Published online May 7, 2025. doi: 10.3748/wjg.v31.i17.105411
Revised: March 10, 2025
Accepted: April 16, 2025
Published online: May 7, 2025
Processing time: 98 Days and 3.7 Hours
Intestinal injury is the most common complication of sepsis, and the mitigation of intestinal damage is crucial for treating sepsis.
To examine the use of ozone-rich water and its action in preventing intestinal damage caused by sepsis.
Through histological analysis, immunohistochemistry, immunofluorescence assays, and Western blot detection, we evaluated the therapeutic efficacy of ozone in mitigating intestinal injury during sepsis. Additionally, by conducting 16S rRNA sequencing and untargeted metabolomics analysis on fecal samples, we identified alterations in the gut microbiota and specific metabolites in septic mice following ozone treatment. This comprehensive approach aims to further elucidate the mechanistic underpinnings of ozone therapy in alleviating sepsis-induced intestinal damage.
Our results demonstrate that ozonated water significantly ameliorates pathological damage in intestinal tissues, enhances the expression of tight junction proteins, and inhibits the polarization of intestinal macrophages, thereby reducing the expression of inflammatory cytokines in intestinal tissues of cecal ligation and puncture-induced septic mice. 16S rRNA sequencing analysis revealed that ozonated water increased the abundance of beneficial bacteria and alleviated gut microbiota dysbiosis. Studies using broad-spectrum antibiotic-treated mice indicated that the protective effects of ozonated water on intestinal injury are dependent on the gut microbiota. Furthermore, metabolomic analysis identified an increase in the tryptophan metabolite DL-tryptophan in the ozonated water treatment group. This suggests that ozonated water protects against intestinal injury by activating the aryl hydrocarbon receptor and suppressing necroptosis in intestinal epithelial cells.
Ozone protected against sepsis-induced intestinal injury through regulation of the gut microbiota and tryptophan metabolism, inhibiting necrotic apoptosis of intestinal epithelial cells through activation of the aryl hydrocarbon receptor.
Core Tip: Ozone protected against sepsis-induced intestinal injury through regulation of the gut microbiota and tryptophan metabolism, inhibiting necrotic apoptosis of intestinal cells through activation of the aryl hydrocarbon receptor.
- Citation: Wang Q, Liu CZ, Li BT, Yu XQ, Zhang JY, Wang ZT, Liao LJ, Liu XD. Ozone controls the metabolism of tryptophan protecting against sepsis-induced intestinal damage by activating aryl hydrocarbon receptor. World J Gastroenterol 2025; 31(17): 105411
- URL: https://www.wjgnet.com/1007-9327/full/v31/i17/105411.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i17.105411
Sepsis represents an extreme systemic inflammatory response to infection and may lead to severe organ damage[1]. A frequent complication of sepsis is sepsis-induced intestinal damage, clinically termed acute gastrointestinal injury[2]. This damage can result in increased permeability of the intestinal barrier, allowing the passage of toxins to the bloodstream, potentially leading to organ failure and, ultimately, death. Currently, sepsis-induced intestinal is typically treated with medication, together with dietary assistance, modulation of intestinal microbes, protection of the gut mucosa, and antioxidant therapy for the prevention and management of complications[3,4]. Given the severity of the condition, it is imperative to explore novel strategies to protect against intestinal injury in the prevention and management of sepsis.
Ozone therapy is a therapeutic modality that involves the interaction of medical-grade ozone with blood or bodily fluids to generate biologically active oxygen molecules and ozone-derived metabolites, thereby exerting multifaceted effects including anti-inflammatory, antioxidant, immunomodulatory, and microcirculatory-enhancing properties[5]. Its mechanisms of action include the induction of a mild, transient, and controlled oxidative stress response, which triggers the production of endogenous antioxidants and modulates immune pathways. Ozone therapy improves microcirculation, promotes mucosal repair, and modulates intestinal microbiota composition, partially restoring intestinal barrier integrity[6]. These effects suggest its potential in protecting the intestine from ischemia-reperfusion injury and alleviating systemic inflammatory responses. Given its multifaceted roles in inflammation regulation, organ protection, and immune modu
Many studies have implicated the intestinal microbiota in various aspects of intestinal inflammation, with microbial metabolites playing a significant role in this process[7]. For instance, irritable bowel syndrome has been linked to fecal short-chain fatty acids produced by gut microbes. The aryl hydrocarbon receptor (AhR) can be activated by tryptophan metabolites produced by the microorganisms, which can control the production of interleukin-22, a cytokine crucial to intestinal homeostasis. Tryptophan metabolites produced by the microbiota have been demonstrated to be significant in the mucosal immune response[8].
In the study, we aimed to explore whether ozone-rich water therapy could protect against intestinal injury induced by sepsis through modulation of the intestinal flora and microbial metabolites.
Mice (C57BL/6, male, 10 weeks old, 20-23 g) were obtained from the Animal Center of Shanghai East Hospital Affiliated with Tongji University, Shanghai, China. The protocol was approved by the Animal Ethics Committee of Tongji University (ethics approval number: TJBB07324106). The mice were kept in a constant-temperature environment at 24 ±
The cecal ligation and puncture (CLP) surgical technique was used for sepsis induction. The mice were randomly assigned to 6 groups, namely, the control (n = 20), sepsis (n = 20), sepsis treated with ozone-rich water (40 μg/mL) (n = 20), sepsis treated with ozone-rich water group (80 μg/mL) (n = 20), sepsis treated with DL-tryptophan (Med
To eliminate intestinal symbiotic bacteria, the mice were given drinking water containing antibiotics [ampicillin (Shenggong Biotechnology, A610028-0025, Shanghai, 100 mg/kg), neomycin (Shenggong Biotechnology, A610366-0025, Shanghai, 100 mg/kg), metronidazole (Shenggong Biotechnology, A600633-0025, Shanghai, 200 mg/kg), and vancomycin (Shenggong Biotechnology, A600983-0001, Shanghai, 200 mg/L)] for 7 days.
Twenty-four hours after the surgery, blood, stool, and primary organ samples were collected for analysis immediately.
The ozone-rich water used for the enema was prepared by trained professionals using a German Kater ozone therapy device. First, 500 mL of sterilized injectable water was placed in the ozone water gas fusion device connected to the ozone therapy device. The parameters were set to 80 μg/L and 40 μg/L, with a time duration of 10 minutes. The resulting ozone water was then transferred to a sterile container and immediately administered to the mice, with all enema procedures completed within 5 minutes.
DNA was extracted from feces using a QIAamp® Fast DNA stool mini kit (QIAGEN, Germany). Sequencing was performed by Genesky Biotechnologies Inc. (Shanghai, China) using primers amplifying the V4 region of the genes. After mixing and quality control (QC), the libraries were sequenced using an Illumina MiSeq system. Operational taxonomic units (OTUs) were identified. Alpha diversity indices were obtained from a depth-normalized OTU table, while beta diversity was examined using Bray-Curtis dissimilarities and unweighted UniFrac distances differences in gut microbiota compositions were analyzed using principal component analysis (PCA) and differences in abundance were assessed using linear discriminant analysis effect size (LEfSe) analysis. A false discovery rate less than 0.05 in Kruskal-Wallis tests, together with minimum logarithmic linear discriminant analysis scores of 2.0 were used to determine significance.
The feces were extracted by placing 100 mg of feces in 500 mL of cold water. The material was then vortexed and centrifuged (13000 rpm, 15 minutes, 4 °C), filtered (0.22 μm), and kept at -80 °C. Samples were pooled to produce a QC sample. Measurements were conducted on an AB Sciex AB Triple TOF 6600 mass spectrometer, using electro-spray ionization sources in both positive and negative ion modes and TOF settings as described. All reagents used were high performance liquid chromatography grade. Data were analyzed using progenesis QI software (Waters Corporation, MA, United States) to identify metabolites. Variations between metabolite groups were determined using the Hotelling T2 area 95% confidence interval indicating significance[10].
Intestinal tissues were fixed (4% paraformaldehyde, 24 hours), followed by dehydration in an ethanol gradient, clearing using xylene, and paraffin-embedding before sectioning (3 μm). Tissues were stained with hematoxylin and eosin (HE), evaluated by a pathologist, and scored according to the degree of tissue damage and histopathological changes. The scores were between 0 and 12, and were determined based on dysplasia of intestinal epithelial cells, reduced numbers of goblet cells, infiltration of inflammatory cells, and submucosal sclerosis.
Tissue sections underwent dewaxing and rehydration, followed by antigen retrieval using citrate buffer in a pressure cooker. The sections were cooled to room temperature, followed by incubation with 3% hydrogen peroxide for 15 minutes and three washes with phosphate buffered saline with tween 20 (PBST). The tissues were then blocked at room temperature for 20 minutes with 5% bovine serum albumin (BSA) and treated overnight with primary antibodies against interleukin-6 (IL-6) (1:500, WL02841 wanlei) and tumor necrosis factor-alpha (TNF-α) (1:500, WL01896 wanlei) at 4 °C. After three washes with PBST, the sections were treated for 1 hour at room temperature with horseradish peroxidase-conjugated secondary antibodies, followed by further washing and incubation with diaminobenzidine (Maxim) and counterstaining with hematoxylin. Following dehydration and mounting with neutral resin, the tissues were scanned and imaged using a pathology slide scanner. Images were analyzed with ImageJ.
Colonic tissues embedded in optimal cutting temperature compound were sectioned into 5 μm slices using a cryostat. After equilibration to room temperature, they were fixed in 4% paraformaldehyde for 10 minutes, followed by three washes with PBST, blocking for 30 minutes with 10% goat serum and 5% BSA, and overnight treatment with antibodies against zonula occludens-1 (ZO-1) (Affinity, AF5145, 1:500), occludin (Proteintech, 27260-1-AP, 1:500), cluster of differentiation (CD) 206 (CST, E6T5J, 1:500), CD86 (wanlei, WL05184, 1:1000), and F4/80 (Affinity, DF2789, 1:500). This was followed by three washes with PBST, incubation with Alexa Fluor 488-conjugated secondary antibodies (1 hour, room temperature), three more washes with PBST, and mounting with antifade mounting medium containing 4’,6-diamidino-2-phenylindole. A confocal microscope (LAX DMi 3000) was used for evaluation and imaging.
Colonic tissues were collected and lysed for protein extraction. Proteins were quantified and electrophoresed in loading buffer on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, followed by transfer to polyvinylidene difluoride membranes (Bio-Rad, United States) and blocking for 1 hour in 5% non-fat milk. The membrane was incubated overnight at 4 °C with primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (Proteintech, 60004-1-Ig, 1:5000), ZO-1 (Affinity, AF5145, 1:1000), occludin (Proteintech, 27260-1-AP, 1:5000), CD206 (CST, E6T5J, 1:1000), CD86 (Wanlei, WL05184, 1:1000), AhR (Immunoway, YT0145, 1:1000), caspase-3/cleaved caspase-3 (Wanlei, WL02117, 1:500), Phospho-mixed lineage kinase domain-like protein (MLKL) (Ser345) (CST, D6E3G, 1:1000), MLKL (Proteintech, 21066-1-AP, 1:2000), receptor-interacting protein (PhosphoSer166) (Immunoway, YP1467, 1:1000), receptor-interacting protein kinase (RIPK) 1 (Proteintech,17519-1-AP, 1:2000), Phospho-RIP3 (Thr231/Ser232) (CST, E7S1R, 1:1000), and RIP3 (Proteintech, 17563-1-AP, 1:2000). This was followed by treatment with secondary antibodies for 1 hour at room temperature, visualization with an enhanced chemiluminescence kit (Thermo Fisher), imaging (Tanon 5200, Tanon, China), and analysis with ImageJ 1.43.
The data from this study were statistically analyzed using the software GraphPad Prism 9.5. The data are shown as the mean ± SE. One-way analysis of variance was used to compare the differences across multiple subgroups, while t-tests were for differences between two groups. P < 0.05 was considered statistically significant.
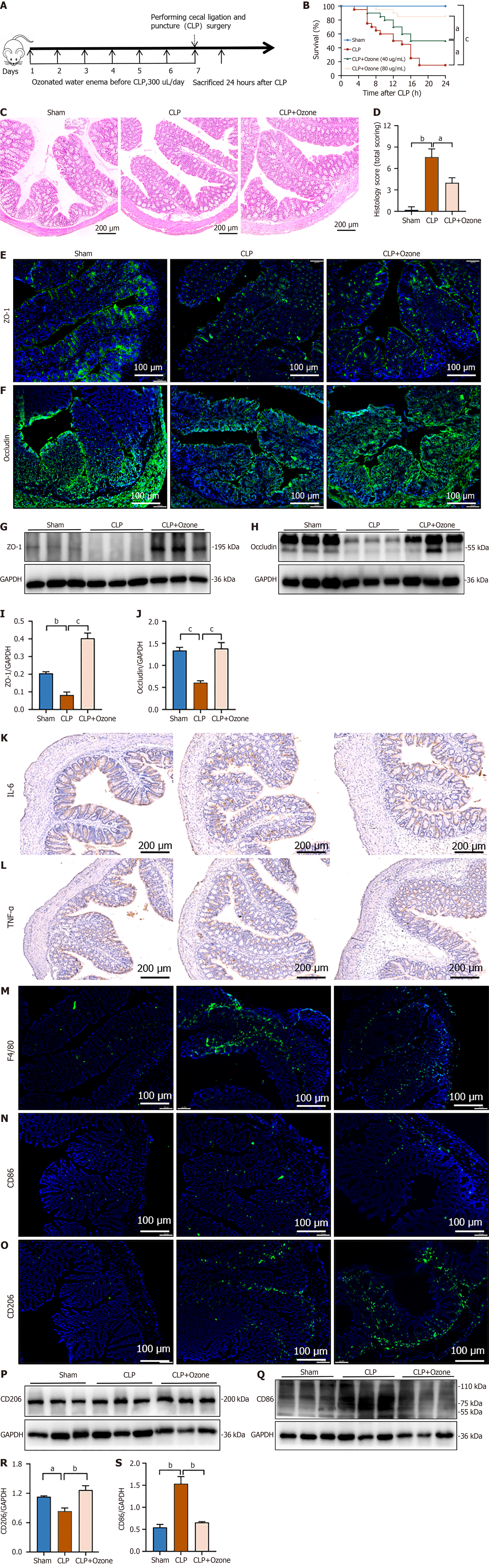
Relative to the sham group (n = 20), mice in the CLP group (n = 20) showed greater mortality. However, treatment with ozone-rich water (80 μg/mL) reduced the mortality rate relative to the CLP (P < 0.001) and CLP plus ozone-rich water (40 μg/mL) (P < 0.05) groups, as shown in ure 1A and B. In the control mice, the epithelial cells appeared normal with no ulceration, together with numerous goblet cells, closely arranged intestinal glands, and normal mucosal morphology. The CLP group had mucosal ulceration accompanied by disappearance of the tissue structure and infiltration of inflammatory cells. CLP mice treated with ozone-rich water showed no infiltration of inflammatory cells, no ulceration, or epithelial cell damage, while goblet cell numbers were decreased, as shown in Figure 1C. The histopathological score of mucosal injuries are presented in Figure 1D (P < 0.05, P < 0.01). The contents of ZO-1 and occludin, both of which are involved in the maintenance of tight-junction integrity in the mucosa, were evaluated by immunofluorescence staining (Figure 1E and F) and Western blotting, showing that the levels of these proteins were increased in the CLP plus ozone-rich water group relative to the CLP group, demonstrating that ozone-rich water protected against damage caused by sepsis (Figure 1G-J, Supplementary Figure 1A and B, P < 0.01, P < 0.001). The immunohistochemical findings indicated marked increases in the contents of the inflammatory cytokines IL-6 and TNF-α in the colons of CLP mice relative to the sham mice, while these levels were reduced following ozone-rich water treatment (Figure 1K and L). F4/80, the mouse homolog of the mucin-like hormone receptor 1, is typically used as a macrophage marker. It was found that F4/80 levels were raised in CLP mice but decreased after ozone-rich water treatment (Figure 1M), while the CD86 level, an M1 macrophage marker, was elevated in as the marker of M1 macrophages, was increased in the CLP group, but was decreased in the CLP plus ozone-rich water group; However, concentrations of the M2 macrophage marker CD206 were decreased in the CLP group, but increased in the CLP plus ozone-rich water group, as shown by immunofluorescence staining (Figure 1N and O) and western blotting (Figure 1P-S, Supplementary Figure 1C and D). Those results suggested that ozone therapy could inhibit intestinal macrophage polarization.
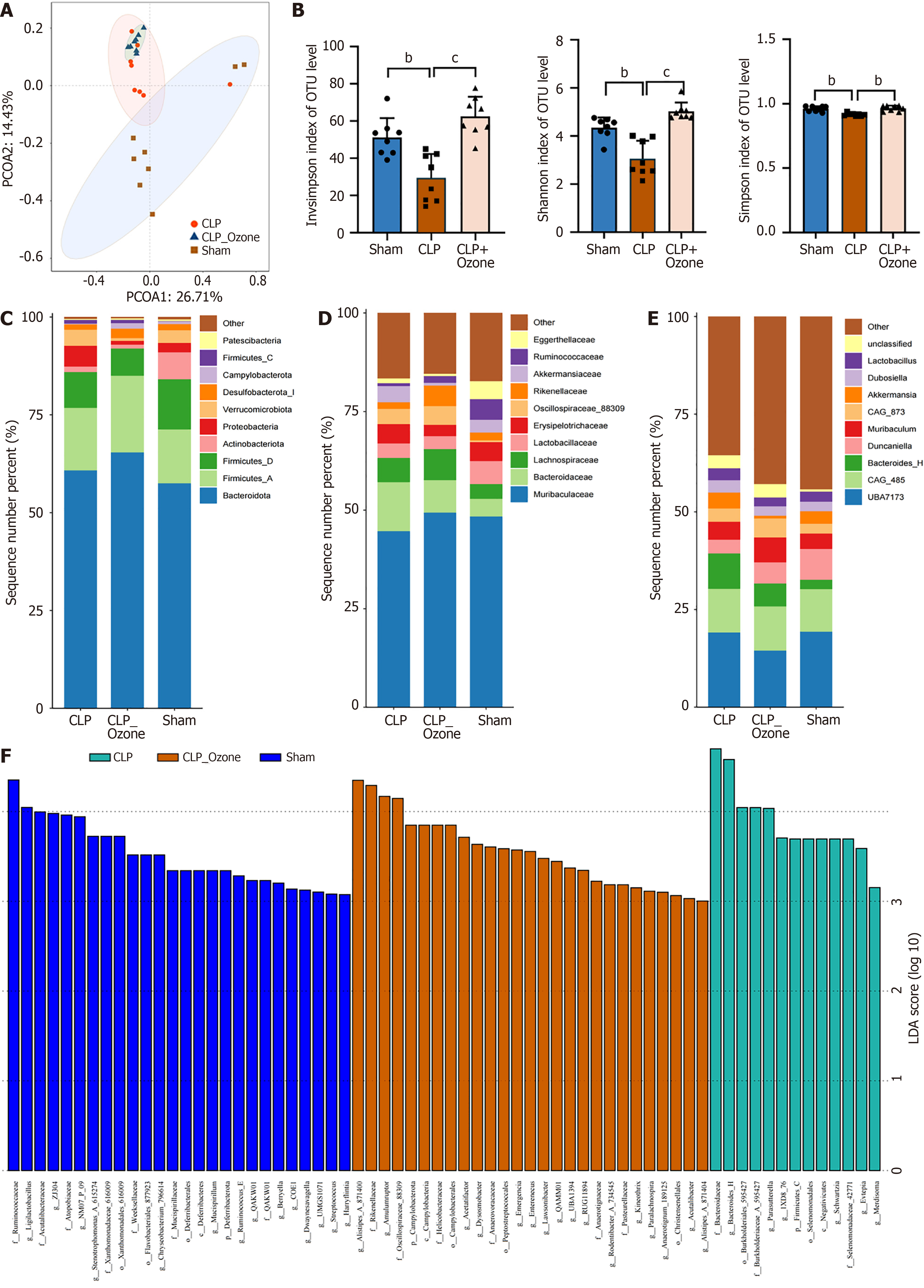
Moreover, the gut flora is known to be involved in the pathogenesis of intestinal injury induced by sepsis. Bacterial community compositions were evaluated with PCA, which revealed separation among the sham (n = 8), CLP (n = 8), and CLP plus ozone-rich water groups (n = 8) (Figure 2A). Analysis of alpha diversity in the samples showed that the InvSimpson, Shannon, and Simpson indices were reduced in the CLP group relative to the sham and the CLP plus ozone-rich water groups, indicating that ozone-rich water could improve the diversity of the flora in the sepsis mouse model (Figure 2B). At the phylum level, it was observed that the Verrucomicrobiota and Proteobacteria were dominant in CLP mice, but were decreased in the CLP plus ozone-rich water and sham groups, while Bacteroidota, Firmicutes, and Campylobacterota were dominant in the CLP plus ozone-rich water group and reduced in the CLP group (Figure 2C). At the family level, Bacteroidaceae was dominant in the CLP group, while Lachnospiraceae and Muribaculaceae dominated in the CLP plus ozone-rich water group (Figure 2D). In terms of genera, UBA7173 was dominant in the CLP group, while Dunaliella was dominant in the CLP plus ozone-rich water group (Figure 2E). LEfSe analysis indicated marked differences in bacterial taxa among the groups (Figure 2F). These results indicate that ozone-rich water therapy could alleviate intestinal flora disturbances induced by sepsis.
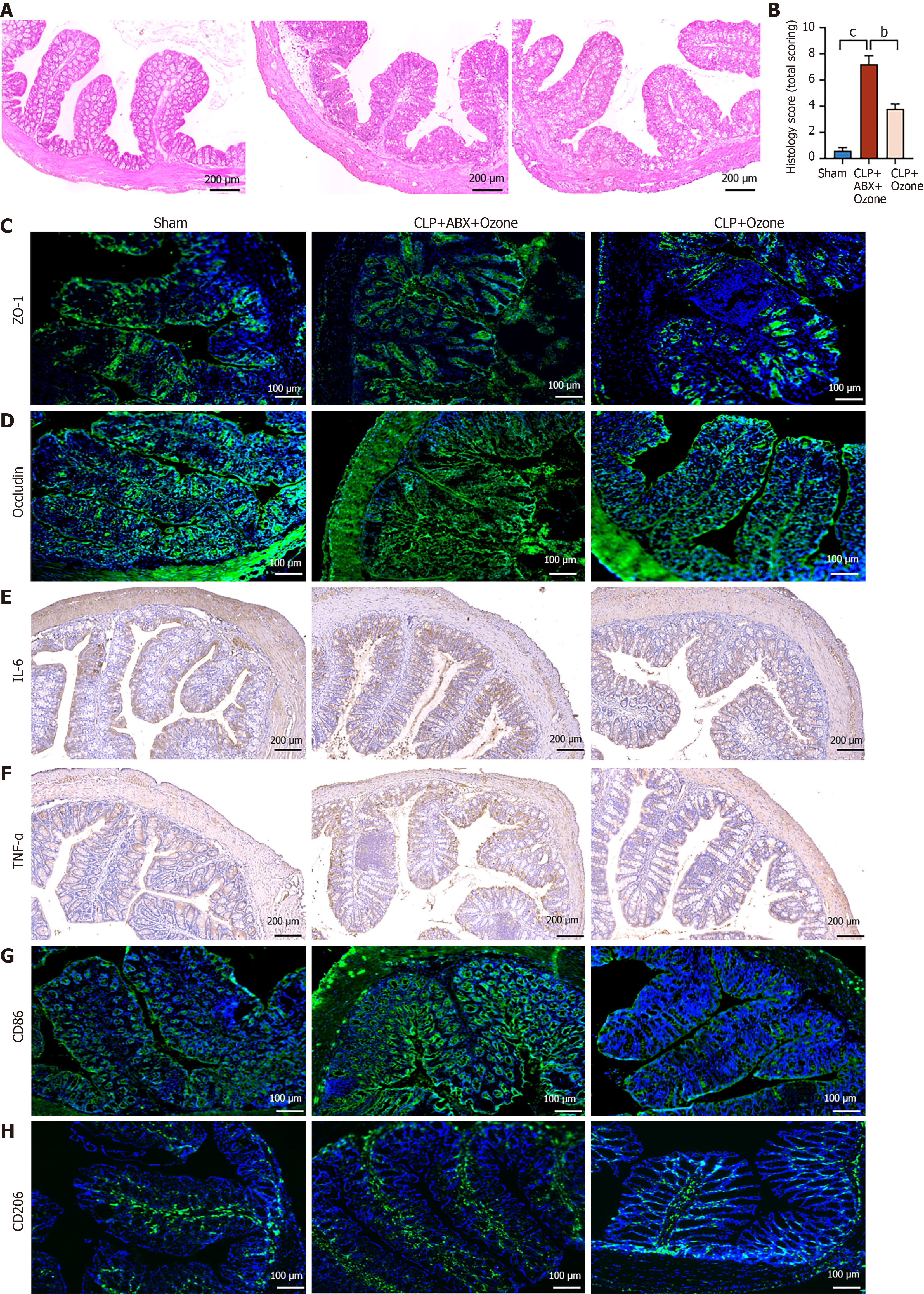
The intestinal tissue of the CLP plus ozone-rich water group showed no infiltration of inflammatory cells, ulceration, or damage to epithelial cells, while goblet cell numbers were reduced compared with the CLP + ABX + ozone-rich water group (n = 10). Chiu mucosal damage scores indicated that ozone-rich water reduced intestinal injury induced by sepsis, as shown in Figure 3A and B. The concentrations of the tight junction proteins ZO-1 and occludin were found to be higher in the CLP treated with ozone-rich water group (n = 10) as opposed to those of the CLP + ABX + ozone-rich water group, as shown by immunofluorescence analysis (Figure 3C and D). The immunohistochemical results indicated that ozone-rich water lowered the contents of IL-6 and TNF-α in the colons of the CLP + ABX + ozone-rich water group (Figure 3E and F). Levels of CD86 were decreased in the CLP plus ozone-rich water group, but were increased in the CLP + ABX + ozone-rich water group (Figure 3G), while CD206 concentrations were increased in the CLP plus ozone-rich water group, but were decreased in the CLP + ABX + ozone-rich water group (Figure 3H). These findings indicate that ozone therapy reduced intestinal injury induced by CLP via the intestinal flora.
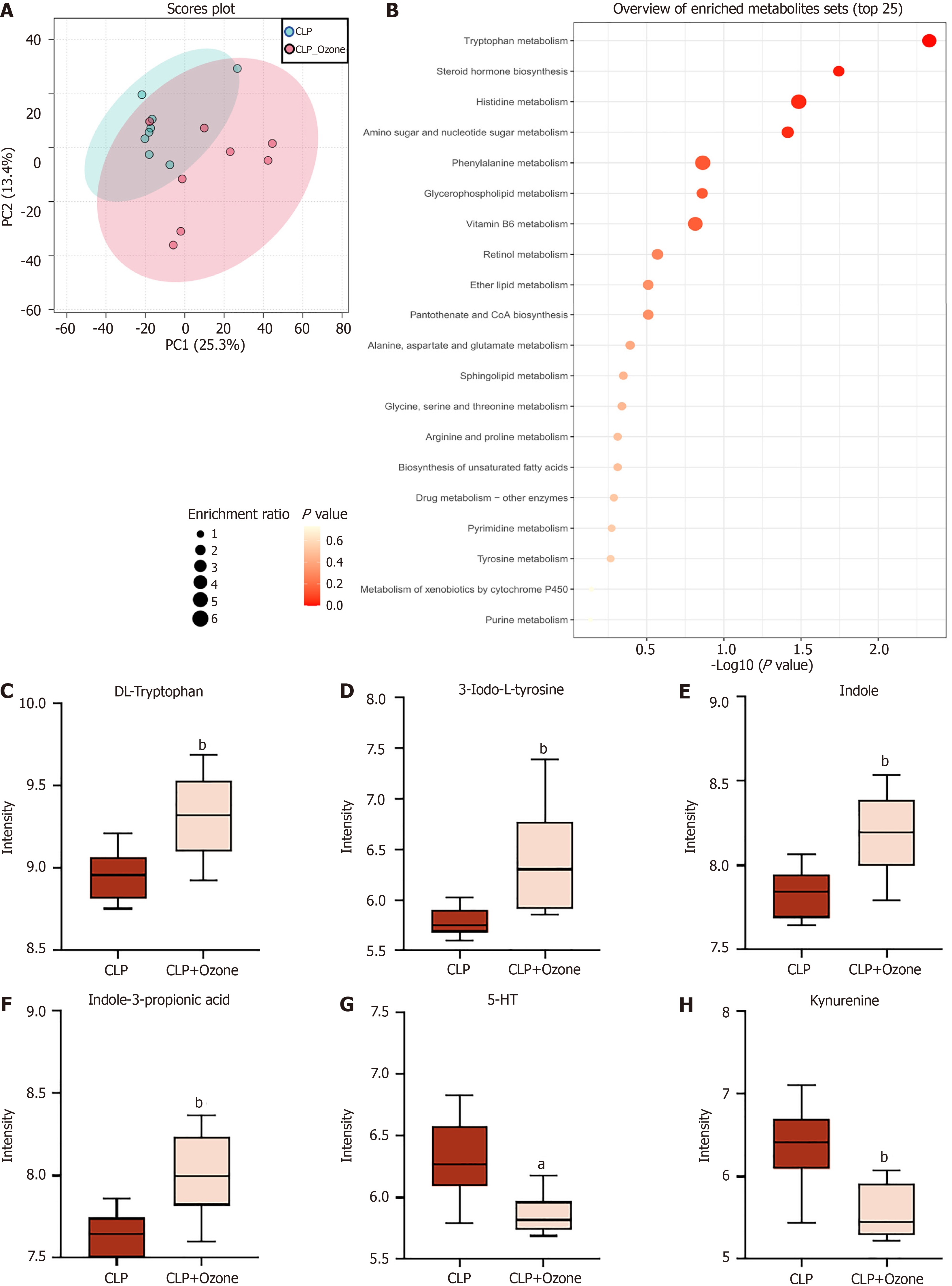
However, metabolic changes are associated with changes in the gut microbiota. The PCA scatter plots indicated clustering of the QC samples, indicative of the quality of the metabolomics analysis (Figure 4A). Using the t-test adjusted P value < 0.05 and variable important in projection ≥ 1, differentially abundant metabolites between the CLP and CLP plus ozone-rich water groups were identified. These metabolites were analyzed by Kyoto Encyclopedia of Genes and Genomes pathway enrichment, with Fisher exact tests revealing that the most significant changes were associated with tryptophan metabolism (Figure 4B). Furthermore, analysis of the most significant tryptophan-associated metabolites showed a marked increase in the levels of various metabolites in the ozone-rich water treatment group, including DL-tryptophan, 3-iodo-L-tyrosine, indol, and indole-3-propionic acid (Figure 4C-E), while in contrast, levels of metabolites related to the kynurenine and 5-hydroxytryptamine (5-HT) metabolic pathways were markedly reduced following ozone-rich water treatment (Figure 4F-H). And expression of DL-tryptophan was significantly increased in the ozone-rich water treatment group. These results suggest that ozone therapy regulates the metabolism of tryptophan in the intestinal flora.
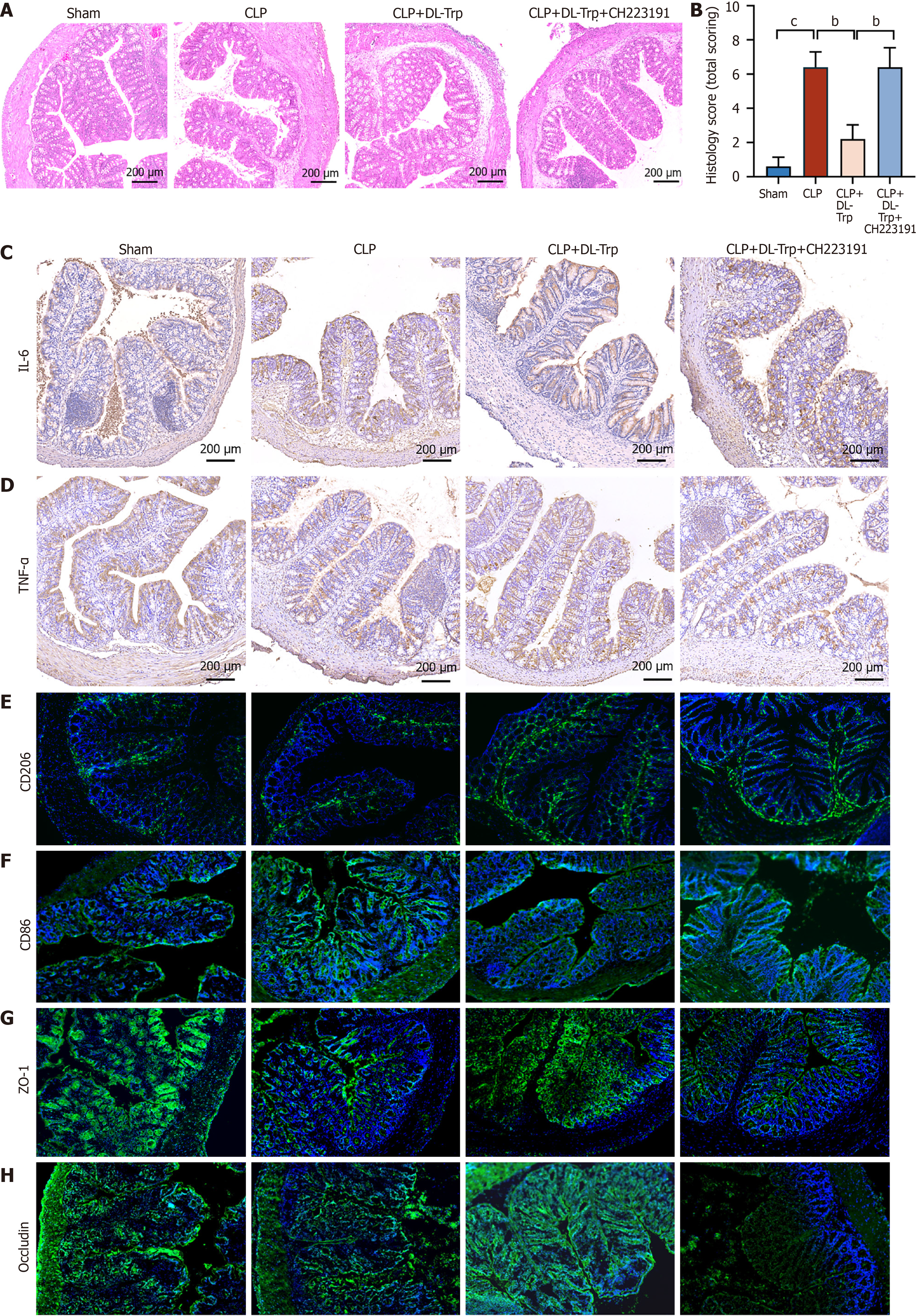
It has been found that tryptophan metabolites can activate AhR to regulate intestinal homeostasis. HE staining of colonic tissue showed that the protective effects of DL-tryptophan on intestinal injury were nullified when treated with the AhR inhibitor (CH223191), which was confirmed by the Chiu score (Figure 5A and B). Treatment with CH223191 increased IL-6 and TNF-α in the ileum and nullified the protective effects of DL-tryptophan (Figure 5C and D). CH223191 also increased CD86, which was decreased in the CLP plus DL-tryptophan water group. CD206 levels were raised in the CLP plus DL-tryptophan group, but were decreased when treated with CH223191, as shown by immunofluorescence (Figure 5E and F). Immunofluorescence indicated marked increases in the levels of ZO-1, and occludin following DL-tryptophan treatment, which was reversed after exposure to CH223191 (Figure 5G and H). These results suggest that DL-tryptophan protects against intestinal injury induced by sepsis and inhibits intestinal macrophage polarization via AhR.
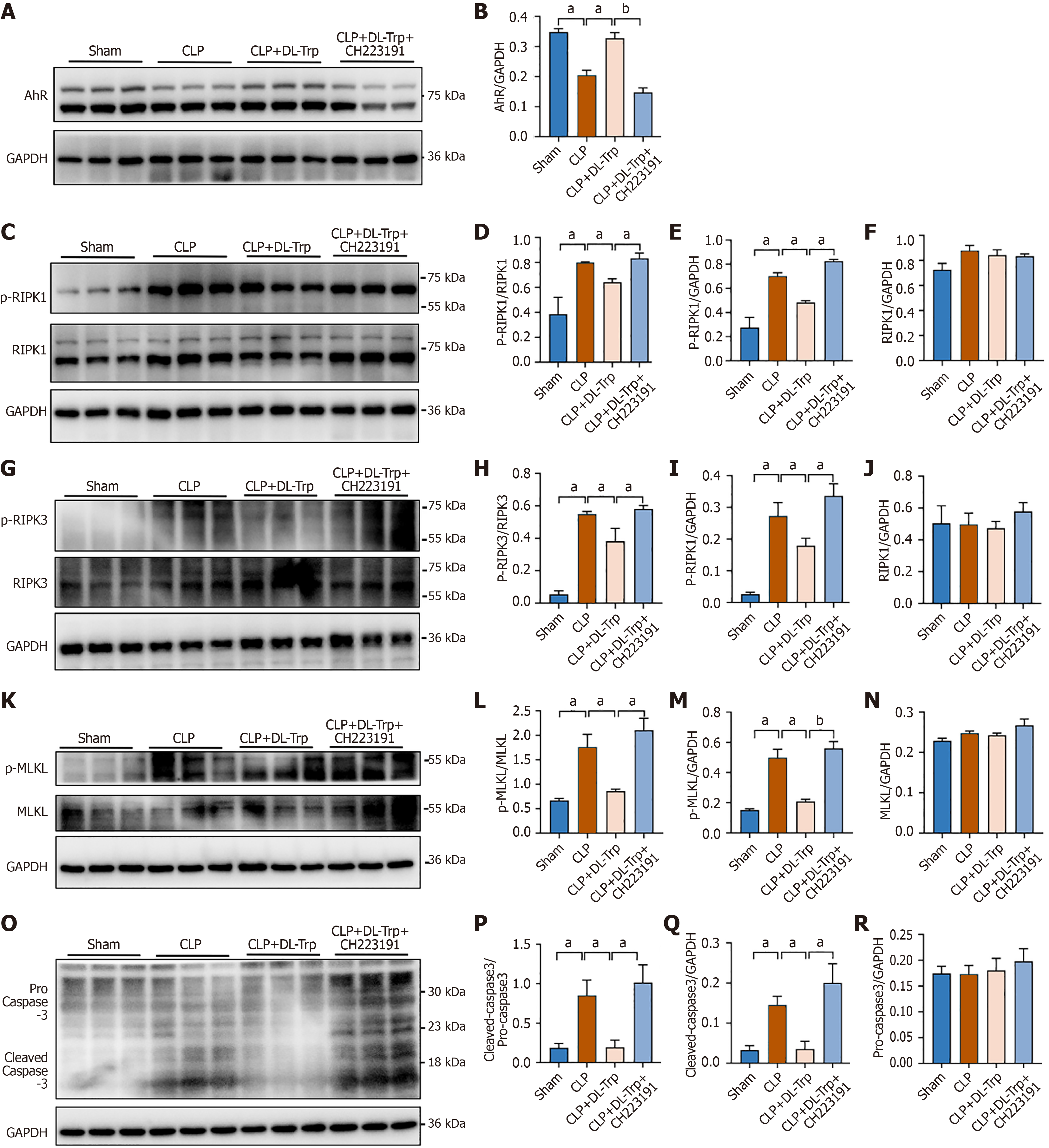
It has been found that protection of intestinal epithelial cells from necroptosis was effective in maintaining the mucosal barrier and homeostasis, while preventing intestinal inflammation. Western blotting was used to examine whether the protective influence of DL-tryptophan on intestinal injury relied on the suppression of necroptosis. It was found that AhR levels were elevated in the CLP plus DL-tryptophan group, but were lowered after exposure to CH223191 (Figure 6A and B, Supplementary Figure 2A). Moreover, the levels of necroptosis-associated proteins (phospho-RIPK1/RIPK1, phospho-RIPK3/RIPK3, phospho-MLKL/MLKL, and cleaved caspase-3/procaspase-3) were elevated in the colonic tissue of the CLP mice. However, these levels were reduced following DL-tryptophan treatment but increased following treatment with CH223191 (Figure 6C-R, Supplementary Figure 2B-E). Overall, these findings suggest that DL-tryptophan protected against intestinal injury by inhibiting the activation of necroptosis via AhR.
Sepsis represents a systemic inflammatory response induced by infection and is commonly seen in intensive care unit patients[11]. Sepsis often leads to intestinal damage, while impairment of the intestinal barrier function can also lead to sepsis, bowel necrosis, and death[12]. Therefore, protection of the intestine from damage is critical in the treatment of sepsis.
Ozone has strong oxidizing properties and has been used as a sterilizing agent[13]. Ozone has antimicrobial, vasodilatory, analgesic, and antioxidant properties, as well as regulating immune function and epigenetics[14]. Low concentrations of ozone were found to mitigate intestinal damage caused by anterior rectal resection through modulation of inflammation and oxidative stress[15]. Although ozone therapy demonstrates potential benefits in modulating inflammation and oxidative stress, it is not without risks. In the treatment of septic patients, ozone therapy at controlled doses may regulate oxidative stress; however, excessive exposure could exacerbate oxidative damage in critically ill sepsis patients, particularly those with hepatic or renal dysfunction. In conditions such as severe anemia, acute respiratory distress syndrome, and uncontrolled hyperthyroidism, ozone therapy may worsen hypoxia or metabolic disturbances. Additionally, in cases of pregnancy, recent myocardial infarction, or active hemorrhagic disorders, the pro-oxidative effects of ozone might outweigh its potential benefits due to dose-dependent risks. Therefore, personalized ozone dosing regimens and real-time monitoring (e.g., vital signs, oxidative stress biomarkers) are critical to minimizing adverse effects[16-18]. Our study revealed that following ozonated water intervention, septic mice exhibited significantly less damage to the colonic tissue morphology. The mRNA expression levels of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β, were markedly downregulated in the colonic tissues, while the expression levels of tight junction proteins, ZO-1 and occludin, were significantly upregulated. Additionally, ozonated water was found to inhibit macrophage polarization. These results demonstrate that ozonated water can alleviate sepsis-induced intestinal injury. This study provides preliminary evidence for the role of ozonated water in mitigating intestinal damage during sepsis and offers a foundation for further exploration of its underlying mechanisms.
The intestinal flora and associated metabolites are closely associated with various diseases[19]. Disruption of the flora alters lipid, carbohydrate, and amino acid metabolism, contributing to intestinal damage[20]. In this study, it was found that ozone could alleviate dysregulation of the intestinal flora by promoting the growth of beneficial bacteria while reducing that of pathogenic bacteria. Tryptophan metabolism was found to be the most significantly enriched pathway. This indicates that ozone regulates the intestinal flora and their metabolites.
Tryptophan is a common aromatic amino acid that is absorbed through the intestines via exogenous supplementation[21]. It can be metabolized through three main pathways, namely, the serotonin (5-HT), kynurenine, and indole deri
AhR is a ligand-activated transcription factor, involved in signal transduction, cell differentiation, and apoptosis[23]. After interaction with ligands (such as dioxins), AhR is heterodimerized with the AhR nuclear transporter and is transported to the nucleus to activate target genes[24]. Interestingly, AhR is also a target for microbially derived indole and eukaryotic tryptophan metabolites such as kynuridine, and AhR-dependent pathways can also influence host-microbial interactions. For example, in the gut, AhR modulates the gut microbiota, immune function, and the intestinal barrier in mice[25]. AhR is also involved in pathways associated with development, metabolic homeostasis, and cell growth in a variety of tissues[26]. Here, it was found that AhR levels were increased after treatment with ozone. Further study showed that ozone could regulate tryptophan metabolism in the intestinal flora to activate AhR for alleviation of intestinal damage caused by sepsis.
A highly complex interaction network exists between ozone, gut microbiota, and tryptophan metabolism. Ozone directly modulates gut microbiota and their metabolic outputs. Studies have demonstrated that ozone selectively inhibits the proliferation of conditional pathogens and harmful bacteria while stimulating the growth of beneficial bacterial species, a finding consistent with our observations in septic mice, where ozone water intervention reduced harmful gut microbiota and increased beneficial taxa. These microbiota alterations further influence tryptophan metabolism, modifying the production of metabolites that exert profound systemic effects. Research indicates that therapeutic interventions such as Wuji Wan significantly reshape gut microbial composition, notably enhancing Lactobacillus abundance and promoting the metabolic conversion of tryptophan into indole-3-acetic acid and indoleacrylic acid[27]. These indole derivatives subsequently activate the AhR pathway, effectively attenuating colonic inflammation and restoring the expression of intestinal barrier proteins. Similarly, our study revealed that ozone intervention altered gut microbiota composition, which in turn modulated tryptophan metabolism. Increased tryptophan metabolites upregulated the expression of intestinal tight junction proteins, suppressed pro-inflammatory cytokine levels, and inhibited macrophage polarization in the gut, thereby exerting protective effects against sepsis-induced intestinal injury. The underlying mechanism involves AhR activation by tryptophan metabolites, which inhibits necroptosis of intestinal epithelial cells, ultimately mitigating sepsis-associated intestinal damage.
Necroptosis is mediated by RIPK1 and RIPK3[28]. The process relies on the activation of the RIPK1/RIPK3 complex upon detection of “death signals”, which recruits and phosphorylates MLKL[29]. Phosphorylated MLKL forms oligomers that bind to phosphatidylinositol, causing translocation from the cytoplasm to the cell membrane and resulting in changes in membrane permeability, leading directly to necroptosis[30]. Currently, little is known of necroptosis in sepsis-induced intestinal dysfunction. However, activation of necroptosis is seen in lipopolysaccharide-induced sepsis models and could induce intestinal damage[31]. Inhibition of RIPK3 and MLKL was able to protect intestinal epithelial cells from necroptosis, thus preserving mucosal barrier integrity and homeostasis and preventing inflammation[32]. Here, it was demonstrated that DL-tryptophan could activate AhR to prevent necroptosis by regulating tryptophan metabolism.
In conclusion, there has been no previous investigation of the effects of ozone in preventing intestinal damage in sepsis. Here, it was found that ozone could inhibit necroptosis to protect against sepsis-induced intestinal injury by regulating tryptophan metabolism-DL-tryptophan in the intestinal flora through activation of AhR. These findings suggest a novel therapeutic approach for preventing intestinal damage in sepsis, and contribute to the application of ozone in the treatment of intestinal damage in sepsis.
| 1. | Zhang H, Li L, Xu L, Zheng Y. Clinical Significance of the Serum lncRNA NORAD Expression in Patients with Neonatal Sepsis and Its Association with miR-410-3p. J Inflamm Res. 2021;14:4181-4188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Wu J, Liu Q, Zhang X, Tan M, Li X, Liu P, Wu L, Jiao F, Lin Z, Wu X, Wang X, Zhao Y, Ren J. The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages. Cell Death Dis. 2022;13:653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 3. | Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y, Rui-Long W, Bin-Qiang X, Jun-Jie F, Chun-Lan H, Yue Z. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes. 2022;14:2112882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Liang L, Shen L, Fu G, Yao Y, Li G, Deng Y, Zhang H, Zhou M, Yang W, Hua G, Zhang Z. Regulation of the regeneration of intestinal stem cells after irradiation. Ann Transl Med. 2020;8:1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Bette M, Cors E, Kresse C, Schütz B. Therapeutic Treatment of Superoxide Dismutase 1 (G93A) Amyotrophic Lateral Sclerosis Model Mice with Medical Ozone Decelerates Trigeminal Motor Neuron Degeneration, Attenuates Microglial Proliferation, and Preserves Monocyte Levels in Mesenteric Lymph Nodes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Scassellati C, Galoforo AC, Esposito C, Ciani M, Ricevuti G, Bonvicini C. Promising Intervention Approaches to Potentially Resolve Neuroinflammation And Steroid Hormones Alterations in Alzheimer’s Disease and Its Neuropsychiatric Symptoms. Aging Dis. 2021;12:1337-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Erb Downward JR, Falkowski NR, Mason KL, Muraglia R, Huffnagle GB. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci Rep. 2013;3:2191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Chu H. Host gene-microbiome interactions: molecular mechanisms in inflammatory bowel disease. Genome Med. 2017;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Yu L, Lu J, Du W. Tryptophan metabolism in digestive system tumors: unraveling the pathways and implications. Cell Commun Signal. 2024;22:174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Mörkl S, Lackner S, Meinitzer A, Mangge H, Lehofer M, Halwachs B, Gorkiewicz G, Kashofer K, Painold A, Holl AK, Bengesser SA, Müller W, Holzer P, Holasek SJ. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr. 2018;57:2985-2997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Schweighöfer H, Rummel C, Mayer K, Rosengarten B. Brain function in iNOS knock out or iNOS inhibited (l-NIL) mice under endotoxic shock. Intensive Care Med Exp. 2014;2:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Yuan Y, Liu S, Ding X, Li Y, Zhang X, Song H, Qi X, Zhang Z, Guo K, Sun T. Early intestinal microbiota changes in aged and adult mice with sepsis. Front Cell Infect Microbiol. 2022;12:1061444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Zeng J, Lei L, Zeng Q, Yao Y, Wu Y, Li Q, Gao L, Du H, Xie Y, Huang J, Tan W, Lu J. Ozone Therapy Attenuates NF-κB-Mediated Local Inflammatory Response and Activation of Th17 Cells in Treatment for Psoriasis. Int J Biol Sci. 2020;16:1833-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Bialoszewski D, Pietruczuk-Padzik A, Kalicinska A, Bocian E, Czajkowska M, Bukowska B, Tyski S. Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Med Sci Monit. 2011;17:BR339-BR344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhang W, Wu M, Chen P, Zhang J, Ma J, Cheng Y, Li X, Hu J, Li W, Du Y, Ding K, Fan Z. Effect of local ozone treatment on rats with anterior rectal resection and the possible mechanisms. Biomed Eng Online. 2021;20:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Grangeat AM, Erario MLA. The Use of Medical Ozone in Chronic Intervertebral Disc Degeneration Can Be an Etiological and Conservative Treatment. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Bocci V, Borrelli E, Travagli V, Zanardi I. The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29:646-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 18. | Caliskan B, Guven A, Ozler M, Cayci T, Ozcan A, Bedir O, Surer I, Korkmaz A. Ozone therapy prevents renal inflammation and fibrosis in a rat model of acute pyelonephritis. Scand J Clin Lab Invest. 2011;71:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Jia X, Chen Q, Wu H, Liu H, Jing C, Gong A, Zhang Y. Exploring a novel therapeutic strategy: the interplay between gut microbiota and high-fat diet in the pathogenesis of metabolic disorders. Front Nutr. 2023;10:1291853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Jia M, Yi B, Chen X, Xu Y, Xu X, Wu Z, Ji J, Tang J, Yu D, Zheng Y, Zhou Q, Zhao Y. Carbon dots induce pathological damage to the intestine via causing intestinal flora dysbiosis and intestinal inflammation. J Nanobiotechnology. 2023;21:167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Stephen-Victor E, Crestani E, Chatila TA. Dietary and Microbial Determinants in Food Allergy. Immunity. 2020;53:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Liao XX, Wu XY, Zhou YL, Li JJ, Wen YL, Zhou JJ. Gut microbiome metabolites as key actors in atherosclerosis co-depression disease. Front Microbiol. 2022;13:988643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Yu W, Lu Y, Shen Y, Liu J, Gong S, Yu F, Huang Z, Zou W, Zhou M, Luo X, You W, Ke C. Exploring the Intestinal Microbiota and Metabolome Profiles Associated With Feed Efficiency in Pacific Abalone (Haliotis discus hannai). Front Microbiol. 2022;13:852460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Wei ZF, Lv Q, Xia Y, Yue MF, Shi C, Xia YF, Chou GX, Wang ZT, Dai Y. Norisoboldine, an Anti-Arthritis Alkaloid Isolated from Radix Linderae, Attenuates Osteoclast Differentiation and Inflammatory Bone Erosion in an Aryl Hydrocarbon Receptor-Dependent Manner. Int J Biol Sci. 2015;11:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Laursen MF, Sakanaka M, von Burg N, Mörbe U, Andersen D, Moll JM, Pekmez CT, Rivollier A, Michaelsen KF, Mølgaard C, Lind MV, Dragsted LO, Katayama T, Frandsen HL, Vinggaard AM, Bahl MI, Brix S, Agace W, Licht TR, Roager HM. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol. 2021;6:1367-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 26. | Shu Y, He J, Zhang H, Liu G, Li S, Deng S, Wu H. Dynamic transcriptome and histomorphology analysis of developmental traits of hindlimb thigh muscle from Odorrana tormota and its adaptability to different life history stages. BMC Genomics. 2021;22:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Jing W, Dong S, Xu Y, Liu J, Ren J, Liu X, Zhu M, Zhang M, Shi H, Li N, Xia P, Lu H, Wang S. Gut microbiota-derived tryptophan metabolites regulated by Wuji Wan to attenuate colitis through AhR signaling activation. Acta Pharm Sin B. 2025;15:205-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Dowling JP, Alsabbagh M, Del Casale C, Liu ZG, Zhang J. TRADD regulates perinatal development and adulthood survival in mice lacking RIPK1 and RIPK3. Nat Commun. 2019;10:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Reilly B, Tan C, Murao A, Nofi C, Jha A, Aziz M, Wang P. Necroptosis-Mediated eCIRP Release in Sepsis. J Inflamm Res. 2022;15:4047-4059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 30. | Khoury MK, Yang H, Liu B. Macrophage Biology in Cardiovascular Diseases. Arterioscler Thromb Vasc Biol. 2021;41:e77-e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Liu AB, Li SJ, Yu YY, Zhang JF, Ma L. Current insight on the mechanisms of programmed cell death in sepsis-induced myocardial dysfunction. Front Cell Dev Biol. 2023;11:1309719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Su YR, Hong YP, Mei FC, Wang CY, Li M, Zhou Y, Zhao KL, Yu J, Wang WX. High-Fat Diet Aggravates the Intestinal Barrier Injury via TLR4-RIP3 Pathway in a Rat Model of Severe Acute Pancreatitis. Mediators Inflamm. 2019;2019:2512687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |