Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.104528
Revised: February 27, 2025
Accepted: March 13, 2025
Published online: April 21, 2025
Processing time: 113 Days and 5.1 Hours
This article discusses a recent study by Wang et al that sheds light on the meta
Core Tip: This article reviews a recent study by Wang et al investigating how carnitine palmitoyltransferase II (CPT II) inactivity drives malignant progression in metabolic dysfunction-associated fatty liver disease (MAFLD) through aberrant lipid metabolism, T-cell dysfunction, and liver cancer stem cell activation. Their work revealed that mitochondrial damage and reduced CPT II activity under lipid-rich conditions may be early indicators of liver cancer risk, influencing immune cell profiles and the stem cell-like properties of hepatocytes. Understanding these processes could pave the way for novel preventative and therapeutic interventions targeting the metabolic and immunological underpinnings of MAFLD-related hepatocarcinogenesis.
- Citation: Cai H, Yang CH, Gao P. Rethinking carnitine palmitoyltransferase II and liver stem cells in metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma. World J Gastroenterol 2025; 31(15): 104528
- URL: https://www.wjgnet.com/1007-9327/full/v31/i15/104528.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i15.104528
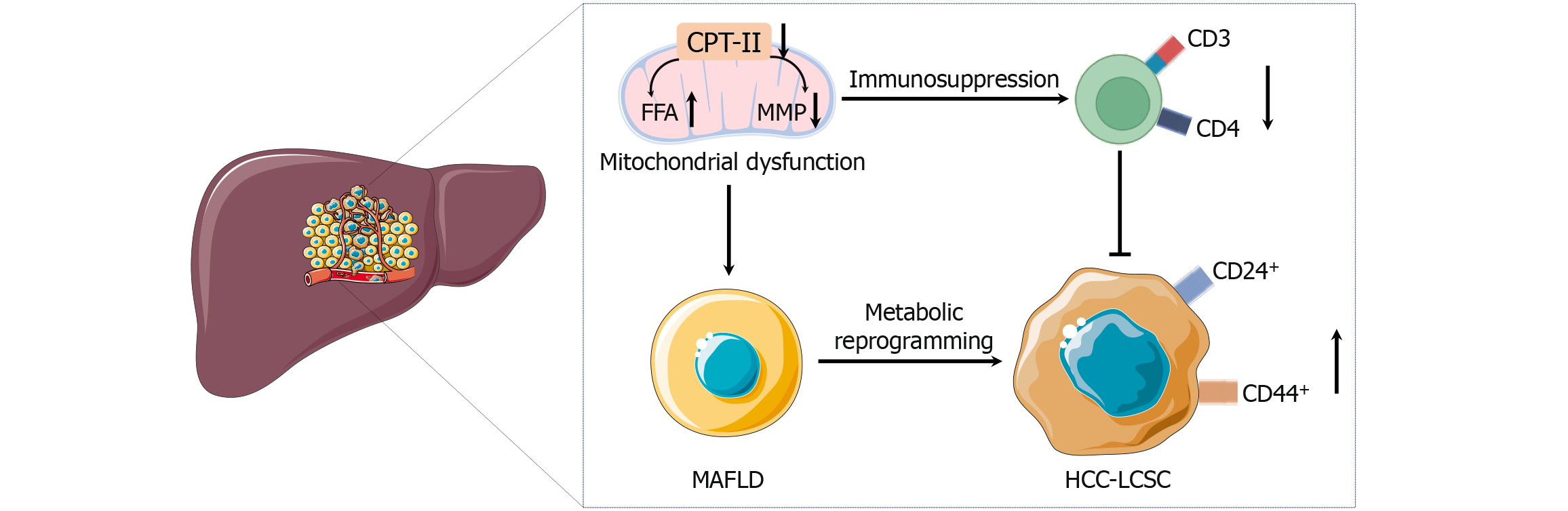
Metabolic dysfunction-associated fatty liver disease (MAFLD) is increasingly recognized as a global health concern. It encompasses a spectrum of hepatic pathologies ranging from simple steatosis to inflammatory stages (metabolic dysfunction-associated steatohepatitis). Uncontrolled metabolic dysfunction-associated steatohepatitis is prone to progressing to cirrhosis, and eventually, to hepatocellular carcinoma (HCC)[1]. While the precise molecular drivers of this progression have yet to be fully elucidated, there is growing evidence that mitochondrial dysfunction, immunosuppression, and the activation of liver cancer stem cells (LCSCs) act in concert to spur malignant changes. The recent article by Wang et al[2] offers a compelling look at how mitochondrial CPT II inactivity and LCSC activation synergize to drive MAFLD progression to HCC (Figure 1). These findings mark a critical step in delineating the metabolic and immunologic cues that facilitate malignant transformation in MAFLD.
Mitochondria serve as central hubs of energy production and lipid metabolism[3]. In the normal liver, efficient mitochondrial fatty acid β-oxidation ensures metabolic homeostasis. However, in MAFLD, particularly under conditions of chronic caloric surplus and lipid accumulation, mitochondrial pathways become dysregulated[4]. A variety of metabolic alterations occur in MAFLD, including increased fatty acid synthesis, reduced fatty acid oxidation, and altered glucose metabolism. These changes lead to an accumulation of lipids within hepatocytes, which can cause lipotoxicity, oxidative stress, and mitochondrial dysfunction. CPT II, an enzyme located on the inner mitochondrial membrane, is essential for the transport of long-chain fatty acids into mitochondria for β-oxidation[5]. In the context of MAFLD, research has shown that CPT II activity significantly decreases as the disease progresses. This decrease in CPT II function leads to impaired mitochondrial fatty acid oxidation, excessive lipid accumulation, and alterations in the mitochondrial membrane potential, collectively creating a metabolically compromised and oxidative stress-rich environment. These metabolic alterations not only disrupt normal liver function but also create conditions that favor the survival and expansion of LCSC, thereby linking metabolic dysfunction directly to oncogenic processes. In MAFLD, these mito
While recent studies highlight the critical role of CPT II in the pathogenesis and malignancy of MAFLD, the precise molecular mechanisms linking CPT II inactivity to specific oncogenic pathways remain underexplored. Wang et al[2] have begun to bridge this gap by associating CPT II inactivity with alterations in key signaling pathways such as p53 and Wnt/β-catenin. The p53 pathway, known for its role in tumor suppression, is often dysregulated in cancerous transformations. CPT II inactivity may exacerbate oxidative stress, leading to p53 mutations or inactivation, thereby undermining genomic stability and promoting tumorigenesis[6]. Additionally, the Wnt/β-catenin pathway, crucial for cell proliferation and differentiation, may be aberrantly activated in the context of impaired fatty acid oxidation. The metabolic stress induced by CPT II inactivity could lead to the stabilization and nuclear translocation of β-catenin, driving the expression of genes that promote cellular proliferation and stemness[7]. Hypotheses for these interactions include the possibility that accumulated lipids and resultant metabolic byproducts directly modify components of these pathways, or that secondary effects of mitochondrial dysfunction, such as altered energy status and redox balance, indirectly influence these oncogenic signaling cascades. Future research should aim to delineate these connections through targeted experiments, such as assessing the impact of CPT II restoration on p53 and Wnt/β-catenin pathway activity, or exploring the use of pathway inhibitors in models of CPT II inactivity.
While mitochondrial dysfunction and CPT II alterations may contribute to carcinogenesis in various liver diseases, the specific metabolic and immunological landscape of MAFLD appears uniquely conducive to HCC development. A more in-depth comparison with other liver diseases, such as alcoholic liver disease (ALD), highlights these distinctions. In ALD, mitochondrial dysfunction is also a prominent feature; however, the primary drivers include ethanol metabolism, acetaldehyde toxicity, and oxidative stress from reactive oxygen species. Unlike MAFLD, where chronic lipid accumulation is central, ALD is characterized by inflammation driven by different mechanisms, such as acetaldehyde adducts and gut-derived endotoxins[8].
Immunologically, MAFLD is associated with a distinct profile of immune cell infiltration and cytokine production compared to ALD. For instance, MAFLD-related HCC often exhibits a unique immunosuppressive microenvironment marked by reduced CD4+ T-cell populations and increased regulatory T cells, whereas ALD may show more pronounced neutrophilic infiltration and different cytokine profiles[9]. These differences suggest that CPT II inactivity’s role in MAFLD-related HCC is intertwined with specific immunometabolic alterations that are not as prominent or differently manifested in other liver diseases. Therefore, the downregulation of CPT II in MAFLD might represent a unique metabolic vulnerability that, when combined with MAFLD-specific immune dysregulation, particularly fosters an environment conducive to HCC development.
The immune landscape of the liver is complex, integrating innate and adaptive responses. In a healthy liver, T cells maintain a vigilant watch, clearing early neoplastic lesions and preventing malignant outgrowth[10]. However, chronic lipid accumulation and mitochondrial dysfunction appear to erode this immune vigilance. Wang et al[2] reported a reduction in intrahepatic CD3+ and CD4+ T cells as the disease progressed. Losing these T cells means a breakdown in adaptive immunity, providing a permissive niche for cancer initiation and progression[11]. This T-cell depletion may be rooted in altered metabolism at the cellular level. Like hepatocytes, T cells rely heavily on mitochondrial function for energy generation[12]. Mitochondrial damage and reduced CPT II function can impair T-cell fitness, rendering them less capable of mounting an effective antitumor response[13]. Mitochondrial dysfunction can also lead to increased oxidative stress and an environment conducive to tumor-promoting inflammation rather than tumor-destructive immunity[14]. Such a shift would promote the survival and proliferation of LCSC and transformed hepatocytes.
Identifying LCSCs offers crucial insight into how MAFLD progresses to HCC[15]. LCSCs are characterized by markers such as CD44 and CD24, which are indicative of cells possessing heightened self-renewal capacity, resistance to conventional therapies, and the ability to initiate tumors[16]. Wang et al[2] reported that CPT II inactivity is strongly correlated with LCSC activation. These findings extend beyond a simple correlation, indicating that CPT II inactivity is a pivotal initiating factor in metabolic reprogramming within hepatocytes. As CPT II activity decreases, long-chain fatty acid β-oxidation becomes inefficient, leading to the accumulation of lipids, diminished mitochondrial membrane potential, and increased oxidative stress. Within this lipid-rich, mitochondrion-compromised environment, certain hepatocytes may gain selective advantages, adopting characteristics commonly associated with stem-like cells. These LCSCs, characterized by markers such as CD44 and CD24, exhibit enhanced self-renewal capacity, therapeutic resistance, and tumor-initiating potential. Moreover, the study’s insights into immunological alterations, particularly decreased intrahepatic CD3+ and CD4+ T-cell populations, suggest that CPT II inactivity indirectly fosters an immunosuppressive microenvironment. The loss of adaptive immune surveillance creates a niche where LCSCs can thrive, escape immune-mediated destruction, and drive malignant transformation.
As CPT II activity wanes, an environment rich in lipids and prone to oxidative stress emerges. This environment likely provides selective pressure for a subset of hepatocytes, enabling them to acquire stem cell-like properties and evade normal growth control[17]. The inactivity or reduction of CPT II contributes to a lipid-rich, metabolically stressed milieu that, in turn, influences the cellular signaling pathways integral to maintaining a stem-like phenotype. With impaired CPT II activity, cells accumulate lipids and reduce their reliance on mitochondrial β-oxidation. This shift can favor glycolysis and other metabolic pathways associated with rapid proliferation and stress resistance, which are hallmarks of stem-like cells[18]. The imbalance created by lipid accumulation and diminished mitochondrial function elevates oxidative stress. Increased reactive oxygen species and altered mitochondrial membrane potential can activate or repress key transcription factors and signaling pathways (e.g., p53, peroxisome proliferator-activated receptor, and steroid biosynthesis pathways). These specific alterations in p53 signaling can lead to impaired apoptosis and increased cell survival, while changes in steroid biosynthesis may modulate immune responses by altering cytokine profiles and T-cell receptor signaling, thereby contributing to the suppression of intrahepatic CD4+ T cells. These pathways influence the cell cycle, apoptosis, and differentiation states, driving cells toward a more stem-like, undifferentiated phenotype[19]. Reduced CPT II activity is correlated with diminished intrahepatic CD4+ T cells and compromised immune surveillance. This weakened immunological ability allows LCSC to persist, evade immune-mediated destruction, and sustain their “stemness” without the selective pressure normally exerted by a healthy immune system. By linking mitochondrial CPT II inactivity with LCSC activation, this study reveals a key mechanistic nexus bridging metabolic dysfunction, immune evasion, and oncogenic transformation.
In addition to their experimental findings, Wang et al[2] employed transcriptomic analyses to clarify the underlying pathways and gene networks associated with MAFLD progression and the emergence of HCC. Differentially expressed genes in intrahepatic T cells were enriched in lipid biosynthesis-related pathways, steroid biosynthesis, the peroxisome proliferator-activated receptor pathway, and p53 signaling. Steroid biosynthesis and lipid metabolism pathways, in particular, reinforce the notion that metabolic reprogramming can foster a tumor-friendly microenvironment[20]. The genes involved in the p53 and cell cycle pathways highlight how critical tumor suppressor networks become dysregulated during this process. p53, a vital guardian of genomic stability, often becomes subverted in cancerous transformations[21]. Thus, the links among CPT II inactivity, lipid metabolic shifts, and changes in p53 signaling suggest that metabolic dysfunction could undermine one of the cell’s primary tumor suppression mechanisms[22]. Moreover, these findings align with a body of literature indicating that MAFLD pathogenesis involves not only passive lipid accumulation but also active metabolic reprogramming. This reprogramming might be a preamble to oncogenic signaling cascades that culminate in cellular dedifferentiation, stemness, and ultimately malignancy.
Given the growing interest in the role of the tumor microenvironment (TME) in cancer progression, it is essential to consider how metabolic dysfunction and immune dysregulation within the TME contribute to the activation of LCSC. Metabolic alterations such as CPT II inactivity create a hypoxic and nutrient-deprived environment, which can induce stress responses in hepatocytes. These stress responses may lead to the secretion of factors that promote stemness and support the maintenance of LCSCs. Additionally, immunosuppressive cytokines and altered immune cell populations within the TME can further facilitate the survival and expansion of LCSCs, thereby driving HCC development from MAFLD.
The evolving nomenclature shifting from nonalcoholic fatty liver disease to MAFLD emphasizes the metabolic un
Understanding the role of CPT II inactivity in LCSC activation and T-cell dysfunction has several potential clinical applications. First, therapies aimed at restoring CPT II activity or enhancing mitochondrial β-oxidation could help prevent the malignant progression of MAFLD. Nutritional interventions, pharmacological agents targeting mitochondrial function, or gene therapy approaches to restore CPT II expression may represent avenues for slowing or halting disease progression. However, the feasibility of these strategies faces several challenges. Restoring CPT II activity in vivo requires precise delivery mechanisms to effectively target hepatocytes without off-target effects. Pharmacological agents must be both potent and specific to avoid disrupting other aspects of mitochondrial function. Additionally, gene therapy approaches, while promising, are still in their infancy for liver diseases and must overcome hurdles related to safety, delivery vectors, and long-term expression. Second, monitoring CPT II levels and LCSC markers (CD44 and CD24) could improve risk stratification for MAFLD patients. Identifying those at high risk of malignant progression before overt HCC emerges would allow earlier interventions and possibly improve outcomes. Integration of the CPT II status with imaging and other noninvasive biomarkers could form a composite index that physicians might use to predict HCC risk in MAFLD patients. However, establishing standardized assays for CPT II and LCSC markers, along with validating their predictive power in large, diverse cohorts, remains a significant task. Third, the interplay between mitochondrial dysfunction and the immune system revealed by Wang et al[2] suggests that immunotherapies or interventions to restore T-cell function might be more effective if combined with metabolic reprogramming strategies. As checkpoint inhibitors and chimeric antigen receptor T-cell therapies have advanced in the field of HCC, understanding how best to restore a metabolically favorable and immunostimulatory environment in the liver could increase the success of these treatments. However, combining metabolic therapies with immunotherapies introduces complexity in treatment regimens and necessitates careful evaluation of potential interactions and cumulative toxicities.
Wang et al[2] presented an insightful link between CPT II inactivity, LCSC activation, and immunosuppression in MAFLD progression toward HCC. However, several key aspects warrant further investigation. First, while their findings highlight a connection between mitochondrial dysfunction and tumorigenesis, the direct influence of CPT II inactivity on specific oncogenic mutations or tumor suppressor genes remains unclear. Transcriptomic data revealed the enrichment of genes involved in p53 signaling and other pathways commonly disrupted in HCC, such as the Wnt/β-catenin pathway. Future studies should dissect whether CPT II inactivity predisposes hepatocytes to genetic mutations or if it merely enhances the tumorigenic potential of cells already harboring these alterations.
A more granular analysis of transcriptomic and metabolomic data could clarify how CPT II inactivity interacts with well-known oncogenic networks. Investigations that track p53 target gene dysregulation in CPT II-inactive contexts or that identify downstream effectors of Wnt signaling sensitive to metabolic stress may help establish a causal model. In addition, examining the roles of other carnitine palmitoyltransferases, such as CPT I isoforms, could reveal complementary or compensatory mechanisms influencing fatty acid flux and disease progression. Addressing whether other metabolic enzymes, such as CPT I isoforms, may compensate for CPT II inactivity in certain contexts could provide insights into potential redundancy and resilience within metabolic pathways. Pinpointing how these mitochondrial transporters integrate with diverse signaling pathways may provide a more comprehensive view of metabolic repro
Importantly, not all HCCs are driven by CPT II inactivity. Some tumors rely heavily on alternative pathways, such as glycolysis, glutamine metabolism, or aberrant cholesterol synthesis. Identifying distinct metabolic subtypes of HCC and determining where CPT II fits into this landscape could refine risk stratification, guide therapeutic choices, and enhance prognostic models. Furthermore, discussing potential resistance mechanisms that might emerge during therapeutic interventions targeting CPT II or LCSCs is crucial for developing robust treatment strategies. Ultimately, a multipronged research approach encompassing genetic, metabolic, and immunological dimensions is needed to validate the significance of CPT II and to translate these insights into effective targeted interventions for MAFLD-related HCC.
Currently, liver biopsy remains the gold standard for diagnosing advanced liver disease states, but it is invasive and not suitable for routine population-level screening. Noninvasive markers are needed, and Wang et al’s work[2] on CPT II and LCSC markers will potentially contribute to the development of blood-based or imaging-correlated tests. Additionally, the immunological context provided by their study may guide the use of adjunctive therapies designed to restore healthy immune function in the liver microenvironment. Given the increasing global prevalence of metabolic disorders and their hepatic manifestations, the study by Wang et al[2] is timely. As the number of patients with MAFLD continues to increase, the burden of HCC is linked to metabolic dysregulation. By teasing apart the complex relationships among lipid overload, mitochondrial dysfunction, immune dysregulation, and cancer stem cell activation, this research helps establish a path toward more effective prevention and intervention strategies.
Based on the above, we wondered whether it is possible to integrate research on ketogenic diets with the understanding that CPT II activity in the context of tumor metabolism is largely indirect and remains an area ripe for further investigation. A ketogenic diet shifts the body’s energy utilization from carbohydrates to fats, increasing the reliance on fatty acid oxidation and ketone body production. Normal cells can often adapt to this metabolic shift by efficiently using fatty acids and ketones as alternative energy substrates. On the other hand, tumor cells frequently display a rigid dependency on glucose and may have reduced flexibility in adjusting to low-glucose, high-fat conditions. Wang et al[2] suggested that CPT II inactivity plays a pivotal role in promoting LCSC activation and mitochondrial dysfunction in MAFLD-related HCC. If a tumor cell population is compromised in its ability to oxidize long-chain fatty acids due to impaired CPT II function, it may struggle to thrive under the metabolic constraints imposed by a ketogenic diet. Such an inability to properly engage in fatty acid β-oxidation could render tumor cells more vulnerable and less capable of sustaining growth in a low-glucose environment.
Conversely, if interventions were developed to increase CPT II activity or improve mitochondrial fatty acid oxidation, normal cells might better adapt to the high-fat, low-carbohydrate conditions of a ketogenic diet. Moreover, tumor cells potentially harboring genetic mutations or other metabolic inefficiencies might fail to achieve similar metabolic flexibility, thereby revealing a metabolic vulnerability that could be therapeutically exploited. While direct experimental data linking CPT II function, ketogenic diets, and cancer progression are still scarce, the theoretical groundwork suggests that the interplay between these factors could be significant. Future studies are needed to clarify the molecular mechanisms underlying this relationship and to determine whether targeting CPT II-mediated fatty acid oxidation can enhance the tumor-suppressive effects of a ketogenic diet.
From a diagnostic perspective, integrating CPT II levels with imaging modalities such as magnetic resonance imaging-proton density fat fraction or transient elastography could yield a more refined noninvasive assessment of disease progression[25,26]. Although Wang et al[2] did not focus on imaging technologies, one can envision a combined approach where patients at risk of malignant progression are identified early by a signature involving metabolic enzymes, LCSC markers, and imaging-based quantification of steatosis or fibrosis. Several potential strategies have emerged for this purpose. Pharmacological restoration of CPT II activity or mitochondrial function may normalize β-oxidation and reduce lipotoxicity. Agents that enhance T-cell metabolism or block the deleterious effects of a lipid-rich environment on immune function might help maintain surveillance against neoplastic clones[17]. Similarly, targeted therapies against CD44+ or CD24+ LCSCs, either by blocking their survival pathways or by resensitizing them to conventional che
HCC is a notoriously heterogeneous disease with numerous etiological underpinnings, from chronic viral hepatitis to alcohol, induced cirrhosis and metabolic disorders[24]. The rise of MAFLD as a principal driver of HCC, however, necessitates a pivot toward understanding metabolic alterations at the core of tumorigenesis. While significant strides have been made in understanding the roles of inflammation, fibrosis, and oncogenic signaling pathways, relatively less attention has been given to early metabolic disruptions such as CPT II inactivity[13]. Wang et al[2] position CPT II not only as a marker but also potentially as a linchpin in a cascade leading from benign lipid accumulation to malignant transformation. This perspective encourages the research community to explore other mitochondrial enzymes and transporters and their roles in shaping the TME. By doing so, we might uncover a network of metabolic vulnerabilities in HCC that could be therapeutically exploited.
MAFLD-related HCC exemplifies the challenges of a disease deeply rooted in metabolic imbalance and immunological compromise. As global rates of obesity, insulin resistance, and related metabolic disorders continue to increase, we can expect MAFLD- and consequently MAFLD-associated HCC to grow as a public health concern. Identifying early biomarkers and unraveling the molecular mediators of this progression are pressing priorities. CPT II emerges from this study as a critical piece of that puzzle. When viewed alongside LCSC markers and immune cell profiles, CPT II inactivity may serve as both a harbinger and a driver of malignant changes. With further validation and deeper exploration, these insights could pave the way for novel biomarkers and targeted therapeutics aimed at restoring mitochondrial function, supporting effective immune responses, and mitigating LCSC-driven oncogenesis. The study by Wang et al[2] advances our knowledge of how metabolic dysfunction, immune dysregulation, and cancer stem cell biology intersect in the progression of MAFLD to HCC. By demonstrating that CPT II inactivity promotes malignant progression through LCSC activation and impaired T-cell function, the authors provide a valuable framework for future research and clinical strategies.
| 1. | Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19:2172-2181.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Wang LL, Lu YM, Wang YH, Wang YF, Fang RF, Sai WL, Yao DF, Yao M. Carnitine palmitoyltransferase-II inactivity promotes malignant progression of metabolic dysfunction-associated fatty liver disease via liver cancer stem cell activation. World J Gastroenterol. 2024;30:5055-5069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Borcherding N, Brestoff JR. The power and potential of mitochondria transfer. Nature. 2023;623:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 164] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 4. | Badmus OO, Hillhouse SA, Anderson CD, Hinds TD, Stec DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci (Lond). 2022;136:1347-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 5. | Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, Tanaka Y, Tateishi R, Hikiba Y, Misumi K, Tanaka M, Hayashi A, Shibahara J, Fukayama M, Arita J, Hasegawa K, Hirschfield H, Hoshida Y, Hirata Y, Otsuka M, Tateishi K, Koike K. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Wang S, El-Deiry WS. Requirement of p53 targets in chemosensitization of colonic carcinoma to death ligand therapy. Proc Natl Acad Sci U S A. 2003;100:15095-15100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Li H, Chen J, Liu J, Lai Y, Huang S, Zheng L, Fan N. CPT2 downregulation triggers stemness and oxaliplatin resistance in colorectal cancer via activating the ROS/Wnt/β-catenin-induced glycolytic metabolism. Exp Cell Res. 2021;409:112892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, Nasyrova RF, Krupitsky EM, Shalikiani NV, Bakulin IG, Shcherbakov PL, Skorodumova LO, Larin AK, Kostryukova ES, Abdulkhakov RA, Abdulkhakov SR, Malanin SY, Ismagilova RK, Grigoryeva TV, Ilina EN, Govorun VM. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 9. | Soysouvanh F, Rousseau D, Bonnafous S, Bourinet M, Strazzulla A, Patouraux S, Machowiak J, Farrugia MA, Iannelli A, Tran A, Anty R, Luci C, Gual P. Osteopontin-driven T-cell accumulation and function in adipose tissue and liver promoted insulin resistance and MAFLD. Obesity (Silver Spring). 2023;31:2568-2582. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Jett KA, Baker ZN, Hossain A, Boulet A, Cobine PA, Ghosh S, Ng P, Yilmaz O, Barreto K, DeCoteau J, Mochoruk K, Ioannou GN, Savard C, Yuan S, Abdalla OH, Lowden C, Kim BE, Cheng HM, Battersby BJ, Gohil VM, Leary SC. Mitochondrial dysfunction reactivates α-fetoprotein expression that drives copper-dependent immunosuppression in mitochondrial disease models. J Clin Invest. 2023;133:e154684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Sun H, Zhang L, Wang Z, Gu D, Zhu M, Cai Y, Li L, Tang J, Huang B, Bosco B, Li N, Wu L, Wu W, Li L, Liang Y, Luo L, Liu Q, Zhu Y, Sun J, Shi L, Xia T, Yang C, Xu Q, Han X, Zhang W, Liu J, Meng D, Shao H, Zheng X, Li S, Pan H, Ke J, Jiang W, Zhang X, Han X, Chu J, An H, Ge J, Pan C, Wang X, Li K, Wang Q, Ding Q. Single-cell transcriptome analysis indicates fatty acid metabolism-mediated metastasis and immunosuppression in male breast cancer. Nat Commun. 2023;14:5590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 12. | Han S, Georgiev P, Ringel AE, Sharpe AH, Haigis MC. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023;35:36-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, Heinrich B, Yu SJ, Zhang Q, Wilson A, Shi ZD, Swenson R, Greten TF. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Mansouri A, Gattolliat CH, Asselah T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology. 2018;155:629-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 551] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 15. | Hernaez R, Peck-Radosavljevic M. MAFLD, HCC and the dilemma of (changing) terminology in liver diseases. Gut. 2023;72:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Lee D, Na J, Ryu J, Kim HJ, Nam SH, Kang M, Jung JW, Lee MS, Song HE, Choi J, Lee GH, Kim TY, Chung JK, Park KH, Kim SH, Kim H, Seo H, Kim P, Youn H, Lee JW. Interaction of tetraspan(in) TM4SF5 with CD44 promotes self-renewal and circulating capacities of hepatocarcinoma cells. Hepatology. 2015;61:1978-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | González-Romero F, Mestre D, Aurrekoetxea I, O'Rourke CJ, Andersen JB, Woodhoo A, Tamayo-Caro M, Varela-Rey M, Palomo-Irigoyen M, Gómez-Santos B, de Urturi DS, Núñez-García M, García-Rodríguez JL, Fernández-Ares L, Buqué X, Iglesias-Ara A, Bernales I, De Juan VG, Delgado TC, Goikoetxea-Usandizaga N, Lee R, Bhanot S, Delgado I, Perugorria MJ, Errazti G, Mosteiro L, Gaztambide S, Martinez de la Piscina I, Iruzubieta P, Crespo J, Banales JM, Martínez-Chantar ML, Castaño L, Zubiaga AM, Aspichueta P. E2F1 and E2F2-Mediated Repression of CPT2 Establishes a Lipid-Rich Tumor-Promoting Environment. Cancer Res. 2021;81:2874-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Che L, Chi W, Qiao Y, Zhang J, Song X, Liu Y, Li L, Jia J, Pilo MG, Wang J, Cigliano A, Ma Z, Kuang W, Tang Z, Zhang Z, Shui G, Ribback S, Dombrowski F, Evert M, Pascale RM, Cossu C, Pes GM, Osborne TF, Calvisi DF, Chen X, Chen L. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2020;69:177-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 19. | Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C, Li Y, Prochownik EV, Karin M, Wang F, Li Y. P53 deficiency affects cholesterol esterification to exacerbate hepatocarcinogenesis. Hepatology. 2023;77:1499-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Di Leo L, Vegliante R, Ciccarone F, Salvatori I, Scimeca M, Bonanno E, Sagnotta A, Grazi GL, Aquilano K, Ciriolo MR. Forcing ATGL expression in hepatocarcinoma cells imposes glycolytic rewiring through PPAR-α/p300-mediated acetylation of p53. Oncogene. 2019;38:1860-1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 22. | Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22:4156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 23. | Xia T, Du M, Li H, Wang Y, Zha J, Wu T, Ju S. Association between Liver MRI Proton Density Fat Fraction and Liver Disease Risk. Radiology. 2023;309:e231007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 24. | van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, Brener S, Moher D, Thavorn K. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int. 2017;37:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Barsch M, Salié H, Schlaak AE, Zhang Z, Hess M, Mayer LS, Tauber C, Otto-Mora P, Ohtani T, Nilsson T, Wischer L, Winkler F, Manne S, Rech A, Schmitt-Graeff A, Bronsert P, Hofmann M, Neumann-Haefelin C, Boettler T, Fichtner-Feigl S, van Boemmel F, Berg T, Rimassa L, Di Tommaso L, Saeed A, D'Alessio A, Pinato DJ, Bettinger D, Binder H, John Wherry E, Schultheiss M, Thimme R, Bengsch B. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J Hepatol. 2022;77:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 26. | Mossmann D, Müller C, Park S, Ryback B, Colombi M, Ritter N, Weißenberger D, Dazert E, Coto-Llerena M, Nuciforo S, Blukacz L, Ercan C, Jimenez V, Piscuoglio S, Bosch F, Terracciano LM, Sauer U, Heim MH, Hall MN. Arginine reprograms metabolism in liver cancer via RBM39. Cell. 2023;186:5068-5083.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 133] [Reference Citation Analysis (0)] |