Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.105004
Revised: February 24, 2025
Accepted: March 24, 2025
Published online: April 14, 2025
Processing time: 92 Days and 18.5 Hours
Patients with acute-on-chronic liver failure (ACLF) have a high mortality rate, poor prognosis, and often experience concurrent thrombocytopenia and bleeding events.
To evaluate the efficacy and safety of recombinant human thrombopoietin (rhTPO) in patients with ACLF with concomitant severe thrombocytopenia.
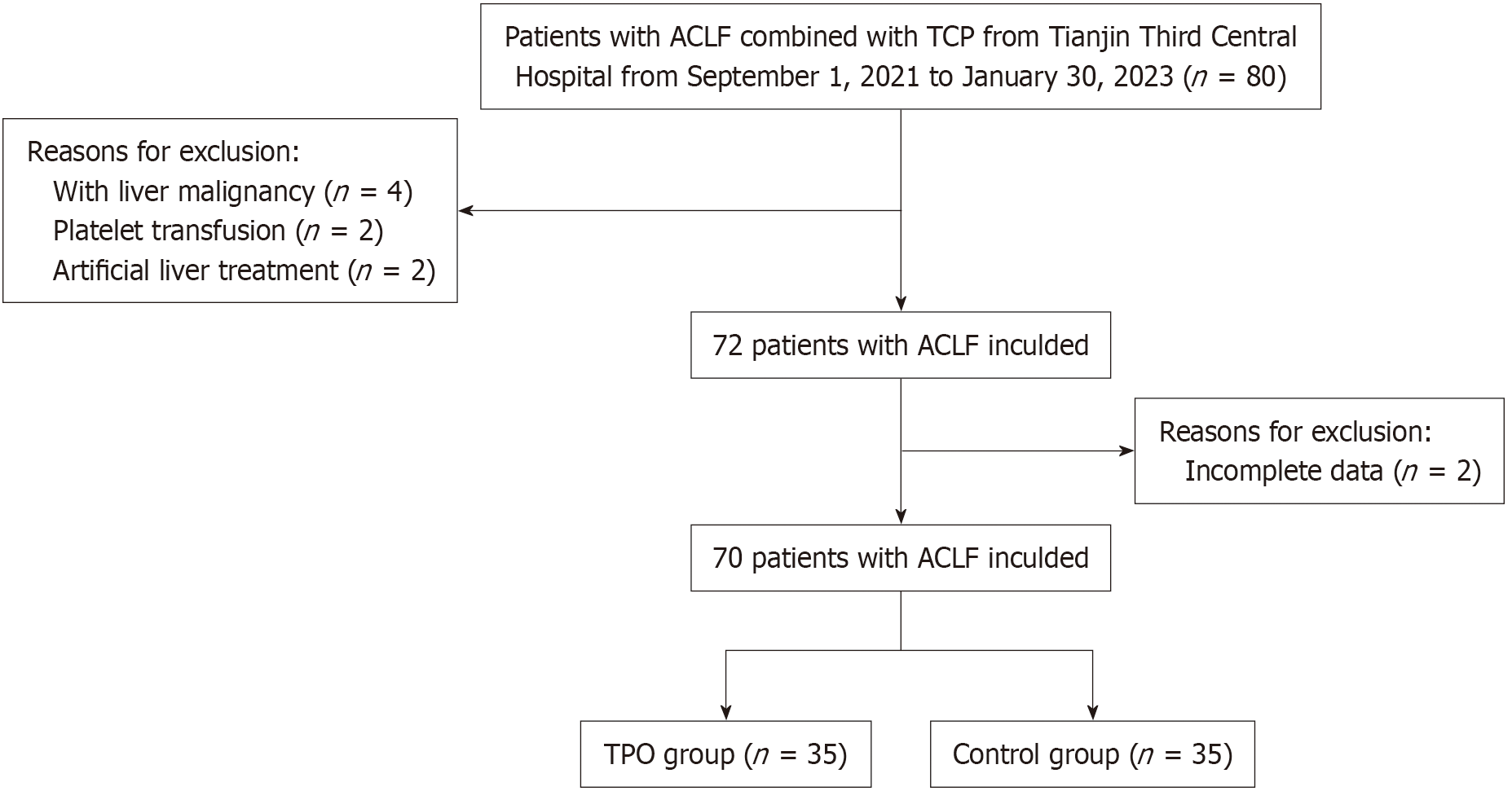
This was a prospective, open-label study. We assigned 70 ACLF patients with severe thrombocytopenia into the rhTPO group and control group, with 35 pa
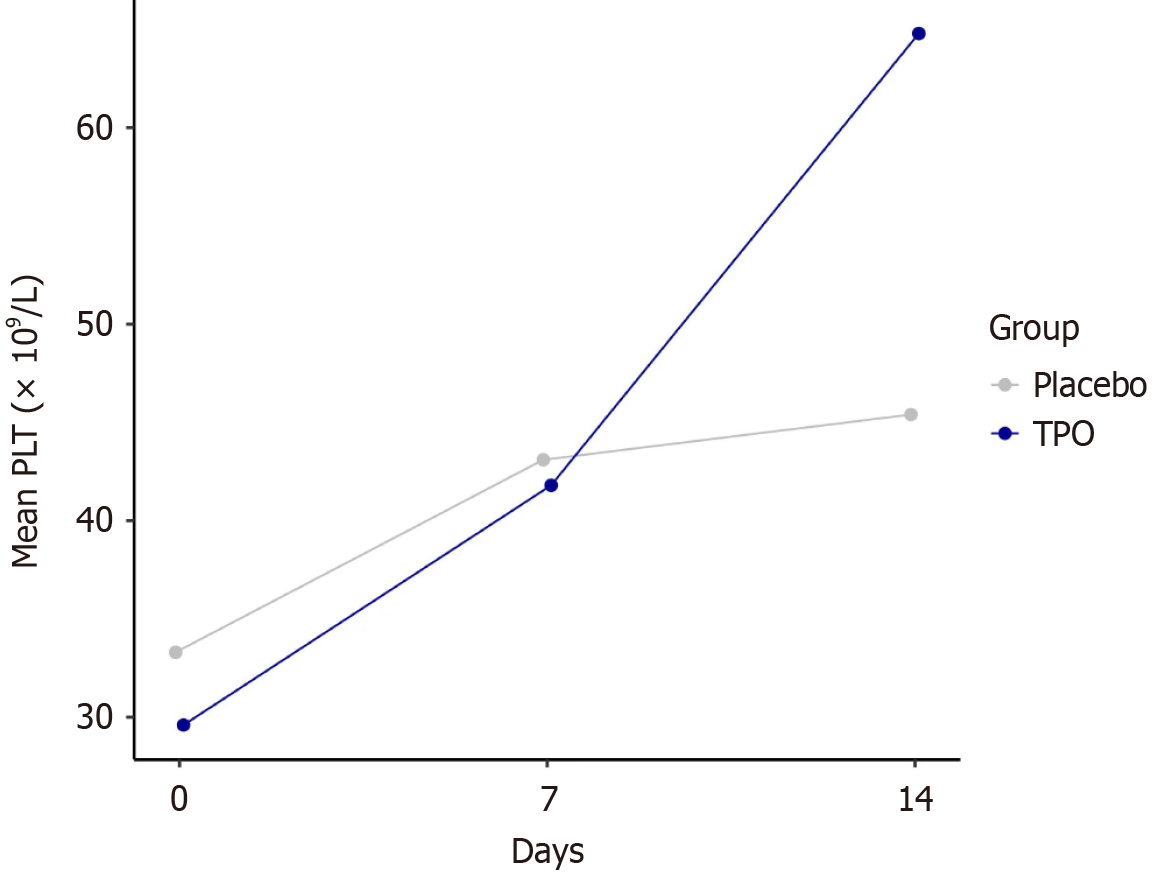
The proportion of patients with platelet count > 50 × 109/L on day 14 was 60.7% in the rhTPO group, which was significantly higher than that (12.0%) in the control group (P < 0.001). The platelet count in the rhTPO group on day 14 was 64 × 109/L, exceeding the baseline of 28 × 109/L. Compared to the control group, the rhTPO group exhibited a significant increase in platelet count from baseline (P < 0.05). Model for end-stage liver disease score, albumin level and international normalized ratio improved significantly from baseline on day 14 after rhTPO injection. The concentrations of serum thrombopoietin and hepatocyte growth factor in the rhTPO group after 7 days were 143.7 and 195.4 pg/mL, respectively, showing a significant increase from baseline (P < 0.05). Eight (22.9%) patients had bleeding events in the control group compared with four (11.4%) in the rhTPO group. The incidence of 90-day mortality was also higher in the control group (6, 17.1%) than that in the rhTPO group (3, 8.6%).
rhTPO significantly increased the platelet count in ACLF patients with thrombocytopenia and reduce the occurrence of bleeding events, with a good safety profile.
Core Tip: Patients with acute-on-chronic liver failure (ACLF) have a high mortality rate and poor prognosis. They often experience concurrent thrombocytopenia and bleeding events. This study aimed to evaluate the efficacy and safety of recombinant human thrombopoietin (rhTPO) in ACLF patients with concomitant severe thrombocytopenia. We assigned 70 ACLF patients with severe thrombocytopenia into the rhTPO group and the control group, with 35 patients in each group. Patients in the rhTPO group received subcutaneous injections of rhTPO for 7 consecutive days, while those in the control group did not receive rhTPO treatment. The platelet count in the rhTPO group increased significantly from the baseline. The model for end-stage liver disease score, albumin level, and international normalized ratio in patients of the rhTPO group improved significantly from the baseline on day 14 after rhTPO injection. In summary, rhTPO can significantly increase the platelet count in ACLF patients with thrombocytopenia, help improve liver function, and reduce the occurrence of bleeding events. It also has a good safety profile.
- Citation: Liu G, Tang F, Wang T, Yan JQ, Li FH, Ha FS, Zhang X, Jing L, Liang J. Efficacy of recombinant human thrombopoietin in patients with acute-on-chronic liver failure and thrombocytopenia: A prospective, open-label study. World J Gastroenterol 2025; 31(14): 105004
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/105004.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.105004
Acute-on-chronic liver failure (ACLF) is a clinical syndrome characterized by severe hepatic dysfunction resulting from acute injury to an underlying chronic liver disease and a substantially high short-term mortality rate[1,2]. Cardinal manifestations are jaundice and coagulopathy, complicated within 4 week by ascites and encephalopathy[2]. Thrombocytopenia has been described in most patients with acute and chronic liver failure and is a cause of bleeding problems in these patients[3]. In addition, bleeding events are common in patients with ACLF and increase with the severity of coagulation disorders[4]. Bleeding complications may be life-threatening. Therefore, bleeding control practices and evidence-based prophylactic strategies are critical for the management of patients with ACLF.
Platelets are an essential factor involved in hemostasis. High platelet counts are beneficial for outcome after liver resection and liver transplantation, not only with regard to bleeding complications, but also because of their ability to promote liver regeneration[5]. Platelet transfusions are commonly used to reduce the risk of bleeding during a procedure, but their short duration of efficacy and the risk of transfusion reactions limit their use[6,7]. Splenectomy and splenic artery embolization are also effective in patients with chronic liver disease and thrombocytopenia. Concerns remain regarding the serious complications following splenectomy and the recurrence of thrombocytopenia following splenic embolization[8,9].
Thrombopoietin (TPO) is a hematopoietic growth factor that exerts its biological effects by binding to specific c-Mpl receptors on the surface of megakaryocytes and platelets. This interaction regulates the proliferation, differentiation, and maturation of megakaryocytes, while also modulating the expression of platelet-specific proteins and controlling the concentration of circulating platelets[10]. Recombinant human TPO (rhTPO) is a full-length glycosylated TPO expressed by Chinese hamster ovary cells and purified via gene recombination technology. Because its characteristics are similar to those of endogenous TPO, rhTPO has similar pharmacological effects on platelet levels. rhTPO is currently available or in development to manage thrombocytopenia in patients with chronic liver disease[11]. However, there is no report on the application of rhTPO in the treatment of ACLF-related thrombocytopenia, nor on its effects on liver function and prognosis in ACLF patients.
The aim of the study was to evaluate the efficacy and safety of rhTPO in patients with ACLF with concomitant severe thrombocytopenia.
This was a prospective, open-label study conducted at the Third Central Hospital of Tianjin in China from September 1, 2021 to January 30, 2023 in compliance with Good Clinical Practice. Patients were assigned in a 1:1 ratio to receive rhTPO (15000 IU/day for 7 days) or no intervention (control group). This study was registered at chictr.org.cn (ChiCTR2100046728) and was approved by the Ethics Committee of Tianjin Third Central Hospital (IRB2121-001-01), and all enrolled patients provided informed consent prior to receiving treatment.
Eligible patients were adults (age > 18 years) with ACLF in compliance with the 2019 Asian Pacific Association (APASL) for the Study of the Liver diagnostic criteria, severe thrombocytopenia during hospitalization (platelet count < 50 × 109/L), and agreement to be followed up for 90 days. The APASL diagnostic criteria for ACLF were acute liver function damage occurring on the basis of previously known or unknown chronic liver disease [total bilirubin (TBIL) ≥ 5 mg/dL and international standardized ratio ≥ 1.5 or prothrombin time activity (PTA) ≤ 40%], combined with the appearance of ascites and/or hepatic encephalopathy within 4 weeks. Exclusion criteria included: (1) Contraindications for rhTPO injection; (2) Malignant liver tumors; (3) Hematological diseases, such as hematological malignancies, immune hemolytic anemia, or idiopathic thrombocytopenic purpura; (4) Having received platelets, glucocorticoids or drugs with bone marrow suppressive activity within the week before admission and during hospitalization; (5) Having received rhTPO agents, leucogen, interleukin-11 and other drugs to increase platelets or antiplatelet drugs within 1 month before admission; (6) History of liver transplantation; having received artificial liver treatment; (7) Portal vein or deep vein thrombosis before treatment; or (8) Loss to follow-up; or incomplete data.
The primary endpoint was the proportion of patients with platelet count > 50 × 109/L on day 14. The secondary efficacy endpoints included changes in platelet count from baseline on days 7 and 14, changes in hepatic function from baseline on days 7 and 14, 90-day mortality, levels of TPO and hepatocyte growth factor (HGF) on day 7, and adverse events. Peripheral blood samples were taken from patients diagnosed with ACLF to assess hepatic and renal function. These assessments included measurements of aspartate aminotransferase (AST), alanine aminotransferase (ALT), g-glutamyl transpeptidase, TBIL, albumin (ALB) and creatinine levels. The model for end-stage liver disease (MELD) score was calculated based on the above tests to evaluate the severity of ACLF and predict short-term mortality risk. Additionally, routine blood tests, PTA, and international normalized ratio (INR) were also assessed.
To estimate the sample size required for analysis of the primary endpoint, we assumed that the proportion of patients with platelet count > 50 × 109/L on day 14 was 50% in the rhTPO group compared with 10% in the control group. A total of 52 patients (26 in each study group) would provide 90% power to detect a significant treatment effect with the use of a two-sided alpha level of 0.05. Considering the dropout rate, the final sample size was 70 patients, with 35 in each group.
Statistical analyses were performed using SPSS version 23.0 (SSPS, Chicago, IL, United States). Continuous variables were expressed as median (Q1, Q3) otherwise specified, while categorical variables were presented as rates. Group comparisons for continuous variables were performed using t-tests and nonparametric rank sum tests, while categorical variables were examined using the χ2 test or Wilcoxon test. P < 0.05 was considered statistically significant.
As of January 30, 2023, 70 patients had been enrolled, with 35 in the rhTPO group and 35 in the control group (Figure 1). The demographic and baseline clinical characteristics of the two groups of patients are shown in Table 1. The rhTPO and control arms were generally well matched. The mean age was 53.5 and 50.1 years, and the male proportion was 91.4% and 74.3% in the rhTPO and control arms, respectively. Regarding medical history, 74.2% and 60.0% had cirrhosis in the rhTPO group and control group, respectively. The mean platelet count was 28.0 × 109/L and 35.0 × 109/L at baseline in the rhTPO and control groups, respectively.
| rhTPO (n = 35) | Control (n = 35) | P value1 | |
| Demographic | |||
| Sex | 0.057 | ||
| Male | 32 (91.4) | 26 (74.3) | |
| Female | 3 (8.6) | 9 (25.7) | |
| Age, mean ± SD, year | 53.5 ± 10.9 | 50.1 ± 12.1 | 0.214 |
| Etiology | |||
| Alcoholic liver disease | 16 (45.7) | 9 (25.7) | 0.073 |
| Hepatitis B | 16 (45.7) | 26 (74.3) | |
| Hepatitis C | 1 (2.9) | 0 (0) | |
| Non-alcoholic steatohepatitis | 1 (2.9) | 0 (0) | |
| Autoimmune liver disease | 1 (2.9) | 0 (0) | |
| Cirrhosis | 26 (74.2) | 21 (60.0) | 0.203 |
| Laboratory findings, median (Q1-Q3) | |||
| WBC, 109/L | 2.5 (1.9, 5.3) | 4.8 (3.1, 8.2) | < 0.001 |
| Hemoglobin, g/L | 108.0 (78.0, 121.0) | 113.5 (98.0, 124.0) | 0.228 |
| Neutrophil, % | 70.8 (63.5, 79.0) | 78.7 (67.3, 87.2) | 0.055 |
| Platelet count, 109/L | 28.0 (19.0, 39.0) | 35.0 (24.0, 42.0) | 0.130 |
| Albumin, g/L | 28.2 (24.4, 31.7) | 27.5 (22.8, 31.8) | 0.742 |
| Alanine transaminase, U/L | 34.0 (20.0, 71.0) | 64.0 (35.0, 148.0) | 0.003 |
| Aspartate transaminase, U/L | 59.0 (36.0, 106.0) | 82.0 (45.0, 173.0) | 0.152 |
| Alkaline phosphatase, U/L | 110.0 (88.0, 161.0) | 70.5 (44.0, 140.0) | 0.037 |
| Gamma-glutamyl transferase, U/L | 47.0 (22.0, 138.0) | 113.0 (56.0, 212.0) | 0.023 |
| Total bilirubin, μmol/L | 88.8 (76.3, 144.1) | 113.9 (95.2, 132.7) | 0.053 |
| Creatinine, μmol/L | 72.0 (59.0, 93.7) | 64.0 (52.0, 81.0) | 0.095 |
| INR | 1.7 (1.5, 2.1) | 2.1 (2.0, 2.6) | 0.088 |
| MELD | 17.0 (9.0, 22.0) | 18.0 (13.8, 23.0) | 0.187 |
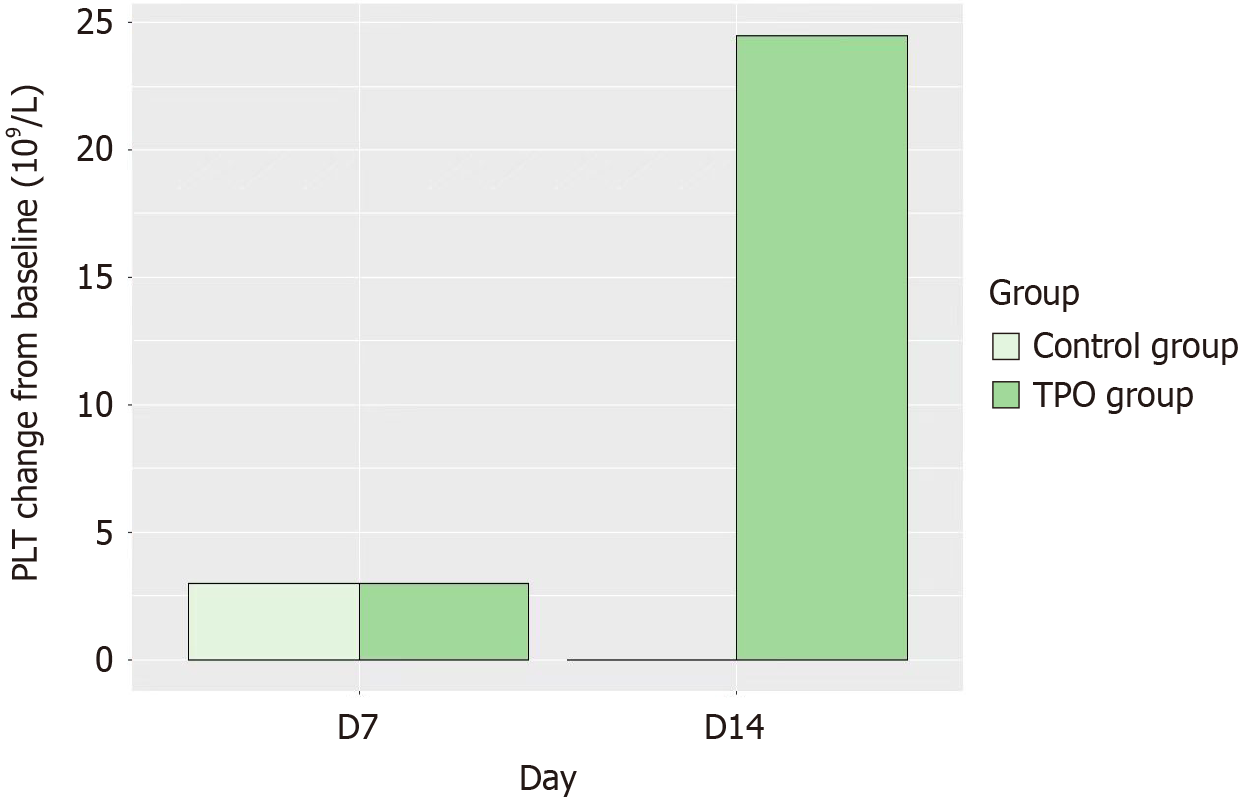
Detailed variations in hematological parameters are presented in Table 2 and Figures 2 and 3. After 7 consecutive days of rhTPO treatment, platelet count in the rhTPO group was 64 × 109/L, exceeding the baseline of 28 × 109/L. While the control group showed no significant differences in platelet count changes. Compared to the control group, the rhTPO group exhibited a significant increase in platelet count on day 14 after rhTPO treatment (P < 0.01; Table 2 and Figure 3). It is worth noting that platelet counts increased over the time from day 7 to 14. The proportion of patients with platelet count > 50 × 109/L on day 14 was 60.7% in the rhTPO group, which was significantly higher than that (12.0%) in the control group (P < 0.001; Table 2). There was a significant difference in the white blood cell count change from baseline between the rhTPO group and the control group on day 14 (P < 0.05).
| Variables | rhTPO (n = 35) | Control (n = 35) | P value1 |
| The proportion of patients with platelet count > 50 × 109/L on day 14 | |||
| Baseline | 3 (8.6) | 2 (5.7) | 1.000 |
| Day 7 | 8 (22.9) | 8 (27.6) | 0.664 |
| Day 14 | 17 (60.7) | 3 (12.0) | < 0.001 |
| Platelet count, 109/L | |||
| Baseline | 28 (19, 39) | 35 (24, 42) | 0.213 |
| Day 7 | 34 (20, 50)a | 37 (27, 53)a | 0.274 |
| Day 7 change from baseline | 3.0 (-3.0, 18.0) | 3.0 (-2.5, 26.5) | 0.956 |
| Day 14 | 64 (33, 91)a | 33 (24, 42) | 0.005 |
| Day 14 change from baseline | 24.5 (4.0, 47.0) | 0 (-11.0, 13.0) | < 0.001 |
| WBC, 109/L | |||
| Baseline | 2.5 (1.9, 5.3) | 4.8 (3.1, 8.2) | < 0.001 |
| Day 7 | 3.0 (2.2, 4.4) | 4.5 (2.9, 6.8) | 0.016 |
| Day 7 change from baseline | 0.21 (-0.45, 0.80) | -0.22 (-2.27, 0.49) | 0.275 |
| Day 14 | 4.0 (2.3, 5.4) | 3.3 (2.6, 5.3)a | 0.695 |
| Day 14 change from baseline | 0.11 (-0.90, 2.24) | -0.67 (-2.14, 0.00) | 0.019 |
After 7 consecutive days of rhTPO treatment, the liver function and coagulation parameters (including ALT, ASB, ALB, TBIL, MELD and PTA) change from baseline in the rhTPO group showed no significant difference compared to the control group on days 7 and 14 (P > 0.05). However, there was still a noticeable improvement trend for liver function and coagulation parameters on days 7 and 14 compared with baseline in both groups (Table 3).
| Variables | rhTPO (n = 35) | Control (n = 35) | P value1 |
| Alanine transaminase, U/L | |||
| Baseline | 34.0 (20.0, 71.0) | 64.0 (35.0, 148.0) | 0.003 |
| Day 7 | 27.0 (18.0, 36.0)a | 46.0 (22.0, 72.5)a | 0.009 |
| Day 7 change from baseline | -8 (-22, -3) | -18 (-50.5, -6.5) | 0.050 |
| Day 14 | 26.0 (15.0, 41.0)a | 35.0 (18.0, 53.0)a | 0.346 |
| Day 14 change from baseline | -9 (-33, 3) | -19.5 (-61, -8) | 0.063 |
| Aspartate transaminase, U/L | |||
| Baseline | 59.0 (36.0, 106.0) | 82.0 (45.0, 173.0) | 0.152 |
| Day 7 | 42.0 (30.0, 71.0)a | 57.0 (31.0, 97.0)a | 0.209 |
| Day 7 change from baseline | -17 (-36, -2) | -20 (-141, 0) | 0.398 |
| Day 14 | 49.0 (27.0, 71.0)a | 46.0 (27.0, 56.0)a | 0.678 |
| Day 14 change from baseline | -21.5 (-34, 3) | -46 (-106, -15) | 0.056 |
| Albumin, g/L | |||
| Baseline | 28.2 (24.4, 31.7) | 27.5 (22.8, 31.8) | 0.742 |
| Day 7 | 29.6 (26.0, 32.1)a | 29.8 (26.5, 32.0)a | 1.000 |
| Day 7 change from baseline | 1.7 (-1.0, 4.2) | 2.9 (-2.0, 6.7) | 0.611 |
| Day 14 | 32.6 (29.3, 35.3)a | 31.7 (26.3, 33.8)a | 0.318 |
| Day 14 change from baseline | 4.9 (0.8, 8.6) | 4.0 (0.0, 6.6) | 0.323 |
| Total bilirubin, μmol/L | |||
| Baseline | 88.8 (76.3, 144.1) | 113.9 (95.2, 132.7) | 0.053 |
| Day 7 | 91.8 (51.5, 138.9)a | 105.3 (84.3, 119.8) | 0.143 |
| Day 7 change from baseline | -12.5 (-41.8, 6.7) | -10.5 (-31.1, 9.7) | 0.787 |
| Day 14 | 82.4 (49.9, 154.7) | 86.2 (61.1, 121.1)a | 0.920 |
| Day 14 change from baseline | -29.0 (-43.1, 9.0) | -25.6 (-51.6, -4.9) | 0.681 |
| MELD | |||
| Baseline | 17 (9.0, 22) | 18 (13.8, 23) | 0.187 |
| Day 7 | 15 (8.5, 18.5) | 16 (13, 19) | 0.230 |
| Day 7 change from baseline | -1 (-3, 1) | -1 (-5, 1) | 0.791 |
| Day 14 | 15 (10.5, 16.5)a | 15 (11, 21) | 0.087 |
| Day 14 change from baseline | -2 (-6.5, 0) | -2 (-5, 1) | 0.676 |
| INR | |||
| Baseline | 1.7 (1.5, 2.1) | 2.1 (2.0, 2.6) | 0.088 |
| Day 7 | 1.7 (1.5, 2.0)a | 2.0 (1.7, 2.2)a | 0.017 |
| Day 7 change from baseline | -0.085 (-0.330, 0.030) | -0.140 (-0.575, 0.055) | 0.493 |
| Day 14 | 1.5 (1.4, 1.8)a | 2.0 (1.8, 2.3) | < 0.001 |
| Day 14 change from baseline | -0.22 (-0.34, -0.08) | -0.105 (-0.450, 0.035) | 0.293 |
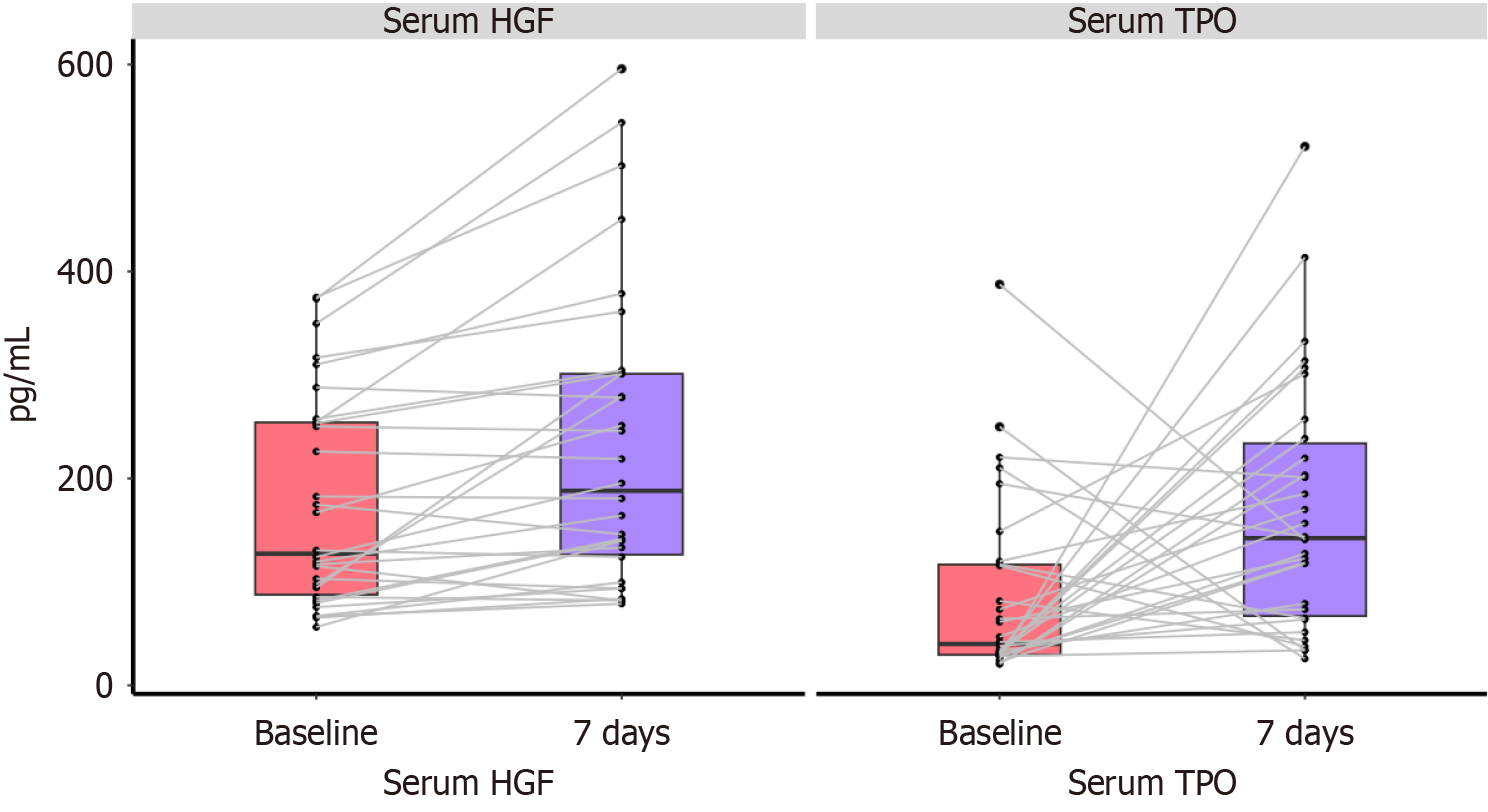
On day 7, the concentrations of TPO and HGF were 143.7 pg/mL and 195.4 pg/mL, respectively, which showed a significant increase compared to the baseline levels (P < 0.05; Figure 4).
At the end of the 90-day follow-up after rhTPO treatment, there were four patients (11.4%) in the rhTPO group experiencing bleeding events, including two with gastrointestinal bleeding, one with skin bleeding, and one with nasal bleeding. The control group had eight patients (22.9%) with bleeding events, including four with gastrointestinal bleeding, three with skin bleeding, and one with gingival bleeding. There were three deaths in the treatment group within 90 days, while the control group had six deaths. Mortality rate within 90 days was lower in the rhTPO group than in the control group (8.6% vs 17.1%) (Table 4). No thrombotic events occurred in either group of patients. During the treatment period, two patients (5.7%) in the rhTPO group experienced transient fever, with no reports of rash, nausea, or diarrhea or any other adverse events (Table 4).
| rhTPO (n = 35) | Control (n = 35) | P value | |
| Fever | 2 (6.7) | 2 (6.7) | 1.000 |
| Thrombophilia | 0 | 0 | 1.000 |
| Bleeding events | 4 (11.4) | 8 (22.9) | 0.205 |
| Death | 3 (8.6) | 6 (17.1) | 0.284 |
Patients with ACLF complicated with thrombocytopenia have a higher risk of bleeding, which may lead to higher mortality. Our study showed that a daily dose of 15000 IU rhTPO for 7 consecutive days increased platelet count and HGF level in patients with ALCF.
At present, treatment for liver failure combined with thrombocytopenia includes platelet transfusion, splenectomy, splenic artery embolization, rhTPO, and new-generation TPO receptor agonists[12]. The delayed peak and magnitude of platelet elevation in patients with ACLF suggest that the speed and extent of TPO-mediated platelet elevation are related to liver functional reserve[13]. In our study, rhTPO had a positive effect on ACLF-associated thrombocytopenia. After rhTPO treatment, the platelet count reached 64 × 109/L, exceeding the baseline of 28 × 109/L. Additionally, serum TPO testing revealed a significant increase in TPO levels after treatment. Previous study on patients with chronic liver disease showed that, following a 5-day treatment of rhTPO, patients exhibited a significant increase in platelet count, surpassing the baseline by 50 × 109/L[11]. Another study of hepatitis-B-related cirrhosis complicated with severe thrombocytopenia showed that initial response was achieved in 65.5%-73.3% of patients after rhTPO treatment[14].
Liver failure is a common clinical syndrome characterized by severe impairment of liver function, and the current treatment options are limited[1]. Liver regeneration plays a crucial role in the recovery and prognosis of liver failure. Current research has found that platelets play a crucial role in liver regeneration by releasing bioactive proteins, including cytokines, chemokines, and growth factors, which promote hepatocyte proliferation[15]. At the same time, the binding of platelets and endothelial cells in liver injury tissues promotes the aggregation of neutrophils and leukocytes, which is important for liver regeneration[16]. Several studies have demonstrated that a significant decrease in platelet count after hepatectomy, particularly a reduction of 40%, is associated with delayed liver function recovery and increased risk of postoperative complications[17,18]. In a mouse model of hepatectomy and partial liver transplantation, the use of TPO increased platelet count and promoted hepatocyte regeneration[19]. Our results suggest that rhTPO enhanced liver cell regeneration and recovery by increasing platelet count and activating related protein pathways and liver gene expression.
Our study followed patients with ACLF and severe thrombocytopenia for 90 days, observing dynamic changes in liver function after rhTPO treatment. Compared to the control group, patients treated with rhTPO showed significant improvements in INR levels and a notable decrease in MELD scores. We detected the serum TPO and HGF levels of the rhTPO group before and after treatment. With the increase of TPO levels, the serum HGF concentration significantly increased after treatment. Some studies have found that platelets can promote liver cell regeneration through HGF[20]. Platelets are the main source of HGF. HGF was first purified from rat platelets in 1986. Some studies have found that platelets play an important role in inducing HGF to promote liver cell proliferation in the early stage of partial hepatectomy in rat models. Another study reported that platelets may be related to the circulating levels of platelet-derived transforming growth factor β1, platelet factor 4, and TPO, as well as liver cell proliferation factors such as HGF and interleukin-6 in the process of liver regeneration after hepatectomy[21]. Therefore, we speculate that rhTPO increases platelets, which may increase the level of HGF and help liver cell regeneration and recovery by activating related protein channels and liver gene expression.
Patients with ACLF often experience bleeding events and a hypercoagulable state due to disorder of coagulation and bleeding functions[4]. It is of clinical interest to investigate whether elevating platelet levels in ACLF patients can reduce bleeding events and the incidence of thrombosis. In this study, patients in the rhTPO group were administered rhTPO injections for 7 days, and a reduction in the rate of bleeding events compared to the control group was observed. Considering the limited sample size of this study and the multifactorial nature of bleeding events in patients with liver failure, including portal hypertension and coagulation dysfunction, further investigation with larger sample sizes and stratified observation is warranted for further elucidation. Besides, the incidence of death was also higher in the control group than that in the rhTPO group (17.1% vs 8.6%). Additionally, thrombotic events were monitored during treatment and follow-up in this study, and no instances of thrombosis were observed in the treatment group, with only a few patients experiencing transient fever, indicating a good safety profile.
This study still had some limitations. As a single-center cohort study, it had a small sample size and lacked long-term follow-up observation. Since the long-term prognosis of ACLF may be affected by multiple factors, more samples and subgroup stratification are needed to further observe the long-term effects of rhTPO on platelet levels, changes in liver function, and survival rate.
In summary, rhTPO can significantly increase the platelet counts in ACLF patients with thrombocytopenia, which helps improve liver function, reduce the occurrence of bleeding events, and demonstrates a good safety profile.
| 1. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 587] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 3. | Lisman T, Luyendyk JP. Platelets as Modulators of Liver Diseases. Semin Thromb Hemost. 2018;44:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Premkumar M, Saxena P, Rangegowda D, Baweja S, Mirza R, Jain P, Bhatia P, Kumar G, Bihari C, Kalal C, Vyas T, Choudhury A, Sarin SK. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: An observational cohort study. Liver Int. 2019;39:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Takahashi K, Liang C, Oda T, Ohkohchi N. Platelet and liver regeneration after liver surgery. Surg Today. 2020;50:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 6. | Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, Mumford AD, Stanworth SJ, Tinegate H; British Committee for Standards in Haematology. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 334] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 7. | Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O'Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AA; AABB. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 640] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 8. | Desai S, Subramanian A. Thrombocytopenia in Chronic Liver Disease: Challenges and Treatment Strategies. Cureus. 2021;13:e16342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Nilles KM, Flamm SL. Thrombocytopenia in Chronic Liver Disease: New Management Strategies. Clin Liver Dis. 2020;24:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29:282-294. [PubMed] |
| 11. | Ding JN, Feng TT, Sun W, Cai XY, Zhang Y, Zhao WF. Recombinant human thrombopoietin treatment in patients with chronic liver disease-related thrombocytopenia undergoing invasive procedures: A retrospective study. World J Gastrointest Surg. 2022;14:1260-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Lim HI, Cuker A. Thrombocytopenia and liver disease: pathophysiology and periprocedural management. Hematology Am Soc Hematol Educ Program. 2022;2022:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 13. | Liang J, Xiang HL, Wang FM, Li Y, Yin WL, Tan F. Short-term clinical evaluation of recombinant humanthrombopoietin treating on thrombocytopaenia in patients with liver cirrhosisassociated with viral hepatitis. Int J Clin Exp Med. 2016;11:22148-22154. |
| 14. | Feng R, Liu Y, Zhu XL, Zhai WY, He Y, Fu HX, Jiang Q, Jiang H, Lu J, Liu H, Wang JW, Wang H, Xie YD, Ma H, Huang XJ, Zhang XH. Recombinant human thrombopoietin increases platelet count in severe thrombocytopenic patients with hepatitis B-related cirrhosis: Multicentre real-world observational study. J Viral Hepat. 2022;29:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 16. | Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (3)] |
| 18. | Takahashi K, Kurokawa T, Oshiro Y, Fukunaga K, Sakashita S, Ohkohchi N. Postoperative Decrease in Platelet Counts Is Associated with Delayed Liver Function Recovery and Complications after Partial Hepatectomy. Tohoku J Exp Med. 2016;239:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Shimabukuro R, Kawanaka H, Tomikawa M, Akahoshi T, Konishi K, Yoshida D, Anegawa G, Uehara H, Hashimoto N, Hashizume M, Maehara Y. Effect of thrombopoietin on platelet counts and liver regeneration after partial hepatectomy in a rat model. Surg Today. 2009;39:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H, Ozaki M. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Frick J, Frobert A, Quintela Pousa AM, Balaphas A, Meyer J, Schäfer K, Giraud MN, Egger B, Bühler L, Gonelle-Gispert C. Evidence for platelet-derived transforming growth factor β1 as an early inducer of liver regeneration after hepatectomy in mice. FASEB J. 2024;38:e70039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |