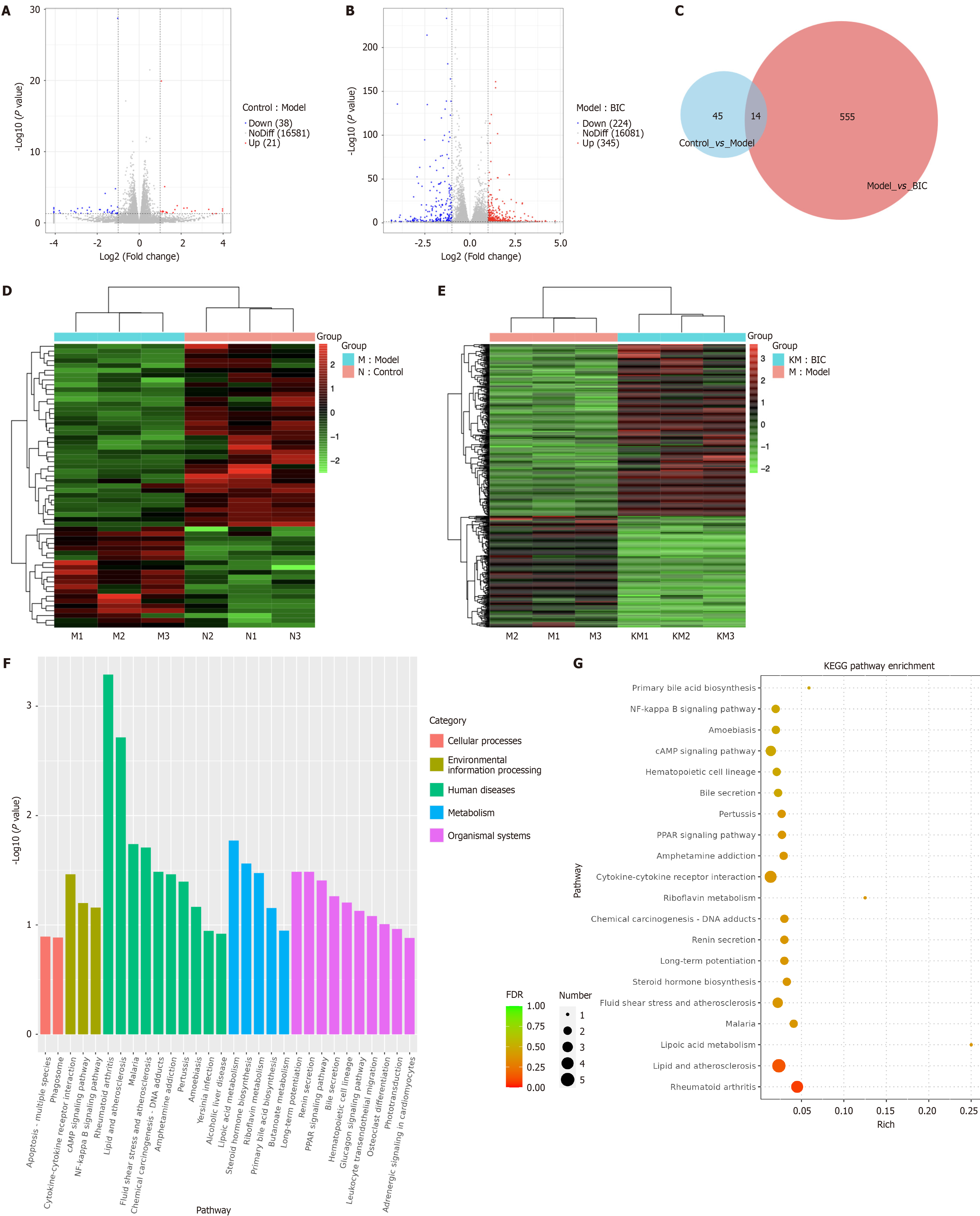
To investigate the mechanisms by which BIC regulates lipid accumulation in MASLD models, we performed RNA-seq analysis on cells from the control, model, and BIC-H groups. As shown in Figure 4A, we identified 59 DEGs between the model and control groups and 569 DEGs between the BIC-H and model groups (Figure 4A-E). Among these genes, 14 exhibited opposite expression trends in the BIC-H group compared with the model group, including 5 upregulated genes and 7 downregulated genes (Figure 4C). GO and KEGG pathway enrichment analyses of these DEGs (Figure 4F and G) revealed that the DEGs were significantly enriched in inflammatory regulation, metabolic reprogramming, and disease-associated pathways. Compared with the BIC group, the model group exhibited pronounced activation of immune-inflammatory responses (e.g., the NF-κB signaling pathway) and activation of pathways associated with lipid metabolism disorders (e.g., primary bile acid biosynthesis and the PPAR signaling pathway). Specifically, the interaction between NF-κB signaling and cytokine networks may drive chronic inflammation, whereas altered bile acid synthesis and PPAR-mediated regulation reflect adaptive shifts in lipid metabolism. These findings suggest that BIC mitigates MASLD by modulating key pathways that are involved in inflammation resolution, lipid homeostasis, and metabolic reprogramming via DEGs. This study provides mechanistic insights into the therapeutic effects of BIC in MASLD pathogenesis.
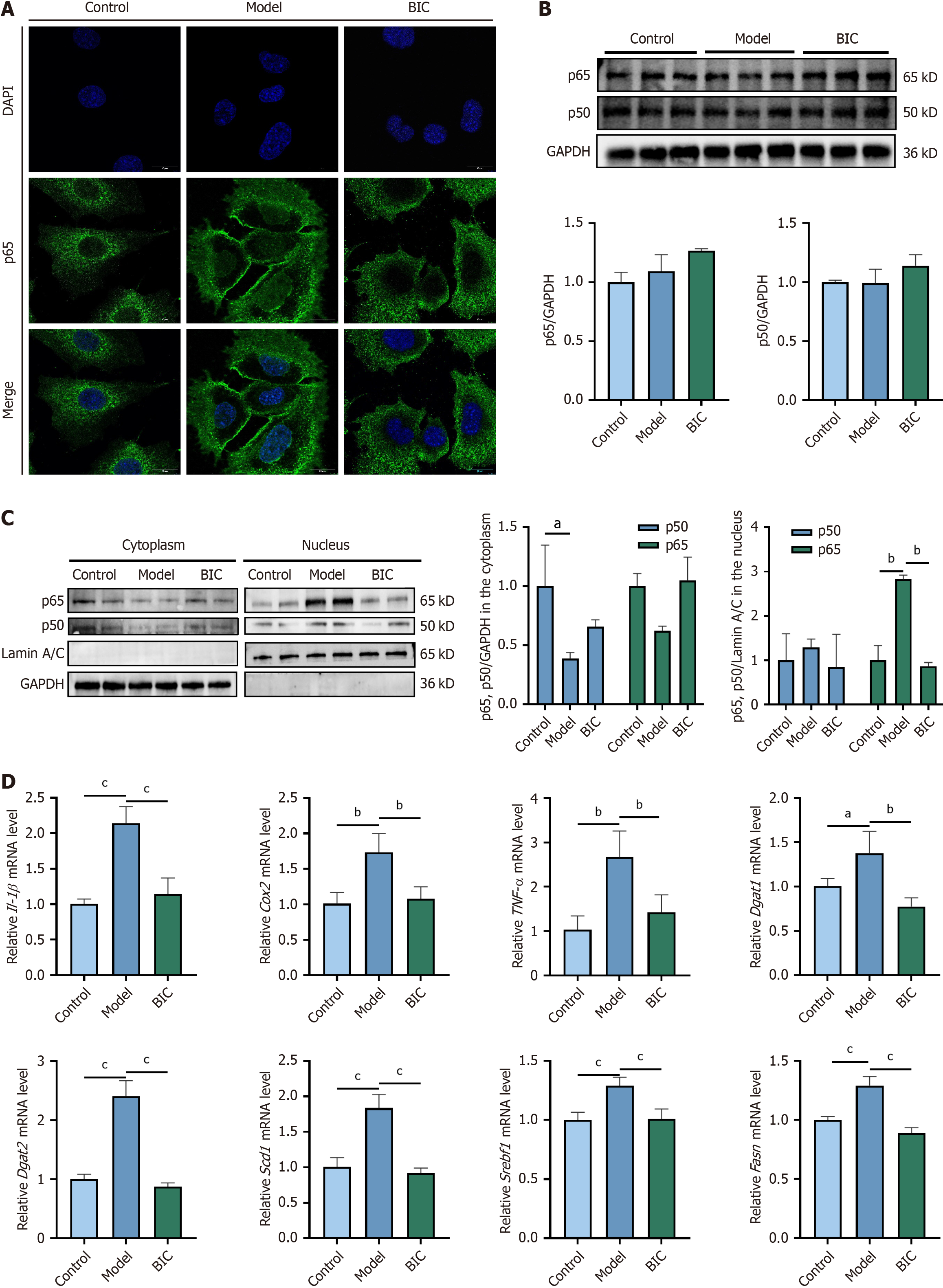
To confirm the transcriptome results, we further explored the mechanism by which BIC inhibits MASLD in the cell model. Immunofluorescence revealed that in AML12 cells, NF-κB was activated and translocated to the nucleus after 48 hours of FFA stimulation. A strong green fluorescence signal of NF-κB (p65) was observed in the nucleus of FFA-exposed cells, indicating that lipid accumulation triggered the translocation of NF-κB (p65) from the cytoplasm to the nucleus (Figure 5A). Notably, BIC treatment reduced NF-κB nuclear translocation.
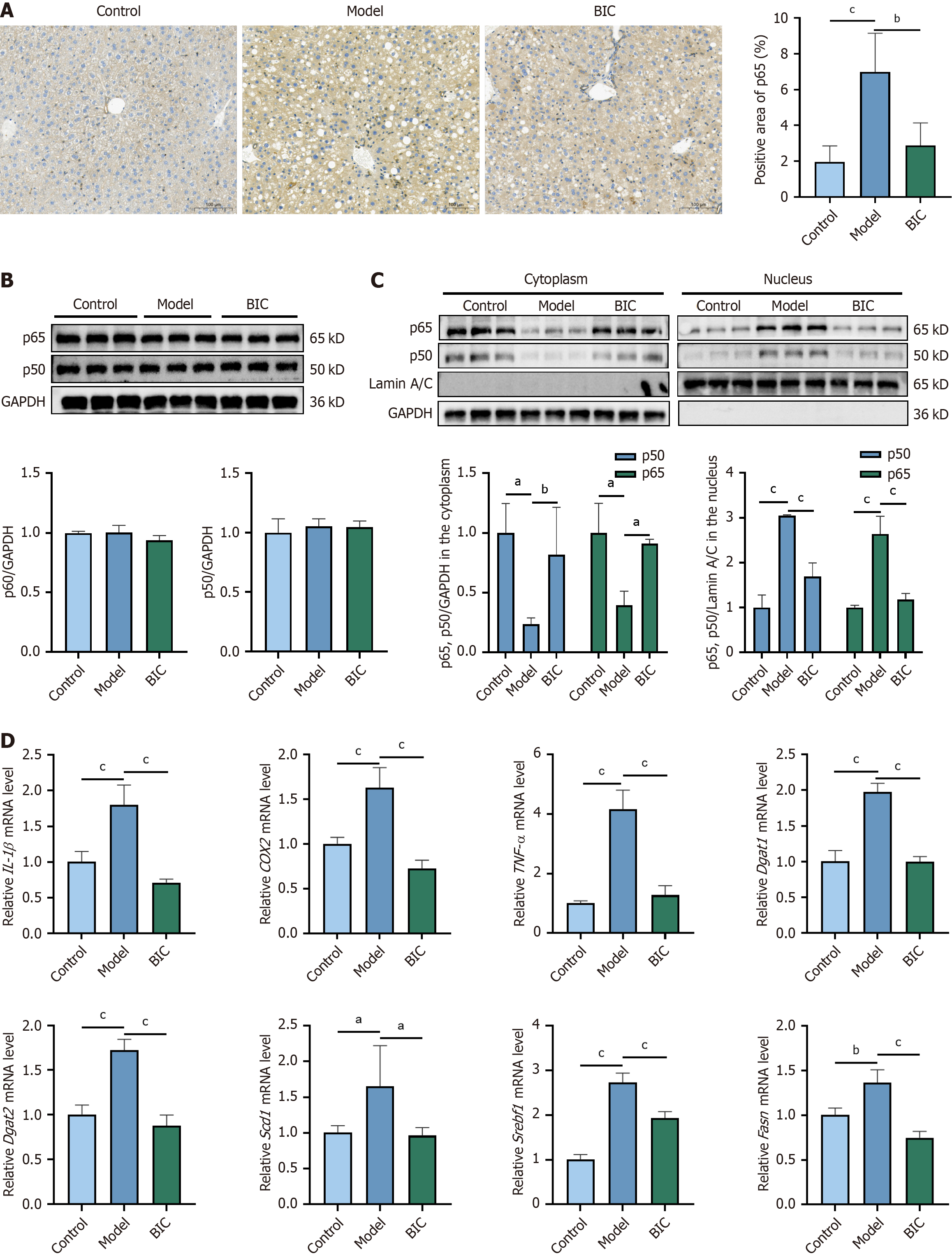
Western blotting analysis revealed no significant difference in total p65 or p50 protein levels across the groups (Figure 5B). However, cytoplasmic and nuclear fractionation revealed that FFAs induced significant nuclear translocation of p65 and p50. Importantly, treatment with 25 μM BIC notably reduced the proportions of p50 and p65 in the nucleus by 44.8% and 183.2%, respectively (Figure 5C). Furthermore, BIC treatment significantly attenuated the overexpression of inflammatory factors such as IL-1β, COX2 and TNF-α, indicating the inhibition of the NF-κB pathway and a reduction in the inflammatory response. RT-PCR analysis of lipid metabolism-related genes revealed increased fatty acid metabolism after BIC treatment, which was specifically manifested as a reduction in Dgat1, Dgat2, Scd1, Srebf1, and Fasn expression (Figure 5D). In conclusion, BIC mitigates the NF-κB activation-induced inflammatory response and ameliorates FFA-induced lipid metabolism disorders, thereby alleviating the progression of MASLD.
To confirm the effectiveness of BIC against MASLD in the zebrafish and cell models, a mouse model of MASLD was established. Male mice were fed a MCD diet for 8 days, which resulted in a condition that effectively mimicked the hepatic steatosis, inflammation, and hepatocyte damage that is observed in human MASLD (Figure 6A). Histological analyses using oil red O and H&E staining revealed excessive lipid droplet accumulation, balloon-like changes, hepato
To further investigate the effects of NF-κB activation on the antilipid deposition and anti-inflammatory effects of BIC, immunohistochemical staining was performed on liver sections from control mice, MASLD model mice, and BIC-treated MASLD model mice. The results revealed a significant increase in NF-κB (p65) nuclear localization in hepatocytes from MASLD model mice, which was indicative of increased NF-κB activation. BIC treatment notably reduced NF-κB acti
In our previous work[7], we demonstrated that EZD, which is a TCM formulation and a source of BIC, has lipid-lowering effects. BIC is widely used to study GABA energy transmission, the role of GABA in the nervous system[17], and the mechanism underlying epilepsy as well as to screen for antiepileptic drugs[18]. In the present study, we report for the first time the beneficial therapeutic effects of BIC on lipid accumulation and hepatic steatosis in both in vivo and in vitro models of MASLD.
Dysfunction of lipid metabolism is a central pathological process in the development of MASLD. Therefore, targeting dysregulated lipid metabolism is a promising strategy for blocking or delaying disease progression. Some natural products[19-21] have shown beneficial effects in ameliorating hepatic steatosis, particularly bioactive molecules with low toxicity and lipid-lowering properties, and these molecules have attracted increasing attention because of their potential for use in the management of MASLD. For example, cordycepin and the Yindanxinnaotong formula reduce the production of harmful lipid metabolites by regulating the AMPK pathway[22,23]. Additionally, Zhi-Kang-Yin has been shown to reduce lipid levels in the liver and serum, alleviating hepatic steatosis[24]. The results of the present study indicate that BIC ameliorated hepatic lipid deposition by downregulating genes that are associated with fatty acid synthesis and upregulating those that are involved in fatty acid degradation (Dgat1, Dgat2, Scd1, Srebf1, and Fasn), as suggested by the results of the GO analysis of DEGs in the transcriptome. In this study, the powerful fatty acid catabolism of BIC was demonstrated by transcriptome sequencing as well as in vivo and in vitro studies.
Transcriptome analysis indicated that the anti-MASLD effect of BIC is closely associated with the inhibition of NF-κB activation, which subsequently reduces hepatic lipid deposition. Currently, the role of NF-κB in disease is receiving increasing attention, and NF-κB is considered a potential new target for treating liver disease. However, the mechanisms underlying NF-κB and lipid metabolism in the liver have not been fully investigated. Current knowledge indicates that classical NF-κB activation requires Scap-mediated Golgi translocation and S1P/S2P-mediated Srebp1 release to play a role in lipid signaling in related diseases[25]. F. nucleatum activates the NF-κB signaling pathway in macrophages, which promotes inflammation, decreases lipid excretion, and promotes lipid deposition. Meanwhile, after CD147 (an upstream protein of NF-κB) was knocked down, the formation of lipid droplets was reduced, suggesting that NF-κB induces cellular lipid deposition through CD147[26]. MASLD increases inflammatory cytokines, which activate the NF-κB signaling pathway and form a feedback loop that exacerbates inflammation and lipid deposition[27]. Similarly, Yanggan Jiangmei formula (YGJMF) can inhibit the NF-κB/NLRP3 signaling pathway to alleviate hepatic steatosis, thereby exerting anti-inflammatory effects on MASLD[28]. Validation studies in cell models and mouse livers have shown that when NF-κB is activated and translocates to the nucleus as a dimer (p65/p50), it may promote lipid metabolism and inflammatory cytokine transcription. BIC effectively alleviates dyslipidemia and hepatic lipid degeneration in the MASLD model by inhibiting NF-κB activation in the liver. This study not only elucidates the intrinsic mechanism underlying the effect of BIC on the NF-κB signaling pathway but also reveals a novel mechanism for treating hepatic steatosis and inflammation.
Moreover, the anti-MASLD effects of BIC are not limited to NF-κB inhibition but also involve a multipathway regulatory network (Figure 4). The downregulation of bile acid synthesis genes (e.g., Cyp7a1) in the BIC-treated group suggested that BIC may restore bile acid homeostasis, thereby reducing endoplasmic reticulum (ER) stress and improving hepatocyte survival. PPARα/γ agonists are well-established modulators of lipid metabolism and inflammation[29,30]. The BIC-induced upregulation of PPAR target genes (e.g., CD36, Fads2) is consistent with increased fatty acid oxidation and decreased lipogenesis, indicating a potential synergistic interplay between NF-κB inhibition and PPAR activation. The hierarchical and interrelated regulatory mechanisms involving the NF-κB, PPAR, and bile acid pathways highlight that BIC shows therapeutic potential by acting on multiple targets. These findings position BIC as a multitarget agent that is capable of simultaneously regulating MASLD-associated inflammation, metabolic dysfunction, and lipotoxicity. These findings suggest that future studies should focus on evaluating the use of BIC in combination with pathway-specific agents (e.g., PPAR agonists) to optimize therapeutic strategies.
In this study, both in vitro and in vivo experiments confirmed the inhibitory effect of BIC on MASLD models. Furthermore, the specific mechanism by which BIC inhibits the NF-κB pathway, thereby reducing inflammation and promoting fatty acid oxidation, was elucidated. These findings suggest that BIC is a promising candidate for the treatment of MASLD and that it potentially overcomes the limitations of currently available treatments. However, further studies are needed to fully elucidate the function of BIC in treating MASLD. The mechanism by which NF-κB inhibition reduces hepatic lipid metabolism has not yet been fully or thoroughly investigated and needs to be further explored. BIC has good therapeutic effects in MASLD models, but the mechanisms remain to be validated and explored in future studies. Although the MCD diet-induced model that was used in this study effectively recapitulates the hepatic pathological features that are characteristic of human MASLD, it does not fully capture the systemic metabolic perturbations that are observed in clinical patients, notably obesity and insulin resistance. To comprehensively validate the anti-MASLD efficacy of BIC, complementary studies utilizing established preclinical models-including long-term high-fat diet-fed mice (16-24 weeks) and genetic models (e.g., ob/ob or db/db mice)-are warranted. These investigations should focus on assessing the capacity of the BIC to induce sustained improvements in liver function biomarkers (ALT/AST), lipid homeostasis, and fibrosis regression. Notably, while our preliminary data indicate no overt toxicity in short-term exposure models, rigorous long-term safety evaluations in chronic MASLD models remain imperative to confirm the translational potential of BIC. To accelerate the clinical translation of BIC for MASLD management, our future research will focus on combining its administration with practical lifestyle interventions (e.g., diet/exercise) and investigating long-term dose-response patterns and organ-specific safety profiles under extended treatment conditions.
In conclusion, a comprehensive approach utilizing zebrafish, cellular, and mouse models was used to investigate the efficacy of BIC in the prevention and treatment of MASLD. In both in vivo and in vitro settings, BIC treatment significantly reduced intracellular lipid accumulation and alleviated MASLD-induced inflammatory responses. Transcriptomic analyses suggested a potential role for the NF-κB pathway in mediating these effects. Validation experiments confirmed that BIC inhibits NF-κB activation, thereby reducing inflammation and lipid deposition and effectively inhibiting MASLD progression.
| 1. | Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J Hepatol. 2023;79:1524-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 2. | Hong T, Chen Y, Li X, Lu Y. The Role and Mechanism of Oxidative Stress and Nuclear Receptors in the Development of NAFLD. Oxid Med Cell Longev. 2021;2021:6889533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1312] [Article Influence: 656.0] [Reference Citation Analysis (1)] |
| 4. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 919] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 5. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 6. | Zhu LR, Li SS, Zheng WQ, Ni WJ, Cai M, Liu HP. Targeted modulation of gut microbiota by traditional Chinese medicine and natural products for liver disease therapy. Front Immunol. 2023;14:1086078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 7. | Zhou Y, Dai Z, Deng K, Wang Y, Ying J, Chu D, Zhou J, Tang C. Eight Zhes Decoction ameliorates the lipid dysfunction of nonalcoholic fatty liver disease using integrated lipidomics, network pharmacology and pharmacokinetics. J Pharm Anal. 2023;13:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Wu C, Yan R, Zhang R, Bai F, Yang Y, Wu Z, Wu A. Comparative pharmacokinetics and bioavailability of four alkaloids in different formulations from Corydalis decumbens. J Ethnopharmacol. 2013;149:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Chen S, Tian CB, Bai LY, He XC, Lu QY, Zhao YL, Luo XD. Thrombosis inhibited by Corydalis decumbens through regulating PI3K-Akt pathway. J Ethnopharmacol. 2024;329:118177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Masiulis S, Desai R, Uchański T, Serna Martin I, Laverty D, Karia D, Malinauskas T, Zivanov J, Pardon E, Kotecha A, Steyaert J, Miller KW, Aricescu AR. GABA(A) receptor signalling mechanisms revealed by structural pharmacology. Nature. 2019;565:454-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 11. | Agüi-Gonzalez P, Guobin B, Gomes de Castro MA, Rizzoli SO, Phan NTN. Secondary Ion Mass Spectrometry Imaging Reveals Changes in the Lipid Structure of the Plasma Membranes of Hippocampal Neurons following Drugs Affecting Neuronal Activity. ACS Chem Neurosci. 2021;12:1542-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 13. | Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol. 2016;22:1664-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43:757-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 293] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 15. | Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 1272] [Article Influence: 254.4] [Reference Citation Analysis (0)] |
| 16. | Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Yamazaki M, Honda S, Tamaki K, Irie M, Mihara T. Effects of (+)-bicuculline, a GABAa receptor antagonist, on auditory steady state response in free-moving rats. PLoS One. 2020;15:e0236363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Vicente-Silva W, Silva-Freitas FR, Beserra-Filho JIA, Cardoso GN, Silva-Martins S, Sarno TA, Silva SP, Soares-Silva B, Dos Santos JR, da Silva RH, Prado CM, Ueno AK, Lago JHG, Ribeiro AM. Sakuranetin exerts anticonvulsant effect in bicuculline-induced seizures. Fundam Clin Pharmacol. 2022;36:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Lan T, Geng XJ, Zhang SJ, Zeng XX, Ying JJ, Xu Y, Liu SY, Li P, Tong YH, Wang W, Mao ZJ, Wang SW. Si-Ni-San inhibits hepatic Fasn expression and lipid accumulation in MAFLD mice through AMPK/p300/SREBP-1c axis. Phytomedicine. 2024;123:155209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Le Y, Guo J, Liu Z, Liu J, Liu Y, Chen H, Qiu J, Wang C, Dou X, Lu D. Calenduloside E ameliorates non-alcoholic fatty liver disease via modulating a pyroptosis-dependent pathway. J Ethnopharmacol. 2024;319:117239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Qiu L, Feng R, Wu QS, Wan JB, Zhang QW. Total saponins from Panax japonicus attenuate acute alcoholic liver oxidative stress and hepatosteatosis by p62-related Nrf2 pathway and AMPK-ACC/PPARα axis in vivo and in vitro. J Ethnopharmacol. 2023;317:116785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Lan T, Yu Y, Zhang J, Li H, Weng Q, Jiang S, Tian S, Xu T, Hu S, Yang G, Zhang Y, Wang W, Wang L, Zhu Q, Rong X, Guo J. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74:686-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 23. | Huang L, Rao Q, Wang C, Mou Y, Zheng X, Hu E, Zheng J, Li Y, Liu L. Multi-omics joint analysis reveals that the Miao medicine Yindanxinnaotong formula attenuates non-alcoholic fatty liver disease. Phytomedicine. 2024;135:156026. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Li Y, Wang H, He X, Zhu W, Bao Y, Gao X, Huang W, Ge X, Wei W, Zhang H, Sheng L, Zhang T, Li H. Zhi-Kang-Yin formula attenuates high-fat diet-induced metabolic disorders through modulating gut microbiota-bile acids axis in mice. Chin Med. 2024;19:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 25. | Fei X, Huang J, Li F, Wang Y, Shao Z, Dong L, Wu Y, Li B, Zhang X, Lv B, Zhao Y, Weng Q, Chen K, Zhang M, Yang S, Zhang C, Zhang M, Li W, Ying S, Sun Q, Chen Z, Shen H. The Scap-SREBP1-S1P/S2P lipogenesis signal orchestrates the homeostasis and spatiotemporal activation of NF-κB. Cell Rep. 2023;42:112586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 26. | Shen S, Sun T, Ding X, Gu X, Wang Y, Ma X, Li Z, Gao H, Ge S, Feng Q. The exoprotein Gbp of Fusobacterium nucleatum promotes THP-1 cell lipid deposition by binding to CypA and activating PI3K-AKT/MAPK/NF-κB pathways. J Adv Res. 2024;57:93-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 27. | Wang Q, Ou Y, Hu G, Wen C, Yue S, Chen C, Xu L, Xie J, Dai H, Xiao H, Zhang Y, Qi R. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br J Pharmacol. 2020;177:1806-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 28. | Wu Y, Zhou J, Zuo X, Kuang Y, Sun L, Zhang X. Yanggan Jiangmei Formula alleviates hepatic inflammation and lipid accumulation in non-alcoholic steatohepatitis by inhibiting the NF-κB/NLRP3 signaling pathway. Chin J Nat Med. 2024;22:224-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, De Bosscher K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev. 2018;39:760-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 30. | Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang H, Lu X. PPAR-γ signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets. Pharmacol Ther. 2023;245:108391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |