TO THE EDITOR
Alcoholic liver disease (ALD), encompassing conditions such as simple steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma (HCC), is the leading cause of chronic liver disease globally. Despite significant advancements in ALD research, its pathogenesis remains poorly understood[1]. Over the past decade, a mixed lineage kinase domain-like protein (MLKL) has been recognized as a critical substrate in the RIPK3-mediated necroptotic pathway[2,3], with substantial evidences positioning it as a promising molecular target for both anti-inflammatory and anticancer therapies. Traditionally, MLKL-driven necroptosis depends on RIPK3-mediated phosphorylation, followed by membrane permeabilization[4]. However, recent findings suggest that MLKL also plays a vital role in non-necroptosis that may have distinct and equally important contributions to the progression of ALD[5]. These non-necroptotic functions, such as modulating inflammatory signaling and immune cell recruitment, may operate independently of canonical necrosome formation yet still promote hepatic injury through alternative pathways, including NF-κB activation. MLKL also promotes hepatic injury by activating NF-κB in a non-necroptotic manner, independent of necrosome formation. These non-necrotic mechanisms are particularly noteworthy because they broaden the therapeutic potential of MLKL beyond cell death inhibition. Targeting MLKL’s immunomodulatory functions could represent a promising strategy to mitigate inflammation-driven liver damage without disrupting the necroptosis-dependent tissue homeostasis. Such an approach would offer a more nuanced and potentially more effective therapeutic avenue for managing ALD.
Xuan Yuan et al[6] employed cell proliferation assays and flow cytometry to investigate the effects of necrosis-inducing stimuli in various cell types. They revealed that MLKL ATP-binding pocket inhibitor, CPD4, reduced the expression of immune factors such as CXCL2, ICAM, and VCAM through the NF-κB pathway, leading to influence the immune response in ALD[6]. These findings not only deepen our understanding of MLKL’s role in immune regulation but also suggest that the MLKL ATP-binding pocket as a promising target for future anti-inflammatory therapies. Whereas, certain aspects of the study could benefit from further clarification to enhance the robustness of the conclusions.
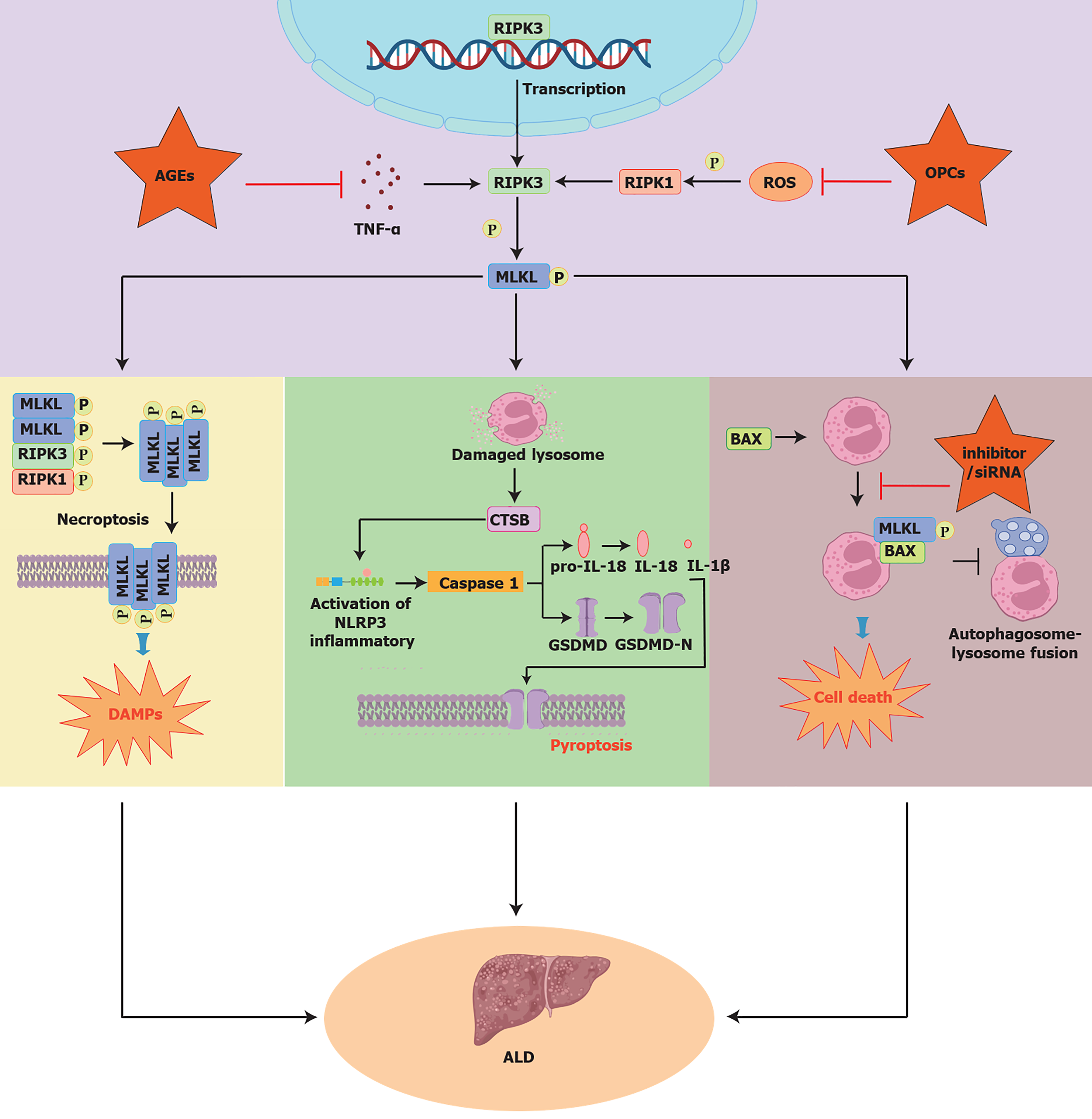
Xuan Yuan et al[6] demonstrated that MLKL influences ALD through its non-necroptotic apoptosis functions and NF-κB-mediated inflammation. However, previous studies suggest that MLKL’s role in ALD is more complex. For instance, AGE regulates MLKL as a downstream target of TLR4/NF-κB/MLKL-mediated necrosis pathway, promoting the progression of ALD[7]. In addition, OPCs inhibit cellular pyroptosis and mitigate acute alcoholic liver injury by disrupting the ROS-MLKL-CTSB-NLRP3 pathway[8]. Further studies showed that activated BAX binds to MLKL, facilitating its translocation to the lysosomal membrane and inducing LMP in response to alcohol-induced lipotoxicity. This process leads to hepatocyte death, inhibits autophagic flux, and exacerbates ALD[9] (Figure 1). These findings suggest that focusing solely on the NF-κB pathway overlooks the broader role of MLKL. Its involvement in multiple signaling pathways, such as necroptosis and inflammation is crucial to ALD pathogenesis. Therefore, targeting multiple MLKL-regulated pathways holds promise for advancing therapeutic strategies in ALD.
Figure 1 Mixed lineage kinase domain-like protein affects alcoholic liver disease through multiple signaling pathways.
Mixed lineage kinase domain-like protein (MLKL) modulates the molecular mechanisms of alcoholic liver disease (ALD) through multiple signaling pathways. From left to right: MLKL, regulated by appendicitis-derived AGEs, acts as a downstream target in the TLR4/NF-κB/MLKL necrosis pathway, driving ALD onset and progression[7]. Procyanidins (OPCs) attenuate acute alcohol-induced liver injury by inhibiting cell pyroptosis through the ROS-MLKL-CTSB-NLRP3 axis[8]. Additionally, activated BAX binds to MLKL, promoting its translocation to the lysosomal membrane and triggering LMP, which worsens alcohol-induced lipotoxicity, induces cell death, and disrupts autophagic flux, thus contributing to hepatocyte death in ALD[9]. ROS: Reactive oxygen species; ALD: Alcoholic liver disease; MLKL: Mixed lineage kinase domain-like protein.
As a chlorophyll-derived metabolite, CPD4 has been shown in multiple studies to possess dual biological effects: Broad-spectrum antiviral activity[10] and the ability to induce apoptosis in specific pathological conditions through modulation of programmed cell death pathways[11]. Notably, Xuan Yuan et al[6] highlighted CPD4’s potential as an MLKL ATP-binding pocket inhibitor to alleviate liver inflammation and improve liver function in ALD patients, positioning it as a novel therapeutic candidate targeting MLKL-mediated non-necrotic apoptosis pathways. Compared to other MLKL inhibitors, such as NSA (targeting the pseudo-kinase domain)[12], TC13172 (binding to the N-terminal coiled-coil domain)[12,13], and NBC1 (allosteric inhibitor of MLKL oligomerization)[14], CPD4’s clinical application faces several limitations. Firstly, its pharmacokinetics, including bioavailability, metabolism, and tissue distribution, remain insufficiently characterized. Secondly, critical toxicological data are currently lacking for CPD4, including dose-response relationships, long-term safety, and organ-specific toxicity. Thirdly, the selectivity of CPD4 in the complex disease microenvironment and its interaction with other cell death pathways require further investigation. Lastly, evidence for CPD4 as an inhibitor of MLKL ATP binding pocket is lacking. In future studies, more attention should be paid to the structural docking between CDP4 and MLKL to deepen our understanding of the role of CPD4 in targeting MLKL. At the same time, preclinical safety evaluation should be combined with adaptive clinical trial design to address these challenges and accelerate their translation to clinical application.
Recent studies have underscored MLKL’s role in non-necrotic apoptosis, particularly in liver diseases such as hepatitis[15,16], fibrosis[17], and HCC[18], highlighting the potential of targeting MLKL-mediated apoptosis as a therapeutic strategy. Future studies should focus on developing different inhibitors and exploring the synergistic effects of MLKL in combination therapies. The specificity of inhibitors for MLKL will be improved by structural biology in the future. In addition, molecular docking, cryoelectron microscopy, surface plasmon resonance and other techniques are essential to solve the off-target effects of existing MLKL inhibitors. Structural studies of MLKL's functional domains, such as the ATP-binding pocket and four-helix bundle, could guide the development of allosteric inhibitors or dual-functional molecules, enabling precise regulation of inflammation and cell death pathways. In conclusion, MLKL-targeted therapies, through interdisciplinary and translational research, may overcome the limitations of single-pathway treatments in ALD and provide personalized, multi-layered solutions for complex liver diseases.
Xuan Yuan et al[6] emphasized the role of MLKL in ALD, particularly its involvement in non-necrotic apoptosis and immune modulation via the NF-κB pathway. Targeting MLKL offers a potential therapeutic strategy for ALD by modulating inflammation and liver injury without inducing necroptosis, providing new perspectives for personalized treatments. Nonetheless, the complex regulation of MLKL-mediated pathways in ALD, along with the specificity of CPD4 in targeting the MLKL ATP-binding pocket, warrants further investigation. Future research should focus on the long-term effects of CPD4 across different pathological contexts and assess its potential toxicity. Additionally, a deeper understanding of MLKL’s regulatory mechanisms in ALD and related liver diseases, alongside the potential for combination inhibitors, is crucial for advancing clinical treatment strategies.